基于脯氨酸的新型手性叔胺N-氧化物合成
2023-10-31田方丽王宇恒刘雄利邓国栋
田方丽, 张 磊, 王宇恒, 刘雄利, 邓国栋
(贵州大学 西南药食两用资源开发利用技术国家地方联合工程研究中心,贵州 贵阳 550025)
叔胺衍生的N-氧化物中,若母体叔胺含有3个不同的基团,则相应的N-氧化物将在氮上含有1个稳定的手性中心。N-氧化物含电子对的独特性质增强了与多种金属形成络合物的可能。部分研究集中于开发用于金属催化反应的新型手性胺N-氧化物配体的合成[1-4]。 FENG课题组以手性脯氨酰胺为起始原料,通过采用m-CPBA(间氯过氧苯甲酸)进行N-氧化,开发了一种新型叔胺衍生的C2对称手性N,N′-二氧化物配位体,在路易斯酸不对称催化领域取得了重大突破[5-7]。然而,开发叔胺衍生的手性N-氧化配体的最大挑战是控制结构中四面体氮的手性。因此,立体选择性合成新型的叔胺衍生的手性N-氧化物配体仍然是不对称催化领域最重要的课题之一。
本文基于脯氨酸为手性源,以光学纯的脯氨酰胺或羟脯氨酰胺1与吡啶-2-甲醛2先发生缩合环化反应,生成中间体3,然后在氧化剂m-CPBA的作用下与中间体3中的氮原子发生氧化反应,合成了24个未见文献报道的手性叔胺N-氧化物4aa~4bk(图1),总产率43%~58%,dr值为10 ∶1~>20 ∶1,其结构经1H NMR,13C NMR和HR-MS(ESI-TOF)表征。该类化合物基于脯氨酸手性源合成了手性N-氧化物,可为金属不对称催化提供新配体筛选[8-16]和生物活性测试筛选[17-20]。
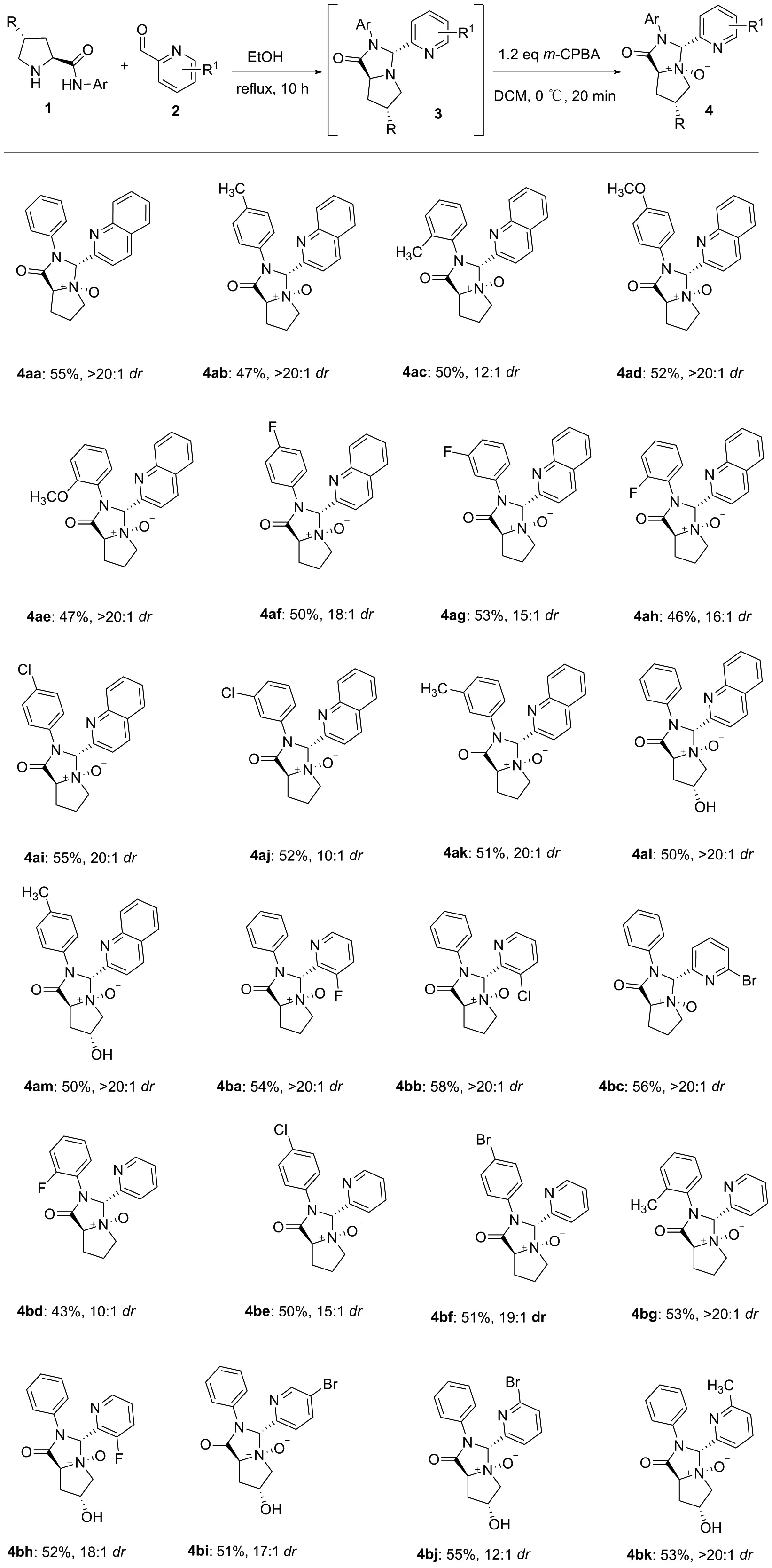
图1 基于脯氨酸新型手性源叔胺N-氧化物的合成路线
1 实验部分
1.1 仪器与试剂
WRS-1B型数字熔点仪;Bruker-400 MHz型核磁共振仪(DMSO-d6为溶剂,TMS为内标);MicroTMQ-TOF型高分辨质谱仪。
所用试剂均为分析纯。
1.2 4aa~4bk的合成(以4aa为例)
参考FENG课题组的合成方法[5-7],在反应管中将原料脯氨酰胺1a(1.1 eq)与吡啶-2-甲醛2a(1.0 eq, 0.3 mmol)溶于2.0 mL无水乙醇中,回流10 h,反应后处理液通过柱层析纯化,得到中间体3。将中间体3(100 mg, 1.0 eq)与m-CPBA(1.1 eq)溶于2.0 mL三氯甲烷中,在0 ℃下反应20 min,反应后处理液通过柱层析纯化,得到N-氧化物4aa,淡黄色固体, m.p.145.1~145.7 ℃,产率55%,dr>20 ∶1;1H NMRδ: 2.06~2.13(m, 1H), 2.38~2.46(m, 1H), 2.53~2.63(m, 2H), 3.93~3.99(m, 2H), 4.63~4.66(m, 1H), 6.20(s, 1H), 7.05~7.09(m, 1H), 7.17~7.21(m, 2H), 7.30(d,J=7.6 Hz, 2H), 7.45~7.49(m, 1H), 7.59~7.64(m, 2H), 7.73(d,J=8.0 Hz, 1H), 7.98(d,J=8.4 Hz, 1H), 8.02(d,J=8.4 Hz, 1H);13C NMRδ: 21.9, 23.8, 70.5, 87.8, 121.3, 122.2, 125.6, 126.5, 126.6, 127.5, 128.3, 128.8, 129.0, 134.8, 135.5, 146.5, 149.9, 168.5; HR-MS(ESI-TOF)m/z: calcd. for C21H19N3NaO2{[M+Na]+}368.1369, found 368.1361。用类似的方法合成4ab~4bk。
4ab:淡黄色固体,m.p.146.1~147.0 ℃,产率47%,dr>20 ∶1;1H NMRδ: 2.07~2.11(m, 1H), 2.16(s, 3H), 2.22~2.29(m, 1H), 2.38~2.51(m, 2H), 3.75~3.79(m, 1H), 4.02~4.09(m, 1H), 4.51~4.54(m, 1H), 6.97(s, 1H), 7.10(d,J=8.0 Hz, 2H), 7.45(d,J=8.8 Hz, 2H), 7.58~7.63(m, 1H), 7.73~7.77(m, 1H), 7.81(d,J=8.4 Hz, 1H), 7.96~8.00(m, 2H), 8.35(d,J=8.0 Hz, 1H);13C NMRδ: 20.9, 23.1, 24.9, 71.7, 77.7, 87.6, 122.8, 124.6, 127.7, 128.3, 128.4, 129.5, 129.8, 130.3, 134.1, 135.9, 136.3, 147.1, 153.4, 169.7; HR-MS(ESI-TOF)m/z: calcd. for C22H21N3NaO2{[M+Na]+}382.1526, found 382.1526。
4ac:淡黄色固体,m.p.158.4~159.2 ℃,产率50%,dr=12 ∶1;1H NMRδ: 2.00~2.07(m, 1H), 2.28(s, 3H), 2.32~2.37(m, 1H), 2.49~2.54(m, 2H), 3.97~4.02(m, 2H), 4.77~4.80(m, 1H), 6.18(s, 1H), 6.89~6.92(m, 1H), 6.98~7.08(m, 3H), 7.40~7.44(m, 1H), 7.48(d,J=8.4 Hz, 1H), 7.58~7.62(m, 1H), 7.65(d,J=8.0 Hz, 1H), 7.97~8.02(m, 2H);13C NMRδ: 18.7, 23.0, 24.5, 71.3, 77.0, 89.6, 123.7, 126.9, 127.1, 127.5, 127.7, 128.5, 128.8, 129.7, 130.0, 131.6, 133.3, 136.0, 136.3, 147.3, 151.2, 169.4; HR-MS(ESI-TOF)m/z: calcd. for C22H21N3NaO2{[M+Na]+}382.1526, found 382.1525。
4ad:淡黄色固体,m.p.156.8~156.8 ℃,产率52%,dr>20 ∶1;1H NMRδ: 2.11~2.15(m, 1H), 2.43~2.47(m, 1H), 2.59~2.66(m, 2H), 3.66(s, 3H), 4.01~4.04(m, 2H), 4.69~4.71(m, 1H), 6.21(s, 1H), 6.74(d,J=7.2 Hz, 2H), 7.23(d,J=7.2 Hz, 2H), 7.51~7.54(m, 1H), 7.63~7.70(m, 2H), 7.77(d,J=6.4 Hz, 1H), 8.05(d,J=6.8 Hz, 1H), 8.14(d,J=6.4 Hz, 1H);13C NMRδ: 23.0, 24.7, 55.5, 71.5, 77.4, 89.4, 114.6, 123.4, 125.0, 127.7, 128.3, 130.0, 130.1, 136.6, 147.6, 151.0, 158.3, 169.6; HR-MS(ESI-TOF)m/z: calcd. for C22H21N3NaO3{[M+Na]+}398.1475, found 398.1473。
4ae:淡黄色固体,m.p.156.2~156.9 ℃,产率49%,dr=19 ∶1;1H NMRδ: 1.97~2.04(m, 1H), 2.29~2.34(m, 1H), 2.46~2.53(m, 2H), 3.74(s, 3H), 3.92~3.97(m, 1H), 4.26~4.33(m, 1H), 4.58~4.60(m, 1H), 6.24(s, 1H), 6.66~6.70(m, 1H), 6.76~6.78(m, 1H), 7.05~7.10(m, 1H), 7.27~7.30(m, 1H), 7.36~7.40(m, 1H), 7.50(d,J=8.4 Hz, 1H), 7.54~7.62(m, 2H), 7.95~8.02(m, 2H);13C NMRδ: 22.8, 24.5, 55.8, 71.1, 76.4, 87.7, 111.6, 121.1, 122.3, 123.8, 127.4, 127.6, 128.4, 129.8, 129.9, 130.2, 136.2, 147.3, 151.1, 154.7, 170.1; HR-MS(ESI-TOF)m/z: calcd. for C22H21N3NaO3{[M+Na]+}398.1475, found 398.1475。
4af:淡黄色固体,m.p.157.3~157.9 ℃,产率50%,dr=18 ∶1;1H NMRδ: 2.07~2.14(m, 1H), 2.37~2.46(m, 1H), 2.54~2.64(m, 2H), 3.96~3.99(m, 2H), 4.62~4.65(m, 1H), 6.18(s, 1H), 6.86~6.91(m, 2H), 7.26~7.29(m, 2H), 7.47~7.51(m, 1H), 7.61~7.66(m, 2H), 7.76(d,J=8.0 Hz, 1H), 7.99(d,J=8.4 Hz, 1H), 8.11(d,J=8.4 Hz, 1H);13C NMRδ: 21.9, 23.7, 70.5, 88.0, 115.1(d,JCF=22.4 Hz), 122.3, 123.8(d,JCF=9.1 Hz), 126.6(d,JCF=11.4 Hz), 128.9(d,JCF=9.1 Hz), 135.6, 146.5, 149.6, 159.7(d,JCF=246.3 Hz), 168.5; HR-MS(ESI-TOF)m/z: calcd. for C21H18FN3NaO2{[M+Na]+}386.1275, found 386.1277。
4ag:淡黄色固体,m.p.152.3~153.6 ℃,产率53%,dr=15 ∶1;1H NMRδ: 2.10~2.15(m, 1H), 2.44~2.50(m, 1H), 2.58~2.65(m, 2H), 3.93~3.99(m, 1H), 4.03~4.05(m, 1H), 4.70~4.73(m, 1H), 6.33(s, 1H), 6.79~6.83(m, 1H), 7.03~7.05(m, 1H), 7.15~7.19(m, 1H), 7.32~7.35(m, 1H), 7.51~7.54(m, 1H), 7.65~7.68(m, 1H), 7.72(d,J=6.4 Hz, 1H), 7.78(d,J=6.4 Hz, 1H), 8.01(d,J=6.8 Hz, 1H), 8.16(d,J=6.8 Hz, 1H);13C NMRδ: 22.9, 24.8, 71.5, 88.2, 109.6(d,JCF=20.1 Hz), 113.4(d,JCF=17.4 Hz), 116.9, 127.7, 127.8, 130.0(d,JCF=7.3 Hz), 130.6(d,JCF=8.1 Hz), 136.8, 137.3(d,JCF=8.3 Hz), 147.6, 150.6, 162.7(d,JCF=196.6 Hz), 169.5; HR-MS(ESI-TOF)m/z: calcd. for C21H18FN3NaO2{[M+Na]+}386.1275, found 386.1279。
4ah:淡黄色固体,m.p.160.2~161.3 ℃,产率46%,dr=16 ∶1;1H NMRδ: 2.09~2.15(m, 1H), 2.36~2.41(m, 1H), 2.55~2.62(m, 2H), 3.99~4.04(m, 1H), 4.15~4.22(m, 1H), 4.59~4.61(m, 1H), 6.19(s, 1H), 6.93~6.97(m, 1H), 7.01~7.06(m, 1H), 7.12~7.17(m, 1H), 7.39~7.43(m, 1H), 7.46~7.50(m, 1H), 7.59(d,J=8.4 Hz, 1H), 7.63~7.67(m, 1H), 7.72(d,J=8.0 Hz, 1H), 8.03~8.09(m, 2H);13C NMRδ: 21.9, 23.5, 70.7, 87.3, 115.4(d,JCF=19.2 Hz), 121.1(d,JCF=12.4 Hz), 122.8, 123.9(d,JCF=4.4 Hz), 126.6, 127.5, 128.8, 128.9, 129.0, 135.3, 146.4, 149.6, 156.8(d,JCF=247.3 Hz), 169.2; HR-MS(ESI-TOF)m/z: calcd. for C21H18FN3NaO2{[M+Na]+}386.1275, found 386.1274。
4ai:淡黄色固体,m.p.150.9~151.2 ℃,产率55%,dr=20 ∶1;1H NMRδ: 2.09~2.13(m, 1H), 2.24~2.30(m, 1H), 2.39~2.53(m, 2H), 3.76~3.80(m, 1H), 4.06~4.13(m, 1H), 4.51~4.55(m, 1H), 7.07(s, 1H), 7.39~7.42(m, 2H), 7.61~7.65(m, 3H), 7.75~7.79(m, 1H), 7.84(d,J=8.4 Hz, 1H), 7.97~8.01(m, 2H), 8.39(d,J=8.4 Hz, 1H);13C NMRδ: 23.1, 24.9, 71.7, 77.6, 87.1, 124.2, 124.7, 127.8, 128.3, 128.4, 129.4, 129.5, 130.4, 130.5, 135.5, 136.4, 147.1, 153.0, 170.0; HR-MS(ESI-TOF)m/z: calcd. for C21H18ClN3NaO2{[M+Na]+}402.0980, found 402.0981。
4aj:淡黄色固体,m.p.158.4~159.5 ℃,产率52%,dr=10 ∶1;1H NMRδ: 2.04~2.12(m, 1H), 2.20~2.27(m, 1H), 2.34~2.49(m, 2H), 3.70~3.74(m, 1H), 4.05~4.12(m, 1H), 4.46~4.49(m, 1H), 7.07(s, 1H), 7.19~7.22(m, 1H), 7.31~7.35(m, 1H), 7.42~7.45(m, 1H), 7.61~7.65(m, 1H), 7.74~7.84(m, 3H), 7.95~8.00(m, 2H), 8.39(d,J=8.4 Hz, 1H);13C NMRδ: 23.1, 25.0, 71.8, 77.6, 86.8, 120.6, 122.1, 124.7, 126.1, 127.8, 128.3, 128.4, 129.5, 130.4, 131.1, 133.7, 136.4, 138.0, 147.0, 153.1, 170.2; HR-MS(ESI-TOF)m/z: calcd. for C21H18ClN3NaO2{[M+Na]+}402.0980, found 402.0983。
4ak:淡黄色固体,m.p.154.9~155.2 ℃,产率51%,dr=20 ∶1;1H NMRδ: 1.99~2.06(m, 1H), 2.15(s, 3H), 2.35~2.41(m, 1H), 2.49~2.56(m, 2H), 3.89~3.95(m, 2H), 4.64~4.67(m, 1H), 6.25(s, 1H), 6.85(d,J=6.8 Hz, 1H), 6.98~7.05(m, 2H), 7.21(s, 1H), 7.41~7.45(m, 1H), 7.56~7.63(m, 2H), 7.68(d,J=8.0 Hz, 1H), 7.96(d,J=8.4 Hz, 1H), 8.04(d,J=8.4 Hz, 1H);13C NMRδ: 20.4, 21.8, 23.7, 70.3, 76.4, 87.6, 118.3, 122.1, 122.2, 126.5, 126.6, 127.5, 128.0, 128.9, 129.0, 134.6, 135.5, 138.3, 146.5, 150.0, 168.3; HR-MS(ESI-TOF)m/z: calcd. for C22H21N3NaO2{[M+Na]+}382.1526, found 382.1525。
4al:淡黄色固体,m.p.152.2~157.8 ℃,产率45%,dr=12 ∶1;1H NMRδ: 2.66~2.71(m, 2H), 3.86~3.90(m, 1H), 4.08(d,J=11.2 Hz, 1H), 4.51(s, 1H), 5.02~5.06(m, 1H), 6.32(s, 1H), 7.03~7.06(m, 1H), 7.14~7.18(m, 2H), 7.27(d,J=8.0 Hz, 2H), 7.43~7.47(m, 1H), 7.58~7.62(m, 2H), 7.69(d,J=8.0 Hz, 1H), 7.98(d,J=8.4 Hz, 1H), 8.11(d,J=8.0 Hz, 1H);13C NMRδ: 34.8, 69.5, 72.3, 74.4, 85.9, 120.6, 121.1, 125.1, 125.8, 126.0, 126.7, 127.5, 128.1, 128.3, 133.5, 135.2, 145.7, 148.2, 166.7; HR-MS(ESI-TOF)m/z: calcd. for C21H19N3NaO3{[M+Na]+}384.1319, found 384.1323。
4am:淡黄色固体,m.p.146.2~147.7 ℃,产率50%,dr>20 ∶1;1H NMRδ: 2.15(s, 3H), 2.70(d,J=6.0 Hz, 2H), 3.90~3.94(m, 1H), 4.08(d,J=6.0 Hz, 1H), 4.55(s, 1H), 5.03~5.06(m, 1H), 6.23(d,J=1.6 Hz, 1H), 6.98(d,J=8.0 Hz, 2H), 7.15(d,J=8.4 Hz, 2H), 7.47~7.51(m, 1H), 7.58~7.66(m, 2H), 7.74(d,J=8.0 Hz, 1H), 8.01(d,J=8.4 Hz, 1H), 8.15(d,J=8.4 Hz, 1H);13C NMRδ: 19.3, 35.1, 69.9, 72.5, 74.7, 86.5, 121.0, 121.2, 126.0, 126.2, 126.9, 128.3, 128.4, 128.5, 131.0, 135.3, 135.5, 146.0, 148.5, 167.0; HR-MS(ESI-TOF)m/z: calcd. for C22H21N3NaO3{[M+Na]+}398.1475, found 398.1477。
4ba:淡黄色固体,m.p.120.1~120.1 ℃,产率54%,dr>20 ∶1;1H NMRδ: 2.03~2.07(m, 1H), 2.17~2.24(m, 1H), 2.36~2.45(m, 2H), 3.71~3.76(m, 1H), 4.05~4.12(m, 1H), 4.31~4.34(m, 1H), 7.03(s, 1H), 7.16~7.20(m, 1H), 7.33~7.37(m, 2H), 7.49~7.53(m, 3H), 7.76~7.81(m, 1H), 8.46(d,J=4.8 Hz, 1H);13C NMRδ: 23.0, 24.9, 71.9, 77.5, 81.6, 122.5, 124.4(d,JCF=21.2 Hz), 126.6, 127.1, 127.2, 129.5, 136.3, 140.3, 140.4, 145.7(d,JCF=5.5 Hz), 160.5(d,JCF=258.0 Hz), 170.0; HR-MS(ESI-TOF)m/z: calcd. for C17H16FN3NaO2{[M+Na]+}336.1119, found 336.1121。
4bb:淡黄色固体,m.p.145.8~146.9 ℃,产率58%,dr>20 ∶1;1H NMRδ: 2.02~2.08(m, 1H), 2.16~2.23(m, 1H), 2.33~2.47(m, 2H), 3.65~3.69(m, 1H), 3.95~4.02(m, 1H), 4.35~4.38(m, 1H), 6.83(s, 1H), 7.17~7.21(m, 1H), 7.34~7.38(m, 2H), 7.49~7.53(m, 3H), 7.68(d,J=7.2 Hz, 1H), 7.88~7.92(m, 1H);13C NMRδ: 23.0, 24.9, 71.9, 77.5, 86.6, 122.6, 125.6, 126.6, 126.8, 129.5, 136.3, 140.5, 150.0, 153.5, 169.7; HR-MS(ESI-TOF)m/z: calcd. for C17H16ClN3NaO2{[M+Na]+}352.0823, found 352.0825。
4bc:淡黄色固体,m.p.150.2~151.1 ℃,产率56%,dr>20 ∶1;1H NMRδ: 2.02~2.09(m, 1H), 2.15~2.23(m, 1H), 2.33~2.47(m, 2H), 3.64~3.68(m, 1H), 3.95~4.02(m, 1H), 4.33~4.36(m, 1H), 6.80(s, 1H), 7.17~7.22(m, 1H), 7.34~7.38(m, 2H), 7.48~7.50(m, 2H), 7.64~7.66(m, 1H), 7.69~7.71(m, 1H), 7.77~7.81(m, 1H);13C NMRδ: 23.0, 24.9, 71.9, 77.5, 86.6, 122.7, 126.7, 127.0, 129.3, 129.5, 136.3, 140.2, 141.0, 154.0, 169.7; HR-MS(ESI-TOF)m/z: calcd. for C17H16BrN3NaO2{[M+Na]+}396.0318, found 396.0315。
4bd:淡黄色固体,m.p.160.5~161.1 ℃,产率43%,dr=10 ∶1;1H NMRδ: 2.06~2.12(m, 1H), 2.34~2.38(m, 1H), 2.55~2.62(m, 2H), 3.94~3.98(m, 1H), 4.07~4.14(m, 1H), 4.61~4.63(m, 1H), 5.99(s, 1H), 6.97~7.01(m, 1H), 7.03~7.08(m, 1H), 7.16~7.25(m, 3H), 7.41(d,J=7.6 Hz, 1H), 7.59~7.64(m, 1H), 8.60~9.61(m, 1H);13C NMRδ: 21.8, 23.6, 70.7, 75.4, 87.4, 87.5, 115.4(d,JCF=20.1 Hz), 121.0(d,JCF=12.1 Hz), 123.9, 124.0, 126.2, 128.6, 129.0(d,JCF=8.4 Hz), 135.4, 148.6, 149.2, 156.8(d,JCF=248.0 Hz), 160.9; HR-MS(ESI-TOF)m/z: calcd. for C17H16FN3NaO2{[M+Na]+}336.1119, found 336.1122。
4be:淡黄色固体,m.p.152.4~153.6 ℃,产率50%,dr=15 ∶1;1H NMRδ: 2.03~2.06(m, 1H), 2.17~2.23(m, 1H), 2.33~2.45(m, 2H), 3.65~3.69(m, 1H), 3.97~4.04(m, 1H), 4.35~4.37(m, 1H), 6.79(s, 1H), 7.37~7.41(m, 3H), 7.55(d,J=8.8 Hz, 2H), 7.66(d,J=7.6 Hz, 1H), 7.82~7.86(m, 1H), 8.56(d,J=4.8 Hz, 1H);13C NMRδ: 22.9, 24.9, 71.7, 77.3, 86.9, 123.9, 125.0, 127.9, 129.4, 130.3, 135.5, 136.8, 149.5, 152.0, 170.1; HR-MS(ESI-TOF)m/z: calcd. for C17H16ClN3NaO2{[M+Na]+}352.0823, found 352.0824。
4bf:淡黄色固体,m.p.148.1~148.9 ℃,产率51%,dr=19 ∶1;1H NMRδ: 2.03~2.05(m, 1H), 2.17~2.23(m, 1H), 2.33~2.43(m, 2H), 3.67~3.72(m, 1H), 3.97~4.04(m, 1H), 4.36~4.38(m, 1H), 6.81(s, 1H), 7.37~7.40(m, 1H), 7.47~7.54(m, 4H), 7.66(d,J=7.6 Hz, 1H), 7.81~7.85(m, 1H), 7.98~8.04(m, 1H), 8.55(d,J=4.4 Hz, 1H);13C NMRδ: 22.9, 24.9, 71.6, 77.3, 86.8, 118.6, 124.2, 125.0, 127.9, 132.3, 135.9, 136.9, 149.6, 151.8, 169.9; HR-MS(ESI-TOF)m/z: calcd. for C17H16BrN3NaO2{[M+Na]+}396.0318, found 396.0322。
4bg:淡黄色固体,m.p.156.7~157.2 ℃,产率53%,dr>20 ∶1;1H NMRδ: 1.97~2.03(m, 1H), 2.19(s, 3H), 2.27~2.31(m, 1H), 2.44~2.50(m, 2H), 3.96~4.02(m, 2H), 4.75~4.77(m, 1H), 5.98(s, 1H), 6.82(d,J=7.6 Hz, 1H), 6.94~6.98(m, 1H), 7.05~7.16(m, 3H), 7.30(d,J=7.6 Hz, 1H), 7.49~7.53(m, 1H), 8.54(d,J=4.8 Hz, 1H);13C NMRδ: 17.5, 21.8, 23.5, 70.4, 75.9, 88.5, 123.9, 125.9, 126.1, 126.2, 127.8, 130.5, 132.1, 135.0, 135.5, 148.6, 149.7, 168.4; HR-MS(ESI-TOF)m/z: calcd. for C18H19N3NaO2{[M+Na]+}332.1369, found 332.1368。
4bh:淡黄色固体m.p.160.2~161.1 ℃,产率52%,dr=18 ∶1;1H NMRδ: 2.50~2.54(m, 2H), 3.79(d,J=7.6 Hz, 1H), 4.19~4.23(m, 1H), 4.48(d,J=2.8 Hz, 1H), 4.70~4.74(m, 1H), 7.15~7.21(m, 2H), 7.33~7.37(m, 2H), 7.48~7.56(m, 3H), 7.78~7.88(m, 1H), 8.48(d,J=4.4 Hz, 1H);13C NMRδ: 36.7, 71.0, 75.3, 76.8, 81.1, 122.7, 124.5(d,JCF=18.2 Hz), 126.8, 127.4(d,JCF=5.2 Hz), 129.6, 136.0, 139.5, 139.6, 145.8(d,JCF=5.3 Hz), 160.8(d,JCF=258.1 Hz), 169.3; HR-MS(ESI-TOF)m/z: calcd. for C17H16FN3NaO3{[M+Na]+}352.1068, found 352.1071。
4bi:淡黄色固体,m.p.170.2~171.3 ℃,产率51%,dr=17 ∶1;1H NMRδ: 2.50~2.53(m, 2H), 3.73(d,J=9.2 Hz, 1H), 4.14~4.18(m, 1H), 4.46(d,J=3.2 Hz, 1H), 4.71~4.74(m, 1H), 6.91(s, 1H), 7.17~7.20(m, 1H), 7.32~7.36(m, 2H), 7.45~7.46(m, 2H), 7.67(d,J=8.0 Hz, 1H), 8.12~8.14(m, 1H), 8.74(d,J=2.4 Hz, 1H);13C NMRδ: 36.7, 70.9, 75.2, 76.8, 86.2, 121.8, 122.6, 126.7, 129.3, 129.4, 129.5, 136.1, 139.6, 150.5, 167.0; HR-MS(ESI-TOF)m/z: calcd. for C17H16BrN3NaO3{[M+Na]+}412.0267, found 412.0263。
4bj:淡黄色固体,m.p.153.6~154.4 ℃,产率55%,dr=12 ∶1;1H NMRδ: 2.50~2.55(m, 2H), 3.73(d,J=9.2 Hz, 1H), 4.14~4.18(m, 1H), 4.46(s, 1H), 4.73~4.77(m, 1H), 6.90(s, 1H), 7.18~7.22(m, 1H), 7.34~7.38(m, 2H), 7.46(d,J=7.6 Hz, 2H), 7.67(d,J=8.0 Hz, 1H), 7.73(d,J=7.2 Hz, 1H), 7.79~7.83(m, 1H);13C NMRδ: 36.8, 71.0, 75.2, 76.8, 86.1, 122.9, 126.9, 127.1, 129.6, 129.7, 136.0, 140.3, 141.2, 153.2, 168.9; HR-MS(ESI-TOF)m/z: calcd. for C17H16BrN3NaO3{[M+Na]+}412.0267, found 412.0264。
4bk:淡黄色固体,m.p.151.2~153.2 ℃,产率53%,dr>20 ∶1;1H NMRδ: 2.48~2.51(m, 2H), 3.77(d,J=9.2 Hz, 1H), 4.10~4.14(m, 1H), 4.46(d,J=2.8 Hz, 1H), 4.64~4.68(m, 1H), 6.80(br s, 1H), 7.01(s, 1H), 7.13~7.17(m, 1H), 7.28~7.34(m, 3H), 7.41~7.43(m, 2H), 7.65~7.67(m, 1H), 8.40~8.41(m, 1H);13C NMRδ: 18.7, 36.9, 71.3, 74.9, 76.7, 83.1, 122.2, 124.8, 126.3, 129.4, 136.5, 136.6, 138.8, 146.8, 149.7, 169.5; HR-MS(ESI-TOF)m/z: calcd. for C18H19N3NaO3{[M+Na]+}348.1319, found 348.1322。
1.3 单晶培养
将50 mg化合物4ai溶解在10 mL无水乙醇溶剂中,在室温下通过缓慢挥发溶剂,析出白色晶体4ai(CCDC: 2262733)。
2 结果与讨论
2.1 合成分析
本文在对中间体3aa进行氧化反应时发现,如果使用H2O2作为氧化剂不能有效地促进反应进行,反而生成难以处理的混合物。若采用m-CPBA做氧化剂,反应效果最好,反应为20 min时,产率为87%,dr>20 ∶1(表1)。
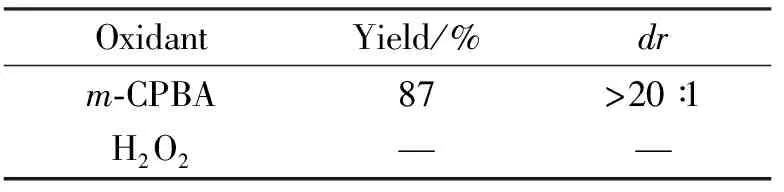
表1 反应条件的优化
2.2 单晶分析
图2为化合物4ai的单晶结构。由图2分析可知,化合物4ai属monoclinic晶系,P21空间群,晶胞参数a=1.07180(5) nm,b=0.70710(3) nm,c=1.27626(6) nm,α=90°,β=07.082(5)°1,γ=90°。
3 结论
本文以L-脯氨酸衍生物作为手性源,采用经济易得的光学纯脯氨酰胺或羟脯氨酰胺1与吡啶-2-甲醛2先发生缩合环化反应生成中间体3,然后3中的氮原子在氧化剂m-CPBA的作用下发生氧化反应合成了24个新型手性叔胺N-氧化物4aa~4bk。该类化合物可为今后不对称路易斯酸催化提供新配体筛选,也可为生物活性测试提供新化合物筛选。
