The Effect and Mechanism of Fructus lycii on lmprovement of Exercise Fatigue Using a Network Pharmacological Approach with in vitro Experimental Verification*
2024-02-26JIXiaoNingLIUZhaoPingZHANGChaoZhengCHENMinLIANGJiangLUJiangandZHANGLei
JI Xiao Ning, LIU Zhao Ping, ZHANG Chao Zheng, CHEN Min, LIANG Jiang,LU Jiang, and ZHANG Lei,#
1. National Resource Center for Chinese Materia Medica, China Academy of Chinese Medical Sciences, Beijing 100700, China; 2. China National Center for Food Safety Risk Assessment, Beijing 100022, China; 3. Chinese Center for Disease Control and Prevention, Beijing 102206, China
Abstract
Key words: Fructus lycii; Exercise fatigue; Network pharmacology
INTRODUCTION
Exercise-induced fatigue is a complex biological phenomenon that significantly impairs athletic performance and health.It is characterized by the inability to sustain a given level of physical activity or maintain a specific exercise intensity, resulting in sluggishness, poor coordination, insensitivity, memory impairment, and difficulty in concentrating.In addition, long-term fatigue has been associated with diseases, such as weakened immunity and cardiovascular diseases.The onset of fatigue is related to several factors,including energy depletion, accumulation of metabolites, oxidative stress, disruption of muscle calcium homeostasis, and central nervous system fatigue[1].The current research indicates that the major mechanisms for alleviating exercise-induced fatigue involve enhancing energy substrate availability through increased glycogen synthesis,clearing fatigue-causing metabolites, improving mitochondrial function, regulating calcium homeostasis, inhibiting inflammatory responses,regulating neurotransmitters, and maintaining muscle proteins[2].Several signal transduction pathways mainly MAPK, P13K-AKT, mTOR, AMPK,and NF-κB signaling pathways might be essential elements in the short- and long-term adaptations of the skeletal muscle to exercise-induced fatigue.The current study suggests that an aberrant MAPK signaling pathway impeding muscle regeneration may disrupt biochemical and metabolic processes in muscles, eventually leading to fatigue[3,4].Additionally, studies have also shown that activation of the PI3K/AKT signaling pathway and increased glucose uptake through the glucose transporter GLUT4 is an effective way to increase energy supply and utilization in muscles[5].Further research on the complex molecular events underlying fatigue will provide new insights into interventions and countermeasures to enhance exercise performance.
Exercise fatigue is inevitable for professional athletes engaged in competitive sports and individuals who engage in regular exercise.Delaying the onset of exercise fatigue is crucial for improving physical performance, optimizing training outcomes, and reducing the risk of sports injuries.Numerous sports supplements are employed to reduce exercise fatigue and enhance exercise capacity.However, some of these supplements may pose potential health risks,such as gastrointestinal distress caused by sodium bicarbonate.Therefore, it is essential to identify antifatigue components or formulations with definite efficacy and fewer adverse effects.Traditional medicines, foods, and naturally active components such asRadix Rehmanniae preparata,Ginseng, andAstragalus membranaceushave been used effectively as healthcare products to relieve exercise fatigue[6,7].The Homology of Medicine and Food, a special food category in China, can be used in both food and medicine.Compared to medicines, they possess more potent nutritional properties and are primarily used as tonics, which are characterized by a mild flavor and gentle nature.They are known to promote recovery,rehabilitation, and overall health.Fructus lycii(goji berry), a medicinal and food product, is usually preserved and consumed in its dried fruit form.Fructus lyciicontains multiple active components,including polysaccharides, carotenoids, flavonoids, and phenolics[8].Studies have confirmed thatFructus lyciihas significant effects on the regulation of immune function, apoptosis, blood lipids, and blood glucose, as well as its anti-tumors, anti-aging, and anti-fatty liver disease properties[9].Lately, researchers have begun to explore its potential to delay fatigue and enhance athletic performance[10].Fructus lyciiis a complex mixture containing multiple compounds that act on various targets; however, the active compounds and their mechanisms of action remain unclear.Network pharmacology, an emerging branch of pharmacology,provides a means to scrutinize the synergistic effects of multiple components and targets, particularly useful for investigating the potential mechanism of traditional Chinese medicine (TCM) in disease treatment[11].
We hypothesized that the presence of multiple active components ofFructus lyciiand their associated mechanisms may contribute to the alleviation of exercise fatigue.Therefore, the main purpose of this study was to explore the complex interplay betweenFructus lyciiand exercise fatigue using network pharmacology.Subsequently,in vitroassays were performed on C2C12 cells to validate the effects and mechanisms of the representative components ofFructus lyciipredicted by network analysis to improve exercise fatigue.
METHODS
Method of Network Pharmacology
Identification of the Compound and Targets of Fructus lyciiThe Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP) was used to identify the major chemical components and their potential targets(accessed November 22, 2022).Relevant active compounds were screened for oral bioavailability(OB) ≥ 30%[12]and drug like index (DL) ≥ 0.18 of component[13].In this study, the predicted targets of the main components ofFructus lyciiwere retrieved from TCMSP.The target protein was mapped to its corresponding gene name using the UniProt human database.
Collection of Targets Associated with Exercise FatigueThe corresponding targets of disease related to fatigue were retrieved from the Therapeutic Target Database (TTD), Online Mendelian Inheritance in Man (OMIM), Drug bank and Gene card using the exercise fatigue-related search terms including “exercise fatigue” “physical fatigue” “fatigue” and “exercise capacity” (accessed on November 22, 2022).
The intersection targets of compounds and disease were considered potential targets ofFructus lyciiin the treatment of exercise fatigue, as represented by the Venn diagram in Supplementary Figure S1, available in www.besjournal.com.
Functional Enrichment and Pathway AnalysisThe intersection targets of the main components ofFructus lyciiand exercise fatigue were imported into the Metascape database (http://metascape.org/gp/index.html).The Gene Ontology (GO)enrichment analysis included three aspects:biological process (BP), molecular function (MF), and cellular component (CC) (accessed November 28,2022).Kyoto Encyclopedia of Genes and Genomes(KEGG) pathway enrichment analysis was used for target pathway enrichment (accessed November 28,2022).GO enrichment and KEGG pathway analyses were carried out withP< 0.05 as the screening condition.Finally, the top 10 GO terms and the top 20 KEGG terms were screened with the lowestPvalue and represented as visual graphs using R 4.1.1 software or Bioinformatics (http://www.bioinformatics.com.cn).Construction of NetworkThe “Network Analyzer”function of Cytoscape 3.8.2 was used to calculate the network parameters of each node, protein, gene,active component, or pathway.The effective active compounds and their corresponding intersection targets of exercise fatigue were imported into Cystoscope 3.8.2, to construct an active compoundtarget network.The intersection targets, effective active compounds, and KEGG pathway enrichment in the Metascape database (P< 0.05) were imported into Cystoscope 3.8.2, to construct an active compound-targets-pathways network.The “degree”value represents the number of one node associated with the other nodes in the network.The larger the degree value, the more important the node is in the network, and the more likely it is to become a core node.
Experimental Verification
Cell CultureSkeletal muscle C2C12 cells(Pythonbio) were cultured in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% FBS(Gibco) and 1% penicillin-streptomycin (Gibco).At 90% confluence, the medium was changed to DMEM plus 2% horse serum (Gibco) and 1% penicillinstreptomycin for 6 days to induce C2C12 cells to differentiate into myotube cells, two days after the medium switch.The cells were cultured at 37 °C in a controlled humidified 5% CO2atmosphere.
Cell ViabilityC2C12 myotubes were seeded in 96-well plates at a density of 10,000 cells per well and incubated overnight.The cells were treated with various concentrations (7.8125–1,000 µmol/L) of quercetin for 24 h.Cell proliferation was measured using the Cell Counting Kit-8 (CCK-8) assay (Dojindo Molecular Technologies, Kumamoto, Japan) by adding 10 µL CCK-8 reagent to each well after incubating at 37 °C for 1 h.Optical density (OD) was measured at 450 nm using a plate reader (BioTek,Synergy 4).
Glucose Uptake AssayC2C12 myotubes were washed with phosphate buffer (PBS) and incubated in serum-free DMEM (Gibco) containing FBS for 4 h.After fasting, the cells were treated with 2-(N-(7-Nitrobenz-2-oxa-1,3-diazol-4-yl)Amino)-2-Deoxyglucose(2-NBDG) (ThermoFisher Science) at a concentration of 400 µm at 37 °C for 30 min[14].The cells were washed twice with PBS and counterstained with Hoechst 33,342 for nucleic acid staining.The mean stained area was analyzed using Image Xpress software (Molecular Devices, San Jose, CA, USA).
Reactive Oxygen Species(ROS)MeasurementAfter treatment, C2C12 myotubes were cultured with 100 µmol/L tert-butyl hydroperoxide (TBHP) as an ROS inducer for 30 min before labeling with CellRox Green reagent (ThermoFisher Science)[14].Then, the cells were stained with CellRox Green reagent for 30 min at 37 °C.The subsequent procedure was similar to that described previously.
Mitochondrial Membrane Potential Measurement and Mitochondrial MassMitochondrial membrane potential and mitochondrial mass were measured using the Image-iT™ TMRM reagent (ThermoFisher Scientific) and MitoTracker™ Green FM (Thermo Fisher Scientific), respectively, according to the manufacturer’s instructions[15].Cells were washed twice with PBS, then loaded with TMRM staining or MitoTracker and Hoechst 33342 at 37 °C avoiding light for 30 min.The subsequent procedure was similar to that described previously.
Validation of Key ProteinsC2C12 cells were fixed with fixative buffer (eBioscienceTMFixation: Fixation/Permeabilization = 1:3) from the eBioscience™ Foxp3(ThermoFisher Science) at 18–22 °C for 30 min.The cells were then washed twice with the permeabilization buffer.The cells were then incubated with the following primary antibodies:p-p38 MAPK (1:200, CST), p-MAPK (Erk1/2, 1:200,CST), p-PI3K (1:200, Affinity), p-AKT (1:400, CST), and p-JNK (1:200, CST) at 18–22 °C for 30 min.After washing twice with PBS, the cells were probed with the following secondary antibodies: Antimouse IgG or Anti-rabbit IgG (1:1,000, CST) for 1 h at 18–22 °C.After washing twice, the cells were stained with Hoechst 33,342 for 5 min, and the mean stained area was assessed using Image Xpress software[16].
Statistical Analysis
The results are expressed as mean and standard deviation (SD).Statistical analyses were performed using GraphPad Prism 7.0 (GraphPad Software Inc.,San Diego, CA, USA).Differences were analyzed using one-way analysis of variance (ANOVA),followed by Fisher’s least significant difference (LSD)multiple comparisons.Statistical significance was considered atP-values less than 0.05.
RESULT
Result of Network Pharmacology
Compound and Targets of Fructus lyciiA total of 188 chemical components ofFructus lyciiwere identified, of which 45 had OB > 30% and DL > 0.18.After eliminating the duplicate targets, 45 components had 188 potential targets.The results are presented in Supplementary Table S1, available in www.besjournal.com.
Targets Associated with Exercise FatigueThere were 6,490 potential targets associated with exercise fatigue in the TTD, OMIM, Drug Bank, and Gene Card databases.
Compound and Targets Associated with Exercise FatigueBy considering the intersection targets ofFructus lyciicomponents associated with exercise fatigue, a total of 144 targets were obtained, as shown in Supplementary Figure S1 and Supplementary Table S2 (available in www.besjournal.com).
Construction of Compound-Targets NetworkThe 144 potential targets of the components ofFructus lyciiassociated with exercise fatigue were imported into Cystoscope 3.8.2 to construct an active compound-targets network, as shown in Figure 1.There were 174 nodes, including 30 nodes for chemical components, 144 nodes for targets, and 530 edges for interactions between nodes.Quercetin (degree = 110), β-sitosterol (degree = 29),stigmasterol (degree = 23), 7-O-methylluteolin-6-Cbeta-glucoside_qt (degree = 21), atropine (degree =19), and glycitein (degree = 15) ofFructus lyciiwere identified as the main active components with an anti-exercise fatigue effect.Quercetin was selected as the representative component for further studies.
GO and KEGG Enrichment AnalysisThe Mediascape database was utilized to analyze 144 key targets, and the results of the GO enrichment analysis were divided into three categories: BP, CC, and MF, as shown in Figure 2A.These targets are highly related to cellular components, including the synaptic membrane.Responses to nitrogen compounds,hormones, lipids, and xenobiotic stimuli are primarily involved in biological processes.In addition, G protein-coupled amine receptor activity,transcription co-regulator binding, DNA-binding transcription factor binding, nuclear receptor activity, and ligand-activated transcription factor activity of the molecular function were closely related to these targets.
A total of 198 pathways withP< 0.05 were identified, and the top 20 pathways are illustrated as enriched dot bubbles in Figure 2B.The top three pathways were hsa05200 (degree = 50), hsa05417(degree = 36), and hsa04933 (degree = 27), all of which were highly associated with cancer-,inflammation-, immune-, and apoptosis-related pathways, as revealed by enrichment analysis.
ConstructionofCompound-Targets-Pathways NetworkTo explain the pharmacological mechanism of anti-exercise fatigue inFructus lyciiin detail, a compound-target-pathway network was constructed.As shown in Figure 3 and Supplementary Tables S3–S4 (available in www.besjournal.com), there were 198 pathways related to 136 of the 144 key targets and eight targets without pathways.The average degrees of the targets, pathways, and components were 17.35,12.28, and 8.83, respectively.Based on the double median degree (degree ≥ 18), 39 targets were selected.Targets with the highest degrees were MAPK1 (117), AKT1 (98), RAF1 (84), RELA (77), TNF(68), PRKCA (66), MAPK14 (62), JUN (57), CHUK (54),IL6 (52), and BAX (52).The top signaling pathways included hsa05200 (pathways in cancer), hsa04933(AGE-RAGE signaling pathway in diabetic complications, degree = 28), hsa04151 (PI3K-Akt signaling pathway, degree = 27), hsa04080(neuroactive ligand-receptor interaction pathway,degree = 25), hsa04657 (IL-17 signaling pathway,degree = 24), hsa04668 (TNF signaling pathway,degree = 23), hsa05022 (pathways of neurodegeneration - multiple diseases) and hsa04010 (MAPK signaling pathway, degree = 23).Among these, the PI3K-Akt and MAPK signaling pathways are closely related to exercise fatigue[17].Moreover, most of the core target proteins identified by network pharmacology analysis were associated with the PI3K-Akt and MAPK signaling pathways.Therefore, in the present study, we first focused on the PI3K-Akt and MAPK signaling pathways.
Results of Experimental Verification
Cell ViabilityUpon treating C2C12 cells with or without quercetin for 24 h, we observed that quercetin reduced cell viability in a dose-dependent manner, as shown by the CCK-8 assay(Supplementary Figure S2, available in www.besjournal.com).Quercetin at 125 µmol/L reduced the viability of C2C12 cells to approximately 80%.The viability of cells treated with 453.5 µmol/L was 50%, which was defined as inhibitory concentration 50 (IC50) of quercetin.We chose a concentration that was around 1/10 of the IC50 as the highest concentration of quercetin, 50 µmol/L.This concentration exhibited no discernible toxicity and was also consistent with commonly used concentration of quercetin in other related experiments.
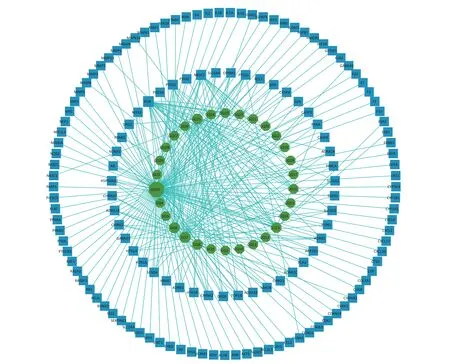
Figure 1.Compound-targets network.GQ1, Sitosterol alpha1; GQ2, Cycloartenol; GQ3, Mandenol; GQ4,Ethyl linolenate; GQ5, LAN; GQ6, Stigmasterol; GQ7, Beta-sitosterol; GQ8, (-)-Hyoscyamine; GQ9,Campesterol; GQ10, Cyanin; GQ11, 24-methylidenelophenol; GQ12, Daucosterol qt; GQ13, Glycitein;GQ14, CLR; GQ15, 14b-pregnane; GQ16, 24-ethylcholesta-5,22-dienol; GQ17, Fucosterol; GQ18, 31-norlanosterol; GQ19, 4,24-methyllophenol; GQ20, Lophenol; GGQ21, 4alpha,14alpha,24-trimethylcholesta-8,24-dienol; GQ22, 4alpha,24-dimethylcholesta-7,24-dienol; GQ23, 4alpha-methyl-24-ethylcholesta-7,24-dienol; GQ24, 6-Fluoroindole-7-Dehydrocholesterol; GQ25, 7-O-methylluteolin-6-Cbeta-glucoside_qt; GQ26, Atropine; GQ27, Physcion-8-O-beta-D-gentiobioside; GQ28, Lanost-8-en-3betaol; GQ29, Obtusifoliol; GQ30, Quercetin.

Figure 2.Enrichment analysis.(A) GO enrichment analysis; (B) KEGG enrichment analysis.BP, biological process; CC, cellular component; MF, molecular function.P < 0.05.
Glucose UptakeTo assess the effect of quercetin treatment on glucose uptake, C2C12 myotubes were treated with different concentrations of quercetin(50, 25, 12.5, 6.25 µmol/L) and then exposed to 2-NBDG, a fluorescently labeled glucose analog.Compared to the control, the glucose uptake of C2C12 myotubes was significantly increased by quercetin (P< 0.01), as shown in Figure 4A.

Figure 3.Compound-targets-pathways network.GQ1, Sitosterol alpha1; GQ2, Cycloartenol; GQ3,Mandenol; GQ4, Ethyl linolenate; GQ5, LAN; GQ6, Stigmasterol; GQ7, Beta-sitosterol; GQ8, (-)-Hyoscyamine; GQ9, Campesterol; GQ10, Cyanin; GQ11, 24-methylidenelophenol; GQ12, Daucosterol_qt;GQ13, Glycitein; GQ14, CLR; GQ15, 14b-pregnane; GQ16, 24-ethylcholesta-5,22-dienol; GQ17, Fucosterol;GQ18, 31-norlanosterol; GQ19, 4,24-methyllophenol; GQ20, Lophenol; GGQ21, 4alpha,14alpha,24-trimethylcholesta-8,24-dienol; GQ22, 4alpha,24-dimethylcholesta-7,24-dienol; GQ23, 4alpha-methyl-24-ethylcholesta-7,24-dienol; GQ24, 6-Fluoroindole-7-Dehydrocholesterol; GQ25, 7-O-Methylluteolin-6-Cbeta-glucoside_qt; GQ26, Atropine; GQ27, Physcion-8-O-beta-D-gentiobioside; GQ28, Lanost-8-en-3betaol; GQ29, Obtusifoliol; GQ30, Quercetin.
ROS GenerationThe effects of quercetin on ROS production were analyzed in TBHP-treated C2C12 myotube cells.Quercetin significantly reduced ROS production in a concentration-dependent manner(P< 0.01; Figure 4B).
Mitochondria FunctionMitochondrial biogenesis is intricately linked to multiple physiological processes and is a crucial factor influencing skeletal muscle function[18].In our study, mitochondrial mass and membrane potential were evaluated using MitoTracker and TMRM, respectively.The results showed that quercetin significantly increased the mitochondrial membrane potential and the mean stained area of TMRM-and Mito Tracker-stained mitochondria in a concentration-dependent manner(Figure 4C and Figure 4D).
Activation of the MAPK and PI3K-Akt Signaling PathwaysThe expression of key targets in the MAPK (p-38 MAPK, p-MAPK, and p-JNK) and PI3K-Akt(p-PI3K and p-AKT) signaling pathways was analyzed based on the results of network pharmacology.The immunofluorescence results (Figure 5A) revealed increased protein expression of p-P38 MAPK, p-MAPK, and p-JNK in the quercetin group compared to the control group.Moreover, the protein levels of p-PI3K and p-AKT markedly increased after quercetin treatment (Figure 5B).
DISCUSSION
A meta-analysis was conducted to investigate the effect ofFructus lyciion exercise fatigue.The findings indicated thatFructus lyciiextended the duration of exhaustive exercise and stored muscle and liver glycogen reserves while decreasing the levels of BUN and lactic acid (Supplementary Tables S6–S7 and Figure S3–S4, available in www.besjournal.com).These indicators are important for evaluating exercise fatigue[19,20], suggesting thatFructus lyciimight have the potential to improve exercise performance.To further investigate the mechanism and identify the primary components responsible for this effect, we conducted a network pharmacology analysis.Six potential active components inFructus lyciias predicted by network pharmacology,emerged, with quercetin exhibiting the highest degree of influence.Other key components included β-sitosterol, stigmasterol, 7-O-methylluteolin-6-Cbeta-glucoside_qt, atropine, and glycitein.Multiple pathways have been identified to be related to antiexercise fatigue, including the AGE-RAGE signaling pathway in diabetic complications, PI3K-Akt signaling pathway, neuroactive ligand-receptor interaction pathway, IL-17 signaling pathway, TNF signaling pathway, and MAPK signaling pathway.Furthermore, quercetin, one of the main active components ofFructus lycii[21], and its two signaling pathways (MAPK and PI3K-Akt) closely related to exercise fatigue were investigated in anin vitrostudy to clarify its function and mechanism.

Figure 5.Protein expression of MAPK and PI3K-AKT signaling pathways.(A) MAPK signaling pathways, the protein expression of p-P38 MAPK, p-MAPK, and p-JNK was detected by a high content imaging analysis system; (B) PI3K-AKT signaling pathway, p-PI3K and p-AKT was detected by a high content imaging analysis system; Left panel: representative images.Data presented were from three biological replicates;Scale bar size: 50 µm; *P < 0.05; **P < 0.01.
Quercetin, a flavonoid, possesses unique biological properties that hold potential for improving mental and physical performance, as well as conferring benefits to overall health and disease resistance.It exhibits anti-inflammatory, antiviral,antioxidant, anticarcinogenic, and psychostimulant activities[22].Several studies have shown that quercetin inhibits LPS-induced mRNA expression of TNF-α, IL-8 and IL-1α[23].In addition, quercetin is widely used as a nutritional supplement and phytochemical therapy for the treatment of various diseases, such as diabetes, obesity, and cardiovascular diseases[24].Recent studies have reported that dietary quercetin supplementation can ameliorate exercise fatigue and enhance performance[25]; however, the underlying mechanism is still unclear.Our study demonstrated that quercetin significantly increased glucose uptake,enhanced mitochondrial function, and decreased ROS levels.Increasing the capacity for glucose uptake and mitochondrial energy production has been suggested as an effective approach to delay exercise fatigue[26,27].The skeletal muscles play an important role in exercise and energy balance.Recently, increasing evidence has revealed that mitochondria are essential for maintaining energy homeostasis, which is highly correlated with muscle function and energy production, resulting in enhanced exercise performance[28,29].Excessive ROS production during exercise can impair mitochondrial and nuclear DNA, and induce mitochondrial dysfunction, which appears to be a key issue during exhaustive exercise.Similar to our findings,quercetin improves mitochondrial regeneration,enhances energy metabolism, and improves exercise endurance by regulating mitochondrial gene expression in mouse muscles[30].In addition, glucose is initially phosphorylated by hexokinase, which traps glucose intracellularly in the skeletal muscle and is then utilized to produce energy[31].Quercetin may facilitate glucose uptake to exert beneficial effects on maintaining glucose homeostasis.This glucose can be stored as glycogen or utilized to produce energy during exercise.Notably, research has underscored quercetin’s role in elevating insulinstimulated glucose uptake and coupled with an improved replenishment function, which is conducive to the rapid restoration of muscle glycogen.This synergistic effect serves to improve time-to-exhaustion during exercise[32].
Previous reports have indicated that the MAPK and PI3K-Akt signaling pathways play crucial roles in various cellular processes, including cell survival,proliferation, differentiation, motility, and apoptosis.Ourin vitroexperiments revealed that quercetin exerts its biological functions by regulating the MAPK and PI3K-Akt signaling pathways.The MAPK signaling pathway regulates energy metabolism by enhancing mitochondrial function and glucose uptake[33,34].The glucose transporter GLUT4 is critical for skeletal muscle glucose uptake and the maintenance of whole-body glucose homeostasis.Similarly, black rice extracts were found to stimulate GLUT4 glucose uptake through the activation of PI3K-Akt and MAPK signaling in C2C12 myotubes[5].Previous studies have shown that activation of the PI3K-Akt and MAPK signaling pathways may help reduce oxidative damage[35,36].Consistent with our results,ginsenoside Rb1 has also been shown to alleviate fatigue syndrome by reducing skeletal muscle oxidative stress through the activation of the PI3KAkt pathway[37].Adaptation and maintenance of the oxidative-antioxidant balance depend heavily on the MAPK signaling pathway.Moreover, the MAPK signaling pathway contributes to antioxidant activity by regulating the levels of SOD, which plays a critical role in anti-fatigue effects[38].Overall, our findings suggest that quercetin exerts its effectsviathe PI3KAkt and MAPK signaling pathways.
It is also noteworthy thatFructus lyciiregulates the inflammatory pathways that are involved in exercise fatigue.Exercise fatigue is often accompanied by increased levels of proinflammatory cytokines[39].It has also been suggested that cytokine release may result in prolonged sickness symptoms or behavioral stillness,which can sometimes be observed in cases of overtraining syndrome in athletes, as well as in a variety of systemic immune and inflammatory conditions.For example, TNF-α, IL-1β, and IL-6 also increased as a result of muscle fatigue[40].Additionally, post-exercise mobilization of Tlymphocytes, particularly CD4+ and CD8+lymphocytes, from peripheral lymphoid compartments into the blood has been observed[41].The IL-17, TNF, and MAPK signaling pathways are the main pathway-linked inflammatory responses, as shown in our study[42].Studies have suggested that regulating the expression of pro-inflammatory and anti-inflammatory cytokines by regulating signaling pathways involved in anti-inflammatory was an effective way to relieve exercise fatigue[2].Hence,future research endeavors should focus on extensively investigatingFructus lycii’spotential in combating to exercise fatigue.
Our study lays the groundwork for potential avenues of deeper exploration into the mechanisms underlying the impacts ofFructus lyciion exercise fatigue.Nonetheless, there exist certain limitations.First, we focused onFructus lyciiand its energy metabolism and antioxidant mechanisms.Moving forward, it is imperative to pay continuous attention to other active ingredients and mechanisms,especially the functions of the inflammatory pathways identified in our study.Second, although we used immunofluorescence staining combined with high-content imaging to display the phosphorylation levels of key target proteins associated with the identified signaling pathways,western blotting can also be used as an auxiliary method to verify the expression and phosphorylation levels of key target proteins.In addition, the mRNA expression should be measured during follow-up.Finally, the study used cultured cells as a model, which does not fully representin vivophysiological conditions.Therefore,in vivostudies are needed to confirm the anti-fatigue activity ofFructus lyciiand its components.
In summary, we conducted a multi-component,multi-target, and multi-pathway exploration of the anti-exercise-fatigue effects ofFructus lycii.The main active components and possible molecular mechanisms underlying the anti-exercise-fatigue activity ofFructus lyciishould be further investigated.Quercetin, a crucial active component inFructus lycii, was shown to enhance energy metabolism and reduce oxidative stress, thereby delaying exercise fatigue.The PI3K-Akt and MAPK signaling pathways might be involved in this process.These results pave the way for subsequent research endeavors aimed at deeper explorations of the mechanisms underlying the effects ofFructus lyciion exercise fatigue.
Fructus lyciisignificantly alleviated exercise fatigue.Six potential active components, including quercetin,sitosterol, stigmasterol, 7-O-methylluteolin-6-C-betaglucoside_qt, atropine, and glycitein, may contribute to the improvement of exercise fatigueviamultiple pathways, including the PI3K-Akt, neuroactive ligandreceptor interaction, IL-17, TNF, and MAPK signaling pathways.Quercetin may play important roles in reducing oxidative stress, boosting glucose uptake,and enhancing mitochondrial function.The underlying mechanism may involve the PI3K-AKT and MAPK signaling pathways.
CONTRIBUTIONS
JI Xiao Ning: methodology, formal analysis,validation, investigation, and writing - original draft;LIU Zhao Ping: conceptualization, methodology,writing - review and editing; ZHANG Chao Zheng:formal analysis, investigation, data curation, writing -review and editing; CHEN Min: conceptualization,investigation, writing - review and editing; LIANG Jiang: conceptualization, writing - review and editing.ZHANG Lei: conceptualization, resources, writing -review and editing; LU Jiang: conceptualization,supervision, writing - review and editing, and project administration.All authors have approved the final version of the manuscript submitted for publication.
CONFLICTS OF INTEREST
There are no conflicts to declare.
Received: June 7, 2023;
Accepted: August 11, 2023
杂志排行
Biomedical and Environmental Sciences的其它文章
- Correlation between Combined Urinary Metal Exposure and Grip Strength under Three Statistical Models: A Cross sectional Study in Rural Guangxi*
- Effects of Bisphenol A and lts Substitute, Bisphenol F, on the Gut Microbiota in Mice*
- The Uptake and Distribution Evidence of Nano- and Microplastics in vivo after a Single High Dose of Oral Exposure*
- Quercetin Alleviates Lipopolysaccharide-lnduced Cardiac lnflammation via lnhibiting Autophagy and Programmed Cell Death*
- Exosome-Transmitted miR-224-5p Promotes Colorectal Cancer Cell Proliferation via Targeting ULK2 in p53-Dependent Manner*
- Risk Factors of Depression Screened by Two-Sample Mendelian Randomization Analysis: A Systematic Review*
