A systematic scoping review of study methodology for randomized controlled trials investigating probiotics in athletic and physically active populations
2024-01-25AlexMohrDviPyneGeovnSilvFogLeiteDeorhAkinsJmiePugh
Alex E.Mohr,Dvi B.Pyne,Geovn Silv Fog¸ Leite,Deorh Akins,Jmie Pugh
a College of Health Solutions,Arizona State University,Phoenix,AZ 85004,USA
b Research Institute for Sport and Exercise,University of Canberra,Canberra,ACT 2617,Australia
c Laboratory of Functional Fermented Food,Faculty of Pharmaceutical Sciences,University of São Paulo,São Paulo 05508-030,Brazil
d Research Institute for Sport and Exercise Sciences,Liverpool John Moores University,Liverpool L3 3AF,UK
Abstract Background:The purported ergogenic and health effects of probiotics have been a topic of great intrigue among researchers,practitioners,and the lay public alike.There has also been an increased research focus within the realm of sports science and exercise medicine on the athletic gut microbiota.However,compared to other ergogenic aids and dietary supplements,probiotics present unique study challenges.The objectives of this systematic scoping review were to identify and characterize study methodologies of randomized controlled trials investigating supplementation with probiotics in athletes and physically active individuals.Methods: Four databases (MEDLINE,CINAHL,Cochrane CENTRAL,and Cochrane Database of Systematic Reviews) were searched for randomized controlled studies involving healthy athletes or physically active individuals.An intervention with probiotics and inclusion of a control and/or placebo group were essential.Only peer-reviewed articles in English were considered,and there were no date restrictions.Results were extracted and presented in tabular form to detail study protocols,characteristics,and outcomes.Bias in randomized controlled trials was determined with the RoB 2.0 tool.Results:A total of 45 studies were included in the review,with 35 using a parallel group design and 10 using a cross-over design.Approximately half the studies used a single probiotic and the other half a multi-strain preparation.The probiotic dose ranged from 2£108 to 1£1011 colony forming units daily,and the length of intervention was between 7 and 150 days.Fewer than half the studies directly assessed gastrointestinal symptoms,gut permeability,or the gut microbiota.The sex ratio of participants was heavily weighted toward males,and only 3 studies exclusively investigated females.Low-level adverse events were reported in only 2 studies,although the methodology of reporting varied widely.The risk of bias was generally low,although details on randomization were lacking in some studies.Conclusion:There is a substantial body of research on the effects of probiotic supplementation in healthy athletes and physically active individuals.Considerable heterogeneity in probiotic selection and dosage as well as outcome measures has made clinical and mechanistic interpretation challenging for both health care practitioners and researchers.Attention to issues of randomization of participants,treatments and interventions,selection of outcomes,demographics,and reporting of adverse events will facilitate more trustworthy interpretation of probiotic study results and inform evidence-based guidelines.
Keywords: Exercise;Experimental methodology;Gastrointestinal symptoms;Gut microbiota;Probiotic supplementation
1.Introduction
Probiotics are defined by the International Scientific Association for Probiotics and Prebiotics as“living microorganisms that when administered in adequate amounts confer a health benefit on the host”.1As a dietary supplement,probiotics have captured the attention of the athletic and exercise communities as a potential agent for promoting ergogenic and health attributes.2Study outcomes have included various indices of health and performance,such as exercise-to-exhaustion time,3duration and severity of upper respiratory tract infections(URTIs),4and incidence or prevalence of gastrointestinal (GI) symptoms.5More recently,investigations in physically active individuals have examined the ability of probiotics in tandem with nutrients,such as plant protein6and iron,7to enhance digestion and absorption.Regardless of the outcomes,randomized controlled trials(RCTs)have been employed widely for probiotic investigations in athletic and physically active populations,and the results have been used to form current evidence-based guidelines.2
In contrast to other commonly used dietary supplements,probiotics offer unique study and data-synthesis (e.g.,metaanalyses) challenges since they are live and highly diverse microorganisms.As more strains are identified and commercialized,researchers,clinicians,and practitioners alike are presented with an array of taxonomically distinct preparations with differing functions and indications.Although probiotics may have some shared functions,different strains possess unique transcriptomes,which have different mechanisms of action and possibly different physiological and clinical effects.8Moreover,these preparations interact with highly complex systems,including the gut microbiota(GM)and gutassociated lymphoid tissue,which may be different in physically active compared to secondary individuals.9Furthermore,probiotic preparations often vary substantially in dosage and may contain a single bacterial strain or multiple strains from different species.Thus,probiotic research has important experimental nuance,and investigations should employ sound research methodology to reflect this reality.
To synthesize the current body of evidence on probiotics,several systematic reviews10-13and meta-analyses14,15have been published recently.Important questions have arisen from these works:Were the included RCTs similar enough to allow for accurate comparison across studies? That is,was a full description of the probiotic strain,dose,and viability(e.g.,live counts of the probiotic independently verified at the start and end of the intervention) provided for each study? How were important aspects of the research design constructed,implemented,and reported across studies,such as the control and proper description of confounding medications and dietary supplements? Answers to these questions are critical for planning and conducting future RCTs and other experimental research,for synthesizing the literature and developing best-practice guidelines,and,ultimately,for translating outcomes to clinical implementation.Therefore,to better map key study elements,provide evidence to inform study practice,and assess the rigor of research in this area,we conducted a systematic scoping review of RCTs investigating the methodology of probiotic supplementation in athletic and physically active populations.A systematic scoping review is appropriate for identifying knowledge gaps and clarifying concepts and methodological approaches as a precursor to a traditional systematic review.16We employed a review framework that has been previously established.17Specifically,we sought to better characterize gaps in research conduct and methodology and to develop a set of best practice guidelines for conducting studies of probiotics in athletes and physically active populations.
2.Methods
This scoping review was conducted according to the requirements of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews.18The protocol was registered with the Open Science Framework on December 14,2021 (osf.io/gfrs4).Articles eligible for inclusion and evaluation in this review were required to meet the pre-established population,intervention,comparator,outcome,and study design (PICOS) criteria (Supplementary Table 1).
2.1.Eligibility criteria
In brief,inclusion of RCTs on probiotics and exercise was limited to (a) studies involving healthy humans defined as athletes or those who are physically active,(b) interventions with probiotics,(c) inclusion of a control and/or placebo group,(d) outcomes not previously defined per the methodology-based nature of this review (as an open question;all outcomes evaluated by included studies were reported),and(e)RCTs with no date restrictions.Due to resource constraints,only peer-reviewed articles in English were considered.
2.2.Search strategy
The literature search was performed on June 29,2022 using the electronic databases MEDLINE(Ovid),CINAHL(Ebsco),Cochrane CENTRAL (Ovid),and Cochrane Database of Systematic Reviews (Ovid) to capture as many relevant articles as possible (for the full search strategy,see Supplementary Table 2).References of relevant systematic reviews were hand searched to identify any potentially useful studies that may have been missed by the database searches.
Articles captured from the database searches were then uploaded into Rayyan,19a software program for title/abstract screening,and reviewed independently by 3 of the study authors(AEM,DA,and JP).The full text of included articles was then screened and once again independently reviewed (AEM,DA,and JP).Any discrepancies were discussed and resolved to reach consensus.Data from articles included in the full text review were extracted to a standardized template (Supplementary Table 3) developed from a study framework described previously.17Specifically,a data-charting form was jointly developed by 3 reviewers(AEM,GSFL,and JP)to determine which study characteristics and methodology components to extract.
2.3.Data-charting process
Through an iterative process,data were independently charted,results were discussed,and the data-charting form was updated continuously.Relevant data included bibliographic information,sample characteristics (e.g.,age,sex,race/ethnicity),intervention description (e.g.,intervention duration and follow-up duration),study methodology,comparator description,outcomes reported,drop-out rate,and adverse events.Given all included articles were RCTs,risk of bias was assessed with the RoB 2.0 tool (https://sites.google.com/site/riskofbiastool/welcome/rob-2-0-tool?authuser=0&pli=1),which comprised questions aimed at assessing the potential bias from the randomization process,deviations from the intended intervention,missing outcome data,measurement of outcomes,and selection of reported results.20Articles were assessed independently by 2 reviewers (AEM and DA),with discrepancies settled by discussion.This assessment was reported using the robvis data visualization tool.21Given this is a scoping review,study design and methodology elements extracted from the included articles were synthesized narratively.
3.Results
3.1.Study selection
A total of 45 articles were included in the scoping review(Fig.1).4-7,22-62The initial search yielded 590 records from MEDLINE,CINAHL,and the 2 Cochrane databases.An additional 5 records were identified through alternative methods(hand selection) and added to the pool for screening consideration.After removing duplicate records,386 unique records were screened for eligibility by title and abstract.Of these,318 were excluded as they did not meet the defined inclusion criteria.Major reasons for exclusion included not being an RCT,study participants from the wrong population,use of animal/in vitromodels,or the article was a review.In addition,1 article was not able to be retrieved.Next,the full texts of the remaining 67 articles were retrieved and screened,leaving 45 articles for inclusion.
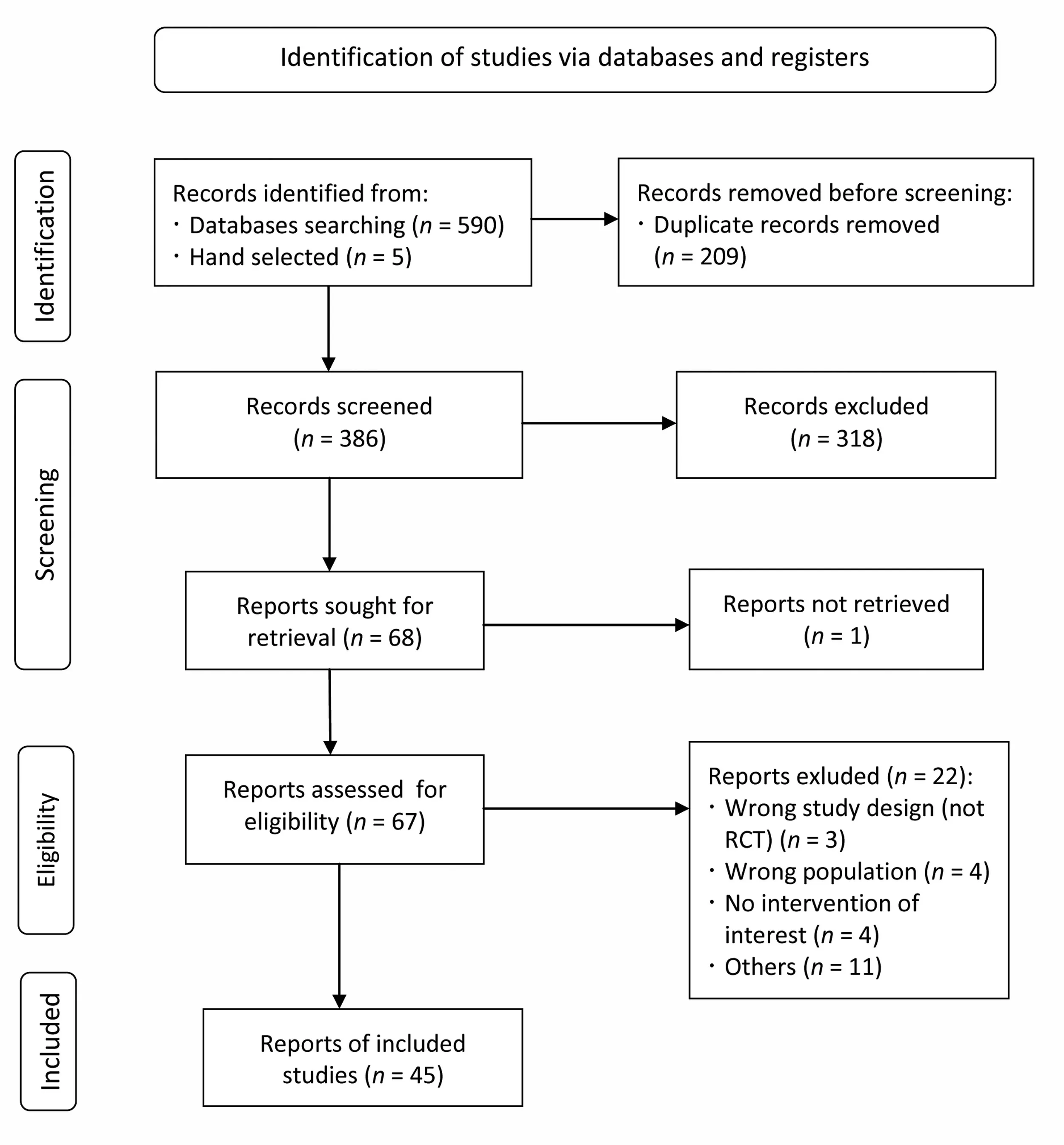
Fig.1.Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram of the literature search and filtering of results for a scoping review examining study methodology of randomized controlled trials with probiotics in athletic and physically active populations.RCT=randomized controlled trial.
3.2.Study characteristics
Individual study characteristics,including the key data elements of probiotic intervention,study design,participant population,outcomes,and safety data,are outlined in Supplementary Table 4.Briefly,35 studies used a parallel group design,5,7,22,23,25-32,35-37,41-47,49-53,55-62whereas 10 used a crossover design.4,6,24,33,34,38-40,48,54The studies were conducted in Finland,22,23,30Australia,24,27,32,34-36,49,57the UK,5,25,28,38,39,43,54,58Italy,26Austria,29,42Iran,31,37New Zealand,33the USA,6,40,47,48,56Serbia,41,44Taiwan,China,45,53Malaysia,46,51,59Brazil,50,52,61Sweden,7Poland,55India,62and Israel.60The majority of included RCTs (27 studies in total)used probiotic preparations in their study arms with a single strain from the generaLactobacillus,4,7,22-25,27,28,30,38,39,41,44,45,48,50,51,59Bifidobacterium,32,35,36,53Bacillus,47,56,62orSaccharomyces.49,57A total of 23 studies implemented multistrain preparations,ranging from 2 to 8 probiotic strains.5,6,26,29,31-37,40,42,43,46,49,52,54,55,57,58,60,61Only 5 RCTs had multiple treatment arms where 1 group received a singlestrain preparation while another received a multi-strain preparation.32,35,36,49,57
Overall,the probiotic dose ranged from 2£108to 1£1011colony forming units daily,and the length of intervention was between 7 days and 150 days.The study cohorts consisted of runners,5,22-24,30,34,38,39,43,50,52,53,55,58,61cyclists,26,27,54,60“endurance athletes” (which often included a combination of runners,cyclists,and/or triathletes),25,28,29,41,42,44,48swimmers,31rugby players,33,49,57recreational exercisers,6,32,35,36,45,46,62bodybuilders/resistance-trained athletes,37,40collegiate athletes,4,47female athletes from a range of sports,7,56soccer players,51and badminton players.59The primary outcomes included URTIs,respiratory symptoms,and/or associated biomarkers,4,22-25,27,28,32,33,35,36,38,41,42,44,47,49,50,52,61endurance performance,31,45,53,55,60oxidative stress,26,30GI symptoms and/or markers of function,5,29,34,39,48strength and related outcomes (e.g.,soreness),40,46,56,57heat shock proteins,43anxiety,51,59iron status,7macronutrient absorption/metabolism,6,37,54,62and blood/muscle metabolome.58Safety data was available for only a few studies,with limited reports of symptoms7,32,52or no adverse events.6,22,30,35,41,45,47,51,53,56In addition,there were a few instances where adverse events were not directly reported but,by default of the assessed outcomes,relevant symptoms were reported,such as those associated with GI complaints,URTI,and/or lower respiratory illnesses.5,27,33,34,38,42,50,54,60-62
3.3.Study methodology elements
Primary data elements related to study methodology,including probiotic intervention,study design,participant characteristics,outcomes,and safety data,are visualized in Fig.2,tabulated in Supplementary Table 5,and described below.
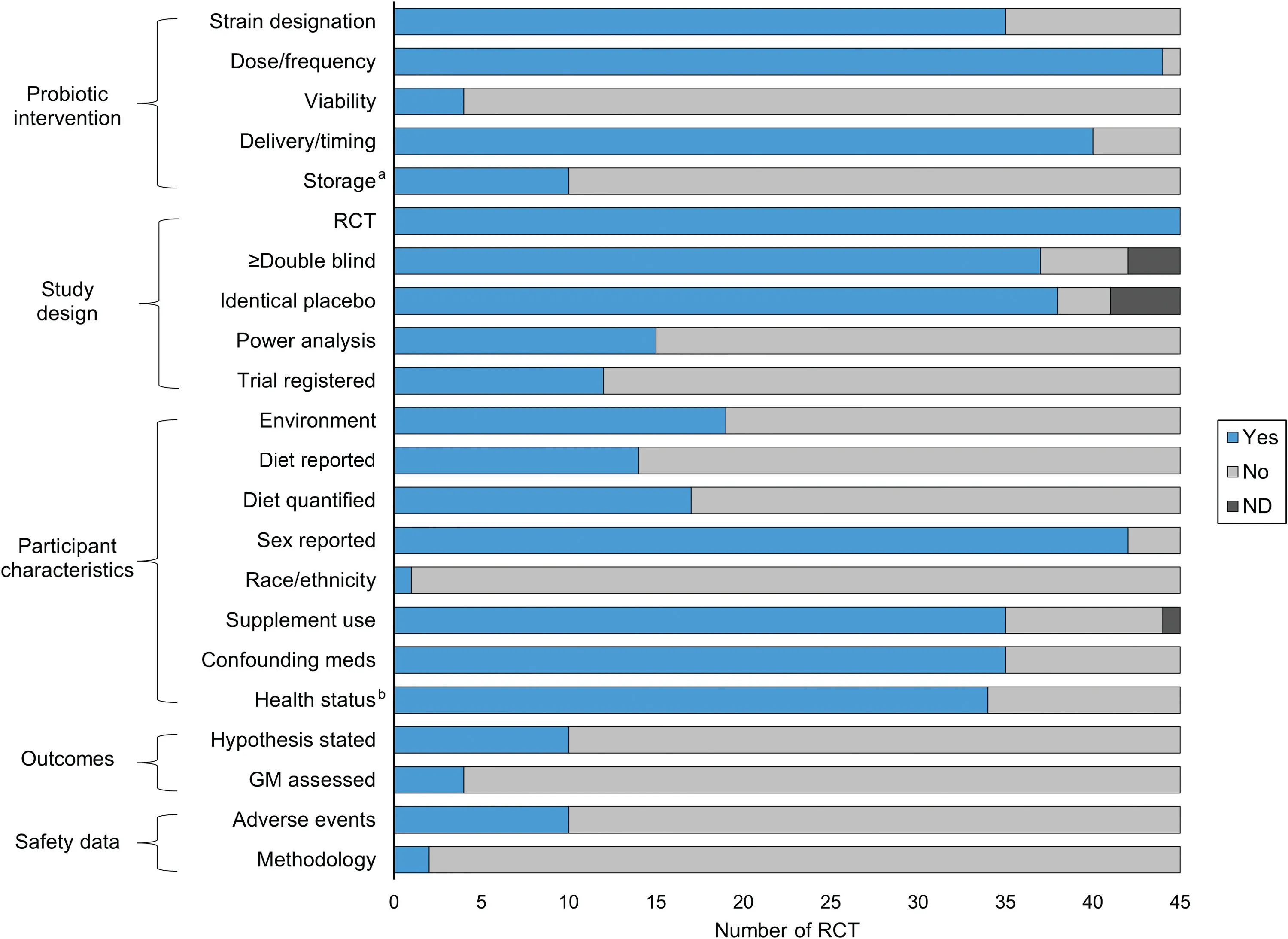
Fig.2.Data elements reported from randomized controlled trials investigating probiotics on undefined outcomes in athletes and physically active individuals.a Description of storage conditions of probiotic.b Screened for confounding preexisting health conditions.GM=gut microbiome;ND=not described;RCT=randomized controlled trial.
3.3.1.Probiotic intervention
Of the 45 included articles,35 articles(~78%)reported the probiotic strain used in the study intervention while 10 articles did not.28,31,33,34,37-39,49,52,57These 10 articles described the probiotic species only in general terms.One article did not adequately report the dose and frequency of use of the probiotic.37Only 4 articles (~9%) reported the viability of the probiotic.38-40,61While 40 articles (~89%) included a description of the delivery and timing of the probiotic,5 omitted these details.22,23,31,40,43Finally,only 10 studies(~22%)reported a description of the storage conditions of the probiotic.4,25,28,33,34,41,44,47,48,51
3.3.2.Study design
In accordance with the inclusion criteria of the scoping review,all included articles were RCTs.Moreover,the majority of the articles (n=37,~82%) had a double-blinded research design,while 5 articles did not,26,33,38,39,43and 3 articles failed to provide a description.31,37,46In addition,38 articles (~84%)provided an identical placebo,while 3 articles did not,23,26,43and it was unclear in 4 other studies.6,40,55,56Only 15 articles(~33%) provided a power analysis for a sample size estimation4,24-29,33-35,42,48,51,54,62and 12 articles (~27%)provided trial registration information.6,7,29,31,35,36,40,49,52,54,57,62
3.3.3.Participant characteristics
Nineteen studies (~42%) described environmental conditions,4,5,22,23,25,29,33,34,38,39,41,43,47-49,58,60-62with season(e.g.,winter,spring,etc.),humidity,and ambient temperature as the most common characteristics detailed.29,34Although 16 studies (~36%) stated that diet was assessed,5,27,29,34,38,39,41,42,45,47,51,54,56,58,59,61only 13 studies(~29%) actually reported this data.5,27,29,34,38,39,42,45,47,54,56,58,61The most common method for recording diet was a 3-day food log.Of the 45 studies,35 studies (~78%)reported dietary supplement use as part of the eligibility criteria or assessed the use of dietary supplements during the intervention period.4-7,22,24-30,33-36,38,40-48,52-54,56,58-62In contrast,10 studies (~22%) did not report controlling for this factor.23,31,32,37,39,49-51,55,57The majority of the included articles were either exclusively or majority male.Five studies were exclusively7,31,56or majority female.28,42Three studies failed to describe the sex of the participants.48,49,59Only a single study reported the participants’ ethnicities.51Screening for prior medication use was part of the eligibility criteria in 35 of the 45 studies (~78%);5-7,22-30,33-36,38,40-46,48,51-54,56,58-62however,10 studies did not report controlling for this confounding factor.4,31,32,37,39,47,49,50,55,57Similarly,34 studies(~76%) reported screening for preexisting health conditions4-7,22,23,25-30,32,35,36,38,41-48,51-54,56,58-62while 11 studies did not(~24%).24,31,33,34,37,39,40,49,50,55,57
3.3.4.
Only 10 studies (~22%) explicitly described a research hypothesis.6,24,34,38,39,42,43,52,54,61Fifteen studies assessed GI symptoms and/or permeability.5,22,27,29,31-35,41,42,48,54,60,62Only 3 studies (~7%) analyzed the GM27,48,53with 2 of them investigating the influence of the probiotic intervention on GM composition.48,53
3.3.5.Safety data
Of the 45 eligible studies,10 studies reported data directly relating to adverse events.6,7,22,30,32,35,41,47,51,56Of these,8 studies indicated that no adverse events were reported by any participant in either probiotic or placebo arms of the trial.6,22,30,35,41,47,51,56Two of the studies reported adverse events.7,32The study of Axling et al.7had a high incidence of adverse events in both probiotic and placebo groups,which may relate to additional supplementation of iron in both intervention groups.Eighteen of 23 participants(~78%)in the placebo group and 15 out of 19 participants (~79%) in the probiotic group reported at least 1 adverse event,although their exact nature was not reported.
While the incidence of adverse events was reported by 10 studies,only 2 of the eligible 45 studies detailed the methods by which adverse events were recorded by participants.6,7Both studies utilized participantesearcher interactions and discussion as opportunities for reporting adverse events.Jger et al.6also reported that participants could spontaneously report adverse events.Axling et al.7stated that study diaries provided the means to report adverse events.
3.4.Risk of bias
The proportion of studies identifying bias in 1 or more design element(s) is presented in Fig.3 and Supplementary Table 6.Principally,concerns of underlying bias were driven by issues arising from the randomization process and deviations from intended interventions,such as lack of clarity regarding blinding(e.g.,whether participants and investigators were aware of their assigned intervention during the trial).However,the risk of bias was low for missing outcome data,which includes attrition bias,measurement of outcomes,and selection of the reported result in all included articles.

Fig.3.Overall risk of bias of articles included in a scoping review of randomized controlled trials investigating probiotics on undefined outcomes in athletes and those who are physically active.
4.Discussion
The objective of this scoping review was to identify and characterize study methodologies of RCTs investigating supplementation with probiotics in athletes and physically active individuals.Using previously established methodology,17we developed and employed a standardized study framework with 5 overarching methodological domains: (a) probiotic intervention,(b) study design,(c) participant characteristics,(d) study outcomes,and(e) safety data.Importantly,this review took the next step in a systematic approach by collating evidence from the current body of RCTs on this subject and applying the conceptual framework laid down in our established narrative work.Within each of these domains,we identified several key elements that were critically lacking in this body of research,such as reporting viability and storage conditions of the probiotic,providing a power analysis for a sample size estimation,including trial registration information,and reporting essential details on dietary practices,usage of dietary supplements,participant sex and race/ethnicity,and safety data.Addressing these deficiencies in future studies will provide the increased rigor needed to synthesize this body of work and form evidence-based guidelines for researchers,clinicians,and other health practitioners.The ultimate aim is to assist health professionals in making informed decisions about probiotic supplementation for athletes and other physically active individuals.
4.1.Probiotic interventions
Under this domain,the 2 intervention elements that were notably absent in most of the extracted articles were the viability and storage conditions of the probiotic.While understandably more complex and perhaps costly,ensuring the viability of the test probiotic is paramount to any investigation that studies these preparations.This issue is of particular concern given that up to a third of commercial products have been independently shown to be below the label claim for colony forming units prior to their expiration date.63Enhancing storage conditions using specialized containment,working with the manufacturer to get viable count data at the time of production,adhering to a reasonable study length for product stability (i.e.,shelf-life),and providing information to participants on best practices for the storage of probiotic formulation (e.g.,refrigeration) are quite reasonable measures for investigators to take.However,these conditions do not always guarantee the survival of the probiotic as it is well established that viability of commercial products can vary widely depending on the length of storage.64,65We consider viable counts of the probiotic product to be critical at both the initiation and conclusion of a study,especially for longer-term investigations with rolling recruitment,where the product could be in storage for more than 6 months.17Where any of the above recommendations are employed,they should be reported.For example,Gill et al.38cultured the speciesLactobacillusto ensure the colony forming units aligned with the manufacturer’s count as well as with the reported counts from the cultured plates in their paper.Other investigators have utilized third parties or independent commercial entities to conduct these analyses.40These options might be useful for laboratories not equipped for enumeration methods.Reporting on the species and strain,and on the delivery and timing,should be sufficiently detailed for other investigators to consider replication studies.
4.2.Study design
Many studies included strong design elements such as double blinding,randomization,and control groups.About one-quarter of studies implemented a crossover design,4,6,24,33,34,38-40,48,54which affords more statistical power but comes at the cost of increased complexity (especially in relation to probiotics).This challenge is exemplified by the inclusion of a washout period,which allows for the probiotic to exit the GI tract and for its effects to dissipate (e.g.,if the probiotic is providing nutrient digestion and absorption effects).Well-executed washout periods were described by Pugh et al.,54who assessed whether a combination of several strains from the speciesLactobacillusandBifidobacteriumincreased carbohydrate absorption and oxidation during a 2-h cycling bout.A washout period of 2 weeks was selected based on data demonstrating the strains used in their study were undetectable in stool samples after this time,meaning there had been a complete cell turnover of the epithelia of the small intestines.
Other important study design considerations that were under-reported include the details of a run-in phase,which are useful for determining compliance to the supplementation regimen and for gauging completion of outcome measures.For example,Axelrod et al.48had participants undergo a 2-week body mass and diet stabilization period prior to randomization.This procedure may not be needed for the majority of these types of studies,but as in the case of Axelrod et al.,48who assessed the GM,such practices are recommended.Surprisingly,only a minority of extracted articles included a power analysis for sample size estimation,and only a minority of studies reported that their study was listed in a trial registry.Finally,none of the studies included in this review were triple blinded.A triple blind experimental design is recommended to increase the power of study results and avoid any analytic bias associated with demand characteristics or the placebo effect.17
4.3.Participant characteristics
Characteristics such as environment,diet,consumption of dietary supplements,sex,and race/ethnicity could influence the outcomes observed in probiotic studies.Thus,given the potential for these to either confound data or provide specific context to any findings,it is important to report or control for them.For example,URTIs,GI symptoms,and/or performance could be influenced by environmental temperature,air humidity,exposure to allergens,etc.17In relation to environmental factors,only a minority of studies reported characteristics such as season,temperature,pollutants,and humidity in experimental trials.Moreira et al.23conducted their study withLactobacillusGG supplementation during a high pollen season (spring).Other studies detailed patterns of illness symptoms in competitive cyclists during a winter training period.27A study of URTI and blood immune markers was conducted during the spring training period and competition.28Experimental control is typically higher in laboratory settings.For example,a study examining gut permeability and blood markers of inflammation during exercise in the heat was conducted in a controlled environment with a temperature of 35˚C and 40%humidity.34Environmental(external)and laboratory (internal) characteristics need to be assessed and reported not only in regard to immune system or gut outcomes,but also in terms of interpreting performance outcomes,energetic metabolism,and self-reported effort perception and affective means(e.g.,anxiety,vigor,stress,fatigue).
The majority of trials controlled for the ingestion of nutritional supplements.Most described either no consumption of any nutritional supplements or ergogenic aids during the study5,27,40or a restriction of vitamins and minerals,additional probiotics,or any fermented dairy products (e.g.,yogurt).28,52An alternative approach is to instruct the participants to maintain their use of the same supplements during the whole study to ensure within-participant standardization.30However,depending on the primary outcome measures,it is important to at least assess the use of dietary supplements and counterbalance participants accordingly in order to ensure there are no confounding effects that could influence study outcomes.
Only 13% of trials included reported data regarding dietary intake of participants.Lamprecht et al.29used a 7-day food record for dietary assessment,including macro and micronutrient values.These specific details will aid in the interpretation of experimental data primarily in relation to nutrient absorption(carbohydrates,protein,amino acids,and micronutrients) and exercise performance.In contrast,Huang et al.45reported the habitual energy intake of their study participants but did not fully report on the methodology,noting only that participants were asked to maintain their normal diet and not consume any other nutritional supplements.It would be more beneficial to fully report on the methods used to assess dietary intake.For example,including information on dietary fiber content would be desirable in studies associated with intestinal permeability,absorption,or GM outcomes.Although the mechanism of action for a probiotic is related to its interaction in the gut(with the microbiota,enterocytes,and immune cells)it is possible that similar species could act differently in participants of different races/ethnicities or if they use different dietary pathways.66Thus,the International Olympic Committee recommends that a complete nutritional assessment of an athlete’s diet should be undertaken before decisions regarding supplement use are made.67
One of the more striking findings was the under-representation of females in the included articles.Not only was the sex ratio overwhelmingly weighted toward males,but only 3 studies exclusively investigated females.7,31,56Given the difference in GI symptomology between males and females,68only 2 studies reported issues related to GI symptoms in females.31,42Clearly there is a need for more equally weighted sex representation,and this requirement should be a prime consideration for future investigations in this area.Relatedly,only 1 study reported working with participants of the same ethnicity,51and all other studies did not identify the race/ethnicity of participants.
Almost a quarter of the studies did not report details about whether prior medication use was controlled for in the data analysis or in subsequent interpretation of study outcomes.There was a similar proportion of studies that did not report screening for pre-existing health conditions.It was not possible to ascertain whether this data was not recorded or not reported.This is a problem because a pre-existing medical condition could influence the underlying adaptative response to probiotic supplementation,thereby confounding experimental results and interpretation.Thus,these issues of experimental control should be considered during the planning and formulation of projects and reviewed regularly during the data collection phase.Involvement of a statistician is recommended during the planning,data analysis,and manuscript preparation phases to ensure trustworthy results and outcomes.
4.4.Outcomes
Surprisingly a substantial proportion of studies did not directly assess GI symptoms,measures of gut permeability,or the GM.While gut permeability is only a specific aspect of this work,and GM assessment requires access to expert personnel and specialized equipment,the omission of selfreported (or physician-verified) GI symptoms is a clear shortcoming.There are several studies that examined GI symptoms in relation to probiotic supplementation,and scrutiny of their methodological approaches offers useful insights for other investigators.69Currently,there is no “gold standard” for the assessment of GI symptoms and markers or downstream consequences of altered GI permeability.An in-depth consideration of these concerns is beyond the scope of the current review.However,we direct readers to a recent discussion of these topics70and to the proposed best practice guidelines for studies involving probiotics.17Studies on GM composition are emerging71,72and offer detailed mechanistic insights into GI physiology and the effects of probiotics at a clinical level.
4.5.Safety data
Microbes have long been consumed safely in the form of both live cultures in fermented foods and commercially produced probiotic supplements.Probiotic supplements have an excellent safety profile,with many clinical trials showing very few side effects or adverse events following probiotic supplementation.While it is clearly beyond the scope of many researchers to assess all potential adverse events that have been raised for concern (e.g.,transfer of antibiotic resistant genes73),researchers should fully consider any commercial probiotic used within studies.Bothin vitroandin vivosubstantiation would be welcome where available.
The majority(~77%)of the studies included in the current review did not include any information or data regarding adverse events.Of the 10 studies that did describe safety data or data relating to adverse events,only 2 studies described the methods used to assess these.While the incidence of adverse events in studies supplementing with probiotics appears to be low,2it is important to continue to report safety data,even in the absence of side effects.Given the heterogeneity of bacterial species and strains used in research,as well as their doses,well-documented safety data can help inform specific practical recommendations.For example,West et al.27reported an increase in incidence of GI symptoms such as bloating and flatulence (although severity decreased) during probiotic supplementation.Other studies using the same probiotic strain(Lactobacillus fermentumVRI-003 (PCC)) have not reported similar data nor any methods used to assess this.24It is therefore important for future studies to employ methodologies that provide study participants with the opportunity to report side effects,whether minor or severe.For example,Jger et al.6utilized participantesearcher interaction points during the study to gather information from participants while also informing them that they could communicate any side effects at any point during the study.This format is recommended for future studies to achieve succinct and clear reporting.
4.6.Guidance for future research
In the context of human research,it is important to remember that a probiotic is a living organism that is entering the digestive system on a routine basis (when consumed in a regimented fashion).Just as all biological life has distinct taxonomy and function,so do these commonly used dietary supplements.Indeed,this is the basis for the mechanisms of action by which probiotics may offer several unique and costeffective strategies for athletes to improve exercise-related outcomes.While not every issue identified in this scoping review is feasible to address or even pertains to the investigator’s research question(s),we urge researchers,practitioners,and the lay public to keep them in mind.
Incorporating a system of best practices in research design and methodology will provide a framework for researchers to better translate outcomes to real-life applications,including support for exercise performance capacity,training adaptations,recovery from exercise,and even GM and immune modulation.For an indepth narrative discussion on this framework,we have presented recommendations for “best practices” in probiotic clinical research for athletes and individuals from other highly active settings previously.17It is appropriate to mention a few additional considerations.First,nearly all the included studies had frequent contact with their study participants,yet many did not report compliance.This simple oversight can easily be remedied with implementation of visual,verbal,and/or diary-type assessments.Second,many of the included studies did not report potential confounding dietary factors such as probiotic-containing foods,including fermented dairy,vegetables,etc.Such factors have been well documented to impact the GM and immunological outcomes.74,75Third,when investigating probiotics in team sport scenarios,where athletes are competing on a regular basis in close proximity(e.g.,sharing bottles,shaking hands),the risk of infection and illness is increased.Potential confounding issues could influence study outcomes and safety data and should be considered during the study design period.Fourth,nuance obviously resides in other related preparations,such as heat-killed probiotic bacteria,synbiotics,and prebiotics;however,many of the elements discussed in this review can be implemented into investigations of those dietary supplements relatively seamlessly.Finally,given the known sex differences with respect to exercise training response,76GI symptoms,68and nutritional and health considerations,77increased representation of females in probiotic research is greatly needed if we are to understand how these preparations might be leveraged toward improving health and exercise performance in women.
As a final note,it is becoming increasingly clear that participant response to probiotic intervention may be highly individual,78,79a consideration not specifically addressed in any of the 45 extracted articles in the present review.The issue of individual responses or responders (sometimes framed as respondersvs.non-responders) is challenging and necessitates consideration of the underlying experimental design and targeted analytical approaches.Differentiation of groupvs.individuallevel responses80to training and dietary interventions is important.A key consideration for researchers is quantifying and reporting the degree of within-subject variability to estimate individual responses to probiotic supplementation.This approach involves isolating the sources of variability in intervention studies via use of a repeated intervention or repeated testing during the intervention.Careful selection of timepoints is required to quantify technical errors of measurement and week-to-week variability over a typical probiotic supplementation period.Use of continuous outcome measures is recommended where possible to avoid shortcomings in dichotomizing or categorizing individuals as responders or non-responders.81
While out of the scope of this review,a major (general)nutrition initiative is the provision of personalized and precision approaches.82For clinical populations,study design,intervention,and participant selection present obvious challenges.In comparison,employing such approaches in relatively healthy athletes(or other physically active occupational settings) may appear less cumbersome.However,heterogenous groups of highly active individuals across multiple athletic disciplines present their own research challenges.Research on dietary supplementation,including probiotic formulations in sports,exercise and occupational settings,will benefit from improved research methodologies and practices.Cross-disciplinary collaboration is highly encouraged and may better inform investigators on participant selection/screening,outcomes,tracking over the study period,and statistical modeling.82,83As an example,the GM and “omic” features can be profiled prior to the intervention to identify so-called responders and non-responders.While not universally feasible,such practices may help distinguish how participants respond to the presence and effects of exogenous probiotic in their resident GM.78Ultimately,this may allow researchers to identify curated strains that promote ergogenic effects on health and performance.Currently,there is no established framework for implementing such a process,though it would likely rely on predictive assessment from several key translational science approaches,includingin silicoandin vitromodeling,in tandem with metadata from the individual (dietary intake,sport,medication use,etc.).82As an unrefined example,a power-based athlete who participates in a team sport may be identified as a candidate for probiotic use during the season if they experience GI distress that negatively impacts their performance.This individual consumes a high-protein,energy dense diet (which is associated with greater bile salt release84),and assessment of their baseline GM is determined to be conducive to engraftment by a narrow range of strains from the generaBifidobacteriumandLactobacillus.85Thus,a collection of probiotics of these strains,displaying high levels of bile salt hydrolase activityin vitro,86and shown to improve GI barrier functionin silicoandin vivoare selected for supplementation.
5.Conclusion
Evidence-based guidelines for probiotic interventions in athletes and physically active individuals are still in their relative infancy,with considerable improvement to be made in terms of study methodology.As with dietary supplements in general,much work is needed to provide a buttress against loose market regulations and build a rigorous foundation of research with vertical advancement in mind.This scoping review highlights many of the key issues and challenges that future investigators should address.Enhanced standardization of study methodology and transparency of reporting are clearly warranted for continued improvements to research in this field.In addition,we advise those interested in future systematic review and meta-analysis efforts to be mindful of the inherent,and in some instances unreconcilable,differences in many of these studies.Depending on the research question(s),a systematic review and/or meta-analysis may not usefully translate to practical implementation in clinical and sports practice settings.
Authors’contributions
AEM conceptualized,designed,screened,and performed quality analysis,data extraction,data analysis,results interpretation,and manuscript preparation;DBP performed results interpretation and manuscript preparation;GSFL preformed quality analysis,data extraction,data analysis,results interpretation,and manuscript preparation;DA designed,screened,and performed data extraction,results interpretation,and manuscript preparation;JP conceptualized,designed,screened,and performed quality analysis,data extraction,data analysis,results interpretation,and manuscript preparation.All authors have read and approved the final version of the manuscript,and agree with the order of presentation of the authors.
Competing interests
AEM is employed by Isagenix International,LLC.Isagenix was not involved in any aspect of the review;JP is a consultant for Aliment Nutrition,Ltd.;JP has previously received grants to evaluate the efficacy of various nutritional supplements including probiotics.All the support had no involvement in the study design and writing of the manuscript or the decision to submit it for publication.The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary materials
Supplementary materials associated with this article can be found in the online version at doi:10.1016/j.jshs.2022.12.012.
杂志排行
Journal of Sport and Health Science的其它文章
- 2024 Adult Compendium of Physical Activities:A third update of the energy costs of human activities
- Older Adult Compendium of Physical Activities:Energy costs of human activities in adults aged 60 and older
- 2024 Wheelchair Compendium of Physical Activities:An update of activity codes and energy expenditure values
- Alexa,let’s train now!—A systematic review and classification approach to digital and home-based physical training interventions aiming to support healthy cognitive aging
- The influence of resistance exercise training prescription variables on skeletal muscle mass,strength,and physical function in healthy adults:An umbrella review
- Associations of daily sedentary behavior,physical activity,and sleep with irritable bowel syndrome:A prospective analysis of 362,193 participants
