Using pipette tips to readily generate spheroids comprising single or multiple cell types
2023-10-24RongPANXiaoyanYANGShimingWUYuanyuanXIEFengCHENKeNINGWeiSUNLingYU
Rong PAN, Xiaoyan YANG, Shiming WU, Yuanyuan XIE, Feng CHEN, Ke NING, Wei SUN, Ling YU
Research Article
Using pipette tips to readily generate spheroids comprising single or multiple cell types

1Key Laboratory of Luminescence Analysis and Molecular Sensing, Ministry of Education, Institute for Clean Energy and Advanced Materials, School of Materials and Energy, Southwest University, Chongqing 400715, China2College of Chemistry and Chemical Engineering, Hainan Normal University, Haikou 571158, China
Three-dimensional (3D) cell culture methods have been validated that can replicate the tumor environment in vivo to a large extent, providing an effective tool for studying tumors. In this study, we demonstrated the use of standard laboratory pipette tips as micro vessels for generating 3D cell spheroids. No microfabrication or wet-chemistry surface modifications were involved in the procedure. Spheroids consisting of single or multiple cell types were generated within 24 h just by pipetting and incubating a cell suspension in pipette tips. Scanning electron microscope and optical microscope proved that the cells grew together tightly, and suggested that while gravity force might have initiated the sedimentation of cells at the bottom of the tip, the active aggregation of cells to form tight cell-cell interactions drove the formation of spheroids. Using common laboratory micropipettes and pipette tips, the rate of spheroid generation and the generation reproducibility was characterized from five boxes each with 80 tips. The ease of transferring reagents allowed modeling of the growth of microvascular endothelial cells in tumor spheroids. Moreover, the pairing and fusion of tumor spheroids could be manipulated in the pipette tips, suggesting the potential for building and assembling heterogeneous micro-tumor tissues in vitro to mimic solid tumors in vivo. This study demonstrated that spheroids can be readily and cost-effectively generated in standard biological laboratories in a timely manner using pipette tips.
Pipette tip; 3D cell culture; Tumor spheroids; Co-culture; In-situ observation
1 Introduction
Cancer is a leading cause of death and a huge barrier to increasing life expectancy worldwide (Bray et al., 2021). Though tremendous efforts have been made, the incidence and fatality rates of cancer are still viewed as one of humanity's most significant challenges. In addition to metastasis of cancer cells and the side effects of chemotherapy, cancer treatment faces considerable limitations due to the tumor environment in vivo, which is difficult to replicate in vitro using current technologies (Li et al., 2021). This makes the detailed study of tumors very difficult. Studying tissue structures and intercellular connections between cells has become essential for understanding the nature of tumors (Leeet al., 2021; Jinet al., 2022). However, to rebuild and replicate the tumor environment is extremely difficult. The most common cell culture method is 2D cell culture in a petri dish (Kadletzet al., 2015). 2D cell culture is used in many cell culture fields because of its convenience and cost-efficiency, but the cell morphology observed in 2D culture is very different from that of a tumor in vivo, which can lead to inconsistency in follow-up studies (Leeet al., 2018). To overcome this deficiency, 3D cell culture was proposed. The development of 3D cell culture is a great leap forward for cell culture technology. It soon became favored by many researchers because the in vivo tumor environment can be simulated to some certain extent (Nuneset al., 2019). Diverse 3D cell culture strategies have been proposed, including scaffold-based methods (Costaet al., 2016; Yanget al., 2019) and non-scaffold-based methods, such as liquid overlay technology (LOT) (Ivascu and Kubbies, 2006; Metzgeret al., 2011; Costaet al., 2014), hanging drop cell culture (Tunget al., 2011; Shriet al., 2017), agitation-based technology (Hanet al., 2006; Liuet al., 2006; Leeet al., 2011), microfluidics (Castiauxet al., 2019; Khotet al., 2020), and layer-by-layer (LbL) (Horiguchi and Sakai, 2015; Fukudaet al., 2018; Wanget al., 2018; Aljadiet al., 2022; Carvalhoet al., 2022) methods. Though succeeding in generating tumor spheroids, these 3D culture methods face various challenges (Breslin and O'driscoll, 2013; Aljadiet al., 2022). For example, a challenge for scaffold-based 3D cell cultures is to find a scaffold which has not only enough mechanical strength but also low toxicity. As for LOT, repeatability is a challenge. One technical problem of hanging drop cell culture is the stability of the drops. Experimenters must be careful to avoid droplets falling or deforming. The size uniformity of spheroids produced by agitation-based technology is of concern. Also, stirring is an essential process for agitation-based technology, but it can cause a certain degree of damage to cells while maintaining them in suspension. Microfluidics need expensive instruments and sophisticated channel designs. A simple, fabrication-free, user-friendly, and high-throughput method is greatly needed for routine use in biological laboratories to reproducibly generate cell spheroids.
Here, we introduce a high-throughput, simple, and time- and cost-effective method to generate cell spheroids. We used pipette tips, the standard commercial and universal laboratory items, as unique cell culture vessels. Pipette tips offer several advantages. First, the pipetting action can precisely aspirate a defined volume of cells in suspension. Second, the defined shape and material of the tip head restrains cell adhesion and promotes cells to form spheroids. Third, a multichannel pipette facilitates the high-throughput generation of cell spheroids. The dynamics of cell spheroid formation at different cell densities was quantified. In addition, the potential for producing spheroids with multiple cell types, spheroid-spheroid pairing, and testing of drug sensitivity on tumor spheroids was demonstrated using the pipette-tip-based 3D culture platform.
2 Materials and methods
2.1 Materials and reagents
Human prostate cancer cells (DU145) and human umbilical vein endothelial cells (HUVECs) were bought from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). The cells were maintained in Dulbecco's Modified Eagle Medium (DMEM, Gibco, Gaithersburg, USA) containing 10% fetal bovine serum (Gibco), penicillin (100 U/mL), and streptomycin (100 μg/mL) at 37 ℃ in a 5% CO2atmosphere. The long-term fluorescence tracer Dio, Calcein-AM, and propidium iodide (PI) were purchased from Beyotime Biotechnology, China. Sterilized pipette tips (10 μL) and gel-loading pipette tips (1–200 μL) were purchased from Biosharp, China. Doxorubicin (DOX) used for drug testing was purchased from Aladdin, China.
2.2 Generation of spheroids of a single cell type in pipette tips
For single cell type spheroid formation, human prostate cancer cells (DU145) were harvested from the culture plate to prepare cell suspensions with different cell densities. The ready-to-go procedure for generating single cell type spheroids is detailed in Fig. 1a. First, a DU145 cell suspension with a cell density of 2×106cells/mL was prepared. Then, 10-μL cell suspension was loaded into the tips. Next, the cell-loaded tips were unloaded and docked onto the tip box, which was filled with 50-mL sterilized deionized water to reduce evaporation of the medium inside the pipette tips. The tip box was placed in a cell incubator (37 ℃ and 5% CO2). The cell culture medium was replaced every 24 h using sharp gel-loading pipette tips. The cell-loaded tips were placed under a microscope (TS100-F, Nikon, Japan), and cell images were directly captured. In addition, after 1 d of incubation, the spheroids were harvested by pipetting. The cell aggregates formed by the cell density of 2×106cells/mL were collected in a 6-well microplate and images were captured using a desktop scanner (V800, Epson, Japan). The number and size of the spheroids were characterized using the particle analysis function of ImageJ software (NIH, USA).
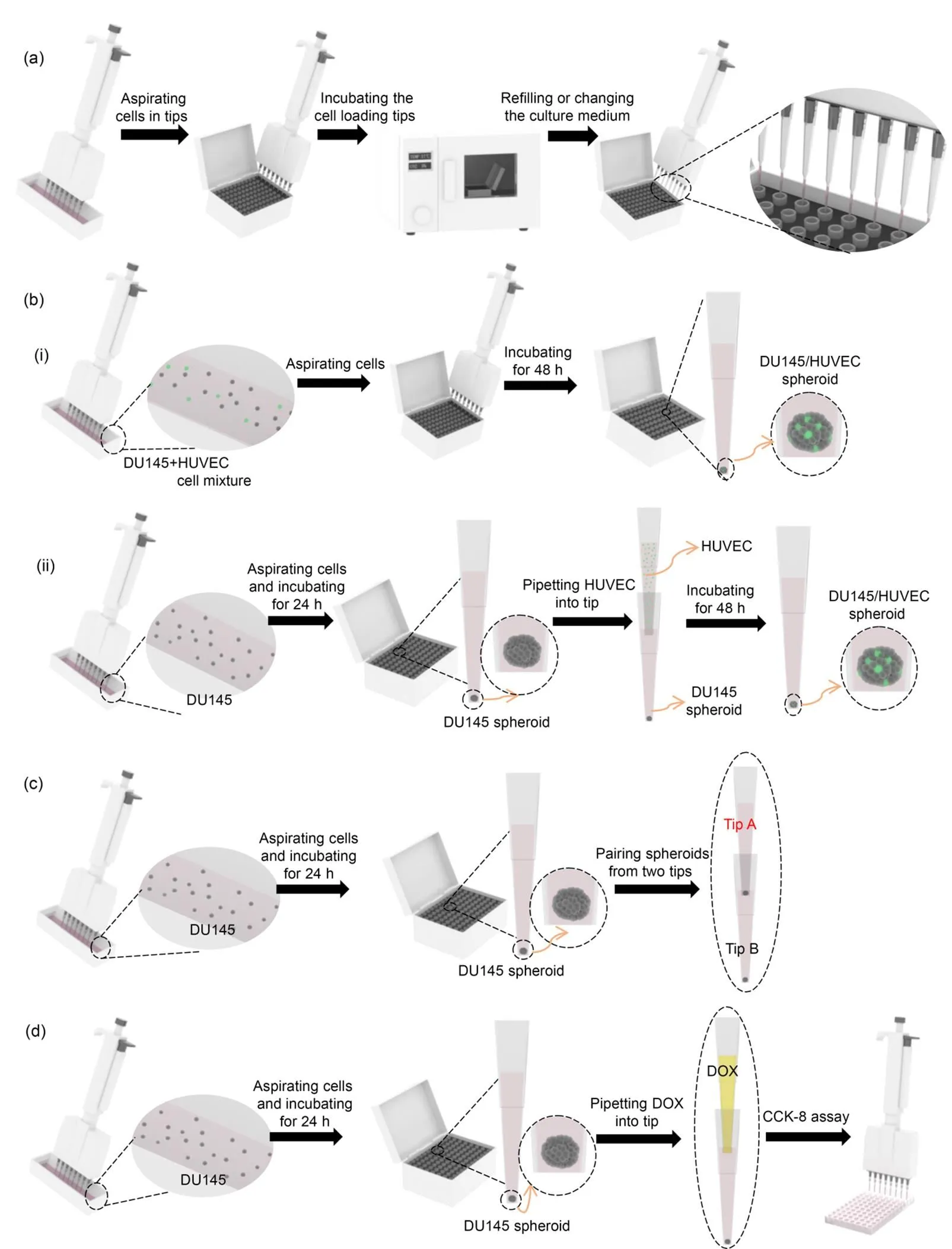
Fig. 1 Ready-to-generate cell spheroids in pipette tips: (a) generating spheroids of a single cell type in pipette tips; (b) generating spheroids with multiple cell types in pipette tips (one-step co-culture method (aspirating cell mixture) (b-i) and two-step co-culture method (adding HUVEC into a tip containing a DU145 spheroid) (b-ii)); (c) assembling and pairing of tumor spheroids; (d) evaluating drug efficacy using tumor spheroids in pipette tips
2.3 Generation of spheroids with multiple cell types in pipette tips
Two procedures were conducted to generate spheroids consisting of multiple cell types. To facilitate the observation and tracing of cells under fluorescent microscope, the live cell fluorescent dye, Dio (10 μg/mL), was used to stain cells according to product protocols. In brief, Dio was added after cells were collected in a 15-mL centrifuge tube. The cells were incubated at 37 ℃ for 10 min and then at 4 ℃ for 15 min. Next, the stained cells were washed three times with phosphate buffered saline (PBS, pH 7.4). Dio-labeled HUVEC cells were adjusted to different cell densities, then two different manipulations were performed (Fig. 1b). (i) One-step co-culture method: DU145 and HUVEC cells were mixed in a 5׃1 ratio. The mixed cell suspension was pipetted into the tips simultaneously. In detail, 200-μL DU145 (1×106cells/mL) and 200-μL HUVEC (2×105cells/mL) were mixed in a container. Then 10-μL mixed cell suspension was aspirated into the tips which were placed in a tip box for cell culture. The process of spheroid formation was monitored under a microscope. (ii)Two-step co-culture method: DU145 (1×106cells/mL) cells of 10 μL were loaded into the tips which were placed in a tip box for cell culture. After 24 h of culturing, 10-μL HUVEC (2×105cells/mL) cells were added to the DU145-preloaded tips from the upper end of the tips and incubated continuously. The process of spheroid formation was monitored under the microscope.
2.4 Pairing and fusion of spheroids in pipette tips
The procedure for the pairing and fusion of two spheroids is shown in Fig. 1c. In brief, 10-μL cell suspension was loaded into the pipette tips (Tip A, Tip B) and incubated for 24 h. Then a cell spheroid formed in Tip A was gently pipetted into Tip B which contained another cell spheroid. The interaction of the two cell spheroids was tracked under the microscope.
2.5 Characterization of the cell spheroids generated in pipette tips
Scanning electron microscope (SEM) characterization: An SEM (SU3500, Hitachi, Japan) was used to examine the cell spheroids collected from the pipette tips. DU145 cell suspension (2.0×106cells/mL) of 10 μL was loaded in the tips and incubated for 24 h in a cell culture incubator. Then, the cell spheroids were collected for fixation and dehydration. In brief, the cell spheroids were fixed in 4% paraformaldehyde solution and then dehydrated successively in a concentration gradient of ethanol solutions (50%, 60%, 70%, 80%, 90%, and 100%). Samples were dried overnight in air after the gradient dehydration was completed. Before SEM measurement, samples were sprayed with platinum for 360 s using an Auto Fine Coater (JEC-3000FC, JEOL, Japan) to facilitate SEM observation.
Live and dead cell staining: DU145 cell suspension (2.0×106cells/mL) of 10 μL was loaded in the tips and cultured in a cell incubator. After 1, 3, or 5 d of incubation, the spheroids were pipetted into a 96-well plate for live and dead cell staining. In brief, a working solution containing 2-μmol/L Calcein-AM and 8-μmol/L PI was prepared in PBS (pH 7.4) for spheroid staining. After incubation for 1 h at 37 ℃, the spheroids were washed with fresh PBS buffer three times to remove the excess dyes. Then, images of the spheroids were captured using an inverted fluorescence microscope at 645-nm excitation for PI and 530-nm excitation for Calcein-AM.
2.6 Three-dimensional tumor spheroids in pipette tips for drug testing
A concentration series of DOX was prepared to treat the cell spheroids. DU145 cell suspension (1.0×106cells/mL) of 10 μL was loaded in the tips and incubated for 1 d. DOX solution of 10 μL was added to each tip. After 2 d of incubation, to quantify the anti-tumor effect of DOX, the percentage of live cells was measured using the CCK-8 assay (Fig. 1d). In brief, spheroids in the pipette tips were digested using AccutaseTMstem cell dissociation reagent. Then, cells were suspended in 100-μL culture medium and placed in a 96-well plate. Next, CCK-8 reagent was added to the cells at a final concentration of 10% and incubated for 2 h at 37 ℃. Finally, the absorbance was measured at 450 nm with a reference wavelength of 630 nm using an ELx800TMmicroplate reader (GENE, Hong Kong, China). The cell viability was calculated as the ratio of the percentage absorbance in DOX-treated cells and untreated cells.
2.7 Statistical analysis
All experiments were performed three times. Data are expressed as the mean±standard deviation. Data were analyzed with the Student's-test using Origin Statistic software (OriginLab, USA).values less than 0.05 were considered to be statistically significant.
3 Results
3.1 Cell aggregation and spheroid formation facilitated by ready-to-go universal pipette tips
Fig. 2a shows images of clear pipette tips (1–10 μL) that are most commonly used in chemistry, biology, forensic, pharmaceutical, and drug discovery labs. The inner diameter of the tip head is 660 μm. The liquid in the tip will not drop out unless pushed (Fig. 2b). The liquid in the tip is much more stable than that of a hanging drop. The 10-μL liquid height in tips was 14 mm. There was space available above the liquid for adding additional cell suspension or cell culture medium from the upper end of the tip using a gel-loading pipette tip. The cells in the clear pipette tips could be observed from different angles and orientations under an optical microscope without perturbation or destruction of cell growth (Fig. 2c). Cells in the suspension gradually settled in the lower part of the tip because of the action of gravity (Fig. 2d). After 18 h of incubation, the shape of the cell aggregate gradually changed due to active cell interactions. Evaluation of cells in the pipette tip suggested that the force of gravity might initiate the settlement of the cells in the tip. However, cell-cell interactions were the driving force promoting cell aggregation and spheroid formation. The spheroid formation process finished after 24 h (Fig. 2d). Compared with previous methods, significantly less time was required to generate the spheroids (Table 1).
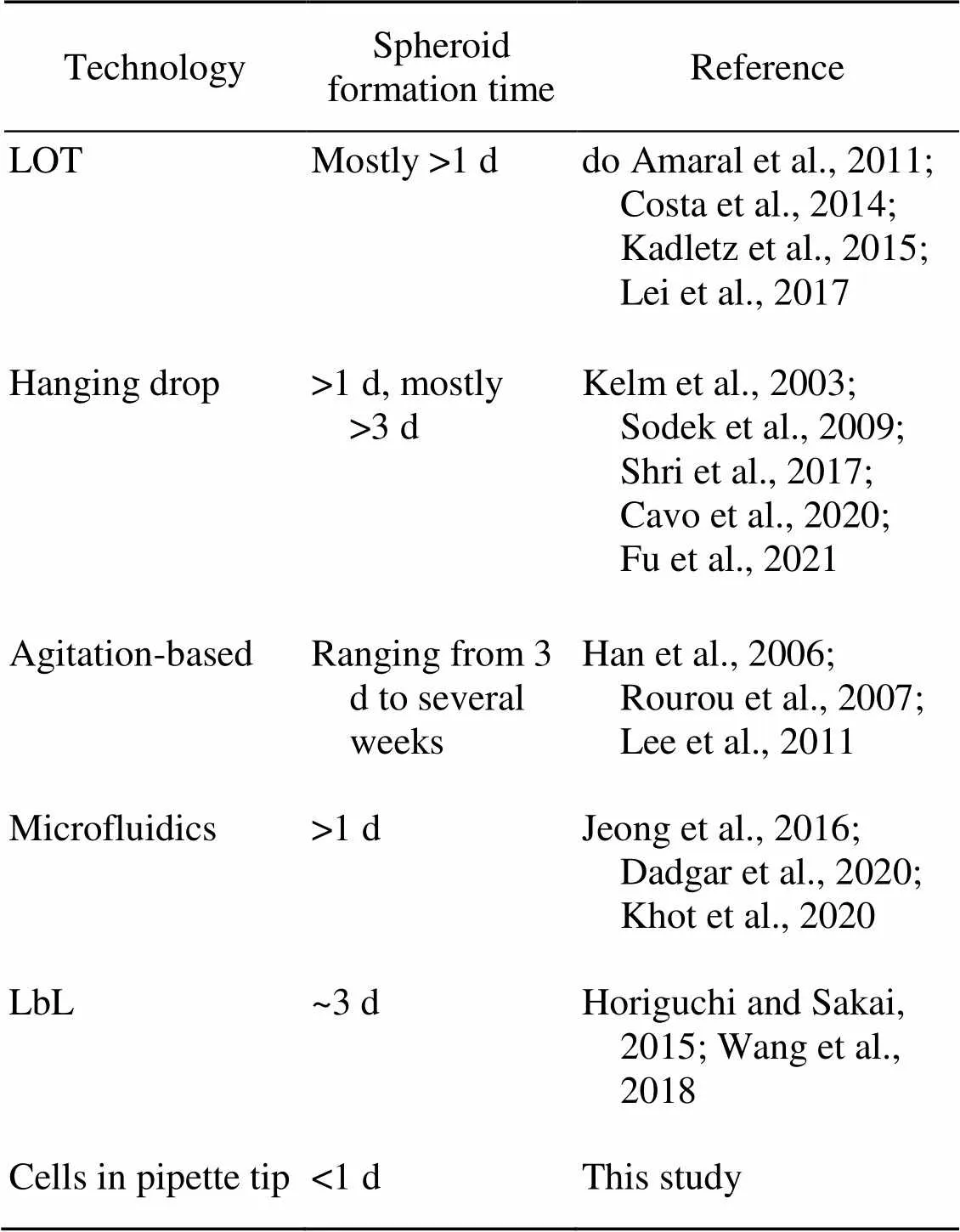
Table 1 Comparison of spheroid formation time for several 3D cell culture technologies
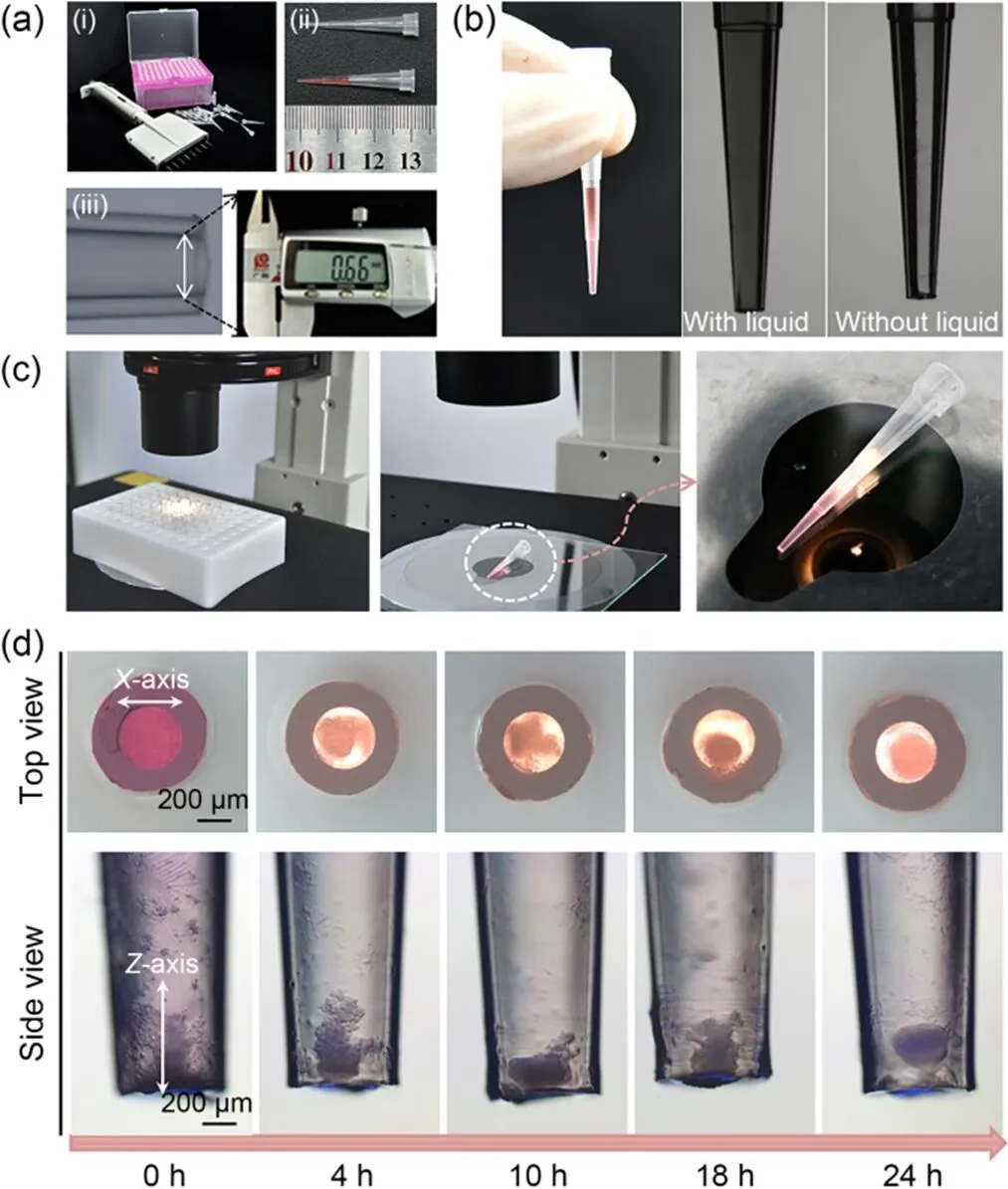
Fig. 2 Pipette tips and cell spheroids in the tips: (a) the features of pipette tips used for this experiment (the pipette and pipette tips (a-i), tips with and without 10-μL solution (a-ii), and micrograph of the tip head (inner diameter ~660 μm) (a-iii)); (b) photos of the 10-μL tip with and without liquid; (c) in-situ observation of the cells in the tip from different angles and orientations; (d) the process of cell spheroid formation (4×104 cells/tip)
Next, the impact of initial cell densities on spheroid formation was studied. With the increase in cell number from 1×104to 4×104cells/tip, the horizontal (-axis) and vertical (-axis) dimensions of the cell aggregates enlarged proportionally (Fig. 3a). For instance, the ratio of the-axis to the-axis for aggregates formed from 1×104cells/tip, 2×104cells/tip, and 4×104cells/tip were 1.067, 1.145, and 1.029, respectively, suggesting a spheroid was formed. When the cell density was further increased to 8×104cells/tip, the cell aggregate had no room to expand horizontally, but the-axis of the aggregate increased, forming a columnar cell structure. When the number of cells exceeded 4×104cells/tip, the shape of the aggregate was no longer spheroid. The ratios of the-axis to the-axis were 0.886 and 0.762 when the numbers of cells were 8×104cells/tip and 1×105cells/tip, respectively. The results indicate that the initial cell numbers determined the size and architecture of the cell aggregates. To prove that cell spheroid did not simply represent the stacking of cells because of gravitational action, but was the result of tight cell-cell interaction, the morphology of the cell spheroids was characterized. SEM characterization showed that cells were closely packed instead of being simply piled up (Fig. 3b). Cells were tightly connected because there were strong interactions between them. Brightfield microscopic images showed that the inner side of the spheroid was darker than the outer part, which is in line with the reported characteristics of spheroids (Fig. 3c). According to previous studies, cells in the inner part of spheroids suffer from nutrient depletion and oxygen scarcity (Costaet al., 2016). The Calcein-AM/PI staining result (Fig. 3c) indicated that the center of the tumor spheroids contained a higher proportion of dead cells, suggesting the formation of a necrotic core characteristic of solid tumors.
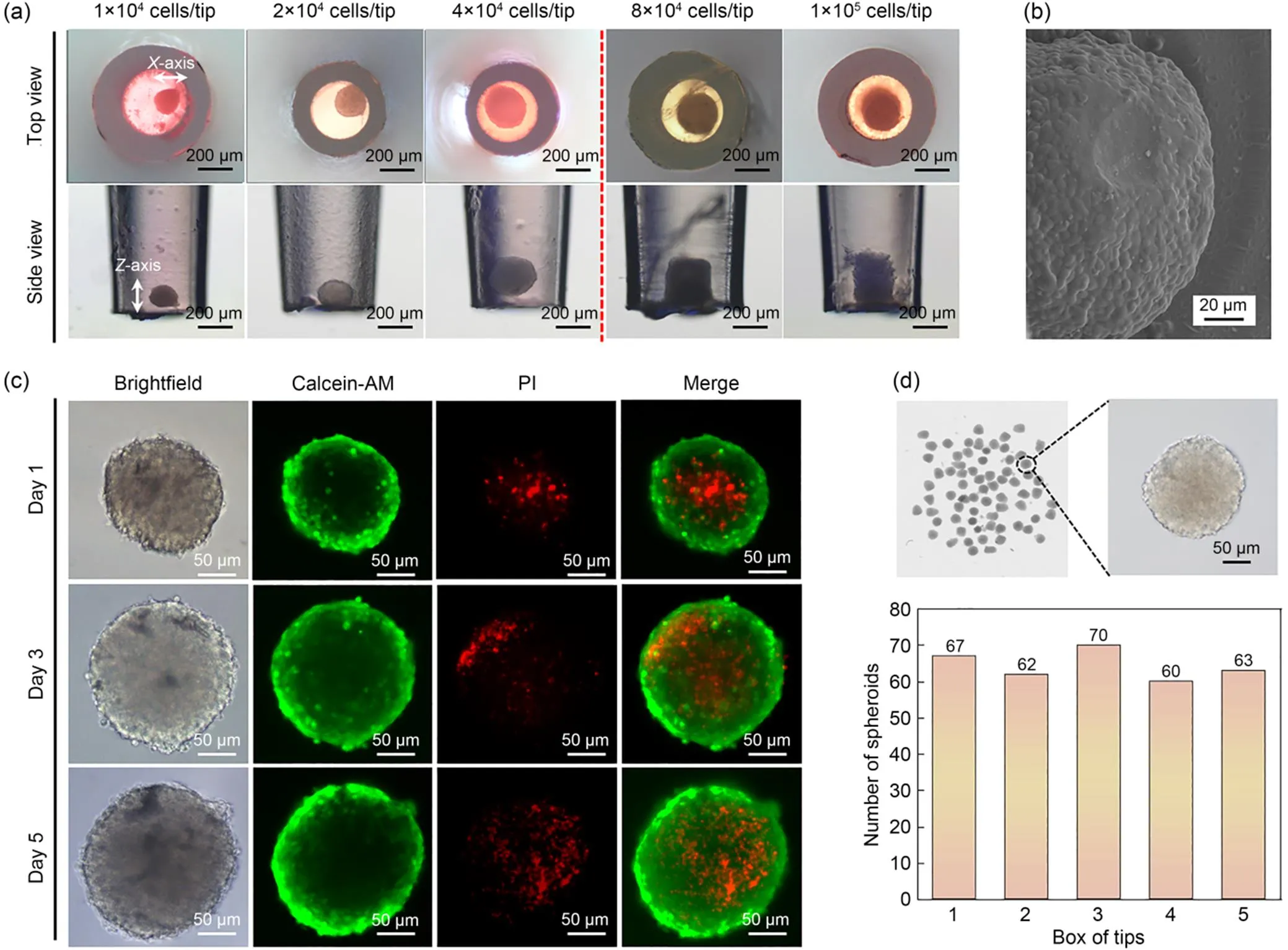
Fig. 3 Dynamics of tumor spheroid formation in pipette tips: (a) micrographs of cell aggregates formed from different initial cell numbers; (b) SEM image of a DU145 spheroid; (c) live/dead cell distribution in tumor spheroids stained using a Calcein-AM/PI kit; (d) scanner images of spheroids generated using a multichannel pipette
SEM and live/dead cell fluorescent staining showed that the proposed pipette tip method could be used to quickly generate tumor spheroids in ordinary laboratories. The high-throughput generation of reproducible spheroids is another advantage. We tested a multichannel pipette that can dock eight tips simultaneously to generate spheroids. For each box of tips, 80 tips were loaded with cells. After 1 d of incubation, cells were pipetted out of the 80 tips and transferred into a 6-well microplate. Particle analysis using ImageJ showed that 70 spheroids formed, and the formation rate was (87.5±4.2)% (Fig. 3d). The average area of the spheroids was (25492.31±2062.95) μm2. Moreover, the reproducibility of the spheroids was characterized by testing five boxes of tips simultaneously. The spheroid formation ratio for the five boxes of tips was (80.5±5.0)%. The results highlighted the potential of the pipette tips for high-throughput generation of cell spheroids.
3.2 Formation of spheroids consisting of multiple cell types in pipette tips
Following the procedure illustrated in Fig. 1b-i, a cell suspension containing tumor cells of DU145 and HUVEC cells was loaded in the pipette tip. After 48 h of incubation, the mixed cells gathered and formed one spheroid (Fig. 4a). In addition, HUVEC cells labeled with the Dio green fluorescent dye were distributed throughout the whole spheroid. A higher density of green HUVEC cells was observed in the inner core of the tumor spheroid. Following the procedure illustrated in Fig. 1b-ii, tumor cells of DU145 were first loaded in the pipette tip to form a spheroid, then HUVEC cells were added to the same tip. After 48 h of co-culture, we found that HUVEC cells had infiltrated the existing DU145 tumor spheroid (Fig. 4b). Both methods proved that co-culture of different cell types could be achieved in the pipette tips.
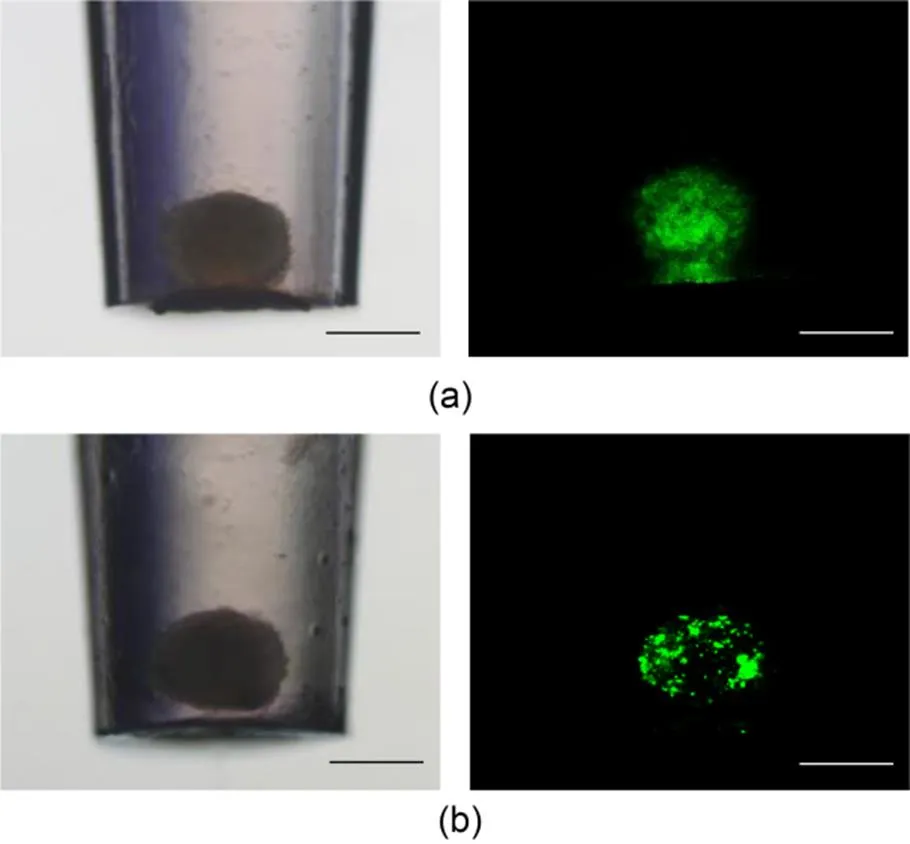
Fig. 4 Generation of spheroids consisting of multiple cell types: (a) one-step method (aspirating a cell mixture containing DU145 and HUVEC cells (green fluorescence) into a tip at the same time); (b) two-step method (aspirating DU145 cells into the tip to form a spheroid, and then adding HUVEC cells into the tip containing DU145 cell spheroids). References to color refer to the online version of this figure (scale bar=200 μm)
3.3 Pairing and fusion of spheroids in pipette tips
Two tumor spheroids could be paired by pipetting one spheroid formed in a tip into another tip containing a spheroid (Fig. 1c). Initially, the newly added spheroid was in the upper part of the liquid inside the tip, which already had a spheroid at the bottom. The upper spheroid gradually migrated down until it met the spheroid at the bottom. The two spheroids began to merge and eventually formed a single bigger tumor spheroid. In addition to gravity, there were complex intercellular and extracellular interactions that made this process happen (Fig. 5a). Although occupied by two spheroids, there was still space available in the tip. Therefore, we conducted pairing experiments with more spheroids (Fig. 5b). All the procedures could be conducted using pipettes and pipette tips, highlighting the practical potential of assembling microtissues by pipetting.

Fig. 5 Assembly of microtumor tissue by pairing spheroids in a pipette tip: (a) fusion of two spheroids; (b) fusion of multiple spheroids (scale bar=200 μm)
3.4 Evaluating anti-tumor efficacy in pipette tips
To verify the drug response of spheroids generated by this new culture platform, DOX was used as a model chemotherapy reagent to treat the spheroids. The morphology of the spheroids was impacted by the drug treatment (Fig. 6a). The CCK-8 results showed that with increasing DOX concentration, the percentage of live cells within the spheroids decreased. In addition, under the same DOX concentration, the cell survival rate of spheroids was higher than that of 2D cultured cells, indicating that cells in 2D culture were more sensitive to DOX treatment (Fig. 6b). The drug resistance characteristics of tumor spheroids generated by this new 3D culture platform were in line with the results of previous studies. The drug testing done in the pipette tips demonstrated that this platform can be used for screening drug efficacy.

Fig. 6 Evaluating DOX anti-tumor efficacy in pipette tips: (a) images of tumor spheroids treated with different DOX concentrations (scale bar=200 μm); (b) the viability of DU145 cells cultured as a 3D spheroid in pipette tips and as a 2D monolayer in a 96-microwell plate
4 Discussion
Tumor spheroids formed successfully in pipette tips, providing a new platform for 3D cell culture. Plenty of spheroids formed in a short period. The critical step in tumor spheroid formation is to make cell–cell connections produced by a wide variety of proteins stronger than cell–environment interaction forces (Białkowska et al., 2020). We hypothesized that cell adhesion into the vertical pristine polyethylene tips was constrained, and the geometrical conical shape of the tips promoted cell contact and even aggregation. The shape of cell aggregates could be adjusted by changing the initial cell densities. With increasing cell number, the shape of cell aggregates gradually changed from spherical to cylindrical because of the geometrical limitations of the tip. We proved that aspirating and incubating cells in a pipette tip could quickly generate tumor spheroids, even micro tumor tissue, to mimic in vivo 3D tissue structures in vitro.
Heterogeneity is one characteristic of cells in vivo (Altschuler and Wu, 2010). Cells in a living body are surrounded with other cells with different functions. They interact to sustain the activity of living organisms (Tang et al., 2020). Thus, the establishment of co-culture systems in vitro is one important strategy to replicate the microenvironment in vivo. For instance, angiogenesis is one of the critical steps in tumor growth and metastasis (Folkman, 1995; Quail and Joyce, 2013). The co-culture of tumor and blood vessel epithelial cells may be helpful for studying their interactions, including gene expression, proliferation rate, and cell functions. Previous studies suggested that the deficiencies in oxygenation in solid tumors stimulate the secretion of hypoxia-inducible factor (HIF)-1α, which controls the up-regulation of a number of factors necessary for solid tumor expansion, including the vascular endothelial growth factor (VEGF) (Ryan et al., 2000). The VEGF cytokine attracts vascular endothelial cells causing them to grow into the tumor tissue and form microvasculature (Hicklin and Ellis, 2005). For the co-culture system in pipette tips, HUVEC showed a preference for gathering in the inner part of the tumor spheroids, mimicking the angiogenesis process. For the one-step pipetting procedure, tumor cells, fibroblast cells, and stromal cells could be put together and aspirated into a tip to generate heterogeneous cell spheroids. The second procedure, involving sequentially introducing cells into an existing spheroid, could mimic the merging and infiltration functions of cells, such as vascular endothelial cells and lymphocytes penetrating solid tumors.
Pairing and fusion of tumor spheroids have been shown to be typical processes in cancer development (Sarkaret al., 2013), and are also two of the self-assembly processes crucial for multicellular architecture engineering (Koshelevaet al., 2020; Bustamante et al., 2021). Multicellular architectures are essential for modeling the construction of living systems, the occurrence of diseases, and the evaluation of therapeutic effects (Muelleret al., 2020; Cuiet al., 2021). The fusion of spheroids is an efficient method of tissue reconstruction (Jakabet al., 2010). Cells undergo various changes during fusion and pairing which are important in cancer treatment studies (Cuiet al., 2021). We performed a spheroid fusion experiment inside the tip. Pipetting a spheroid from one tip into another is a simple operation by which the fusion of two or more spheroids within one tip can be achieved. A drug testing experiment was also conducted using this platform. The drug was added directly into a tip containing a tumor spheroid. The killing process carried out in the tip could be directly observed under the microscope. The above results show that the tip platform has more experimental versatility than other culture platforms. The whole experimental operation was simple, requiring few experimental skills for adding or changing the culture medium or transferring spheroids to other platforms for further analysis. It was extremely convenient without disrupting or disturbing the growth of the spheroids.
5 Conclusions
Tumor spheroid formation was demonstrated by aspirating cells in a pipette tip. A simple aspiration and incubation procedure was applied to generate spheroids consisting of a single or multiple cell types. In addition, pairing and fusion of spheroids to assemble larger cell aggregates were demonstrated, highlighting the potential for generating and culturing mini tumor tissues in the pipette tip. The pipette tip platform also can be applied to drug screening. Adding different DOX concentrations induced spheroid damage in the pipette tip. The small volume of the pipette tip significantly reduced the consumption of reagents. Since biological laboratories are commonly equipped with pipettes and pipette tips, the proposed pipette tip-based spheroid formation platform can eliminate the equipment barrier for resource-limited laboratories to conduct 3D cell culture and research.
Acknowledgments
This work is supported by the National Natural Science Foundation of China (No. 32171401), the Natural Science Foundation of Chongqing (No. CSTB2022NSCQ-MSX0808), and the Specific Research Fund of the Innovation Platform for Academicians of Hainan Province (No. YSPTZX202126), China.
Author contributions
Conceptualization: Rong PAN and Ling YU. Data curation: Rong PAN, Xiaoyan YANG, Shiming WU, and Feng CHEN. Formal analysis: Rong PAN, Yuanyuan XIE, and Wei SUN. Methodology and investigation: Rong PAN, Ke NING, and Xiaoyan YANG. Project administration and supervision: Ling YU. Funding acquisition: Ling YU. Writing–original draft: Rong PAN. Writing–review and editing: Rong PAN, Ling YU, and Wei SUN.
Conflict of interest
Rong PAN, Xiaoyan YANG, Shiming WU, Yuanyuan XIE, Feng CHEN, Ke NING, Wei SUN, and Ling YU declare that they have no conflict of interest.
Aljadi Z, Aval NA, Kumar T, et al., 2022. Layer-by-layer cellulose nanofibrils: a new coating strategy for development and characterization of tumor spheroids as a model for in vitro anticancer drug screening., 22(10):2200137. https://doi.org/10.1002/mabi.202200137
Altschuler SJ, Wu LF, 2010. Cellular heterogeneity: do differences make a difference?, 141(4):559-563. https://doi.org/10.1016/j.cell.2010.04.033
Białkowska K, Komorowski P, Bryszewska M, et al., 2020. Spheroids as a type of three-dimensional cell cultures—examples of methods of preparation and the most important application., 21(17):6225. https://doi.org/10.3390/ijms21176225
Bray F, Laversanne M, Weiderpass E, et al., 2021. The ever-increasing importance of cancer as a leading cause of premature death worldwide., 127(16):3029-3030. https://doi.org/10.1002/cncr.33587
Breslin S, O’driscoll L, 2013. Three-dimensional cell culture: the missing link in drug discovery., 18(5-6):240-249. https://doi.org/10.1016/j.drudis.2012.10.003
Bustamante DJ, Basile EJ, Hildreth BM, et al., 2021. Biofabrication of spheroids fusion-based tumor models: computational simulation of glucose effects., 13(3):035010. https://doi.org/10.1088/1758-5090/abe025
Carvalho BG, Vit FF, Carvalho HF, et al., 2022. Layer-by-layer biomimetic microgels for 3D cell culture and nonviral gene delivery., 23(4):1545-1556. https://doi.org/10.1021/acs.biomac.1c01130
Castiaux AD, Spence DM, Martin RS, 2019. Review of 3D cell culture with analysis in microfluidic systems., 11(33):4220-4232. https://doi.org/10.1039/c9ay01328h
Cavo M, Delle Cave D, D’Amone E, et al., 2020. A synergic approach to enhance long-term culture and manipulation of MiaPaCa-2 pancreatic cancer spheroids., 10(1):10192. https://doi.org/10.1038/s41598-020-66908-8
Costa EC, Gaspar VM, Coutinho P, et al., 2014. Optimization of liquid overlay technique to formulate heterogenic 3D co-cultures models., 111(8):1672-1685. https://doi.org/10.1002/bit.25210
Costa EC, Moreira AF, de Melo-Diogo D, et al., 2016. 3D tumor spheroids: an overview on the tools and techniques used for their analysis., 34(8):1427-1441. https://doi.org/10.1016/j.biotechadv.2016.11.002
Cui HJ, Wang XX, Wesslowski J, et al., 2021. Assembly of multi-spheroid cellular architectures by programmable droplet merging., 33(4):2006434. https://doi.org/10.1002/adma.202006434
Dadgar N, Gonzalez-Suarez AM, Fattahi P, et al., 2020. A microfluidic platform for cultivating ovarian cancer spheroids and testing their responses to chemotherapies., 6:93. https://doi.org/10.1038/s41378-020-00201-6
do Amaral JB, Rezende-Teixeira P, Freitas VM, et al., 2011. MCF-7 cells as a three-dimensional model for the study of human breast cancer., 17(11):1097-1107. https://doi.org/10.1089/ten.tec.2011.0260
Folkman J, 1995. Angiogenesis in cancer, vascular, rheumatoid and other disease., 1(1):27-30. https://doi.org/10.1038/nm0195-27
Fu JJ, Lv XH, Wang LX, et al., 2021. Cutting and bonding Parafilm®to fast prototyping flexible hanging drop chips for 3D spheroid cultures., 14(2):187-199. https://doi.org/10.1007/s12195-020-00660-x
Fukuda Y, Akagi T, Asaoka T, et al., 2018. Layer-by-layer cell coating technique using extracellular matrix facilitates rapid fabrication and function of pancreatic β-cell spheroids., 160:82-91. https://doi.org/10.1016/j.biomaterials.2018.01.020
Han Y, Liu XM, Liu H, et al., 2006. Cultivation of recombinant Chinese hamster ovary cells grown as suspended aggregates in stirred vessels., 102(5):430-435. https://doi.org/10.1263/jbb.102.430
Hicklin DJ, Ellis LM, 2005. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis., 23(5):1011-1027. https://doi.org/10.1200/JCO.2005.06.081
Horiguchi I, Sakai Y, 2015. Alginate encapsulation of pluripotent stem cells using a co-axial nozzle., (101):e52835. https://doi.org/10.3791/52835
Ivascu A, Kubbies M, 2006. Rapid generation of single-tumor spheroids for high-throughput cell function and toxicity analysis., 11(8):922-932.https://doi.org/10.1177/1087057106292763
Jakab K, Norotte C, Marga F, et al., 2010. Tissue engineering by self-assembly and bio-printing of living cells., 2(2):022001. https://doi.org/10.1088/1758-5082/2/2/022001
Jeong SY, Lee JH, Shin Y, et al., 2016. Co-culture of tumor spheroids and fibroblasts in a collagen matrix-incorporated microfluidic chip mimics reciprocal activation in solid tumor microenvironment., 11(7):e0159013. https://doi.org/10.1371/journal.pone.0159013
Jin Z, Li X, Liu B, et al., 2022. Coaxial bioprinted microfibers with mesenchymal stem cells for glioma microenvironment simulation., 5:348-357. https://doi.org/10.1007/s42242-021-00155-2
Kadletz L, Heiduschka G, Domayer J, et al., 2015. Evaluation of spheroid head and neck squamous cell carcinoma cell models in comparison to monolayer cultures., 10(3):1281-1286. https://doi.org/10.3892/ol.2015.3487
Kelm JM, Timmins NE, Brown CJ, et al., 2003. Method for generation of homogeneous multicellular tumor spheroids applicable to a wide variety of cell types., 83(2):173-180. https://doi.org/10.1002/bit.10655
Khot MI, Levenstein MA, de Boer GN, et al., 2020. Characterising a PDMS based 3D cell culturing microfluidic platform for screening chemotherapeutic drug cytotoxic activity., 10(1):15915. https://doi.org/10.1038/s41598-020-72952-1
Kosheleva NV, Efremov YM, Shavkuta BS, et al., 2020. Cell spheroid fusion: beyond liquid drops model., 10(1):12614. https://doi.org/10.1038/s41598-020-69540-8
Lee HJ, Mun S, Pham DM, et al., 2021. Extracellular matrix-based hydrogels to tailoring tumor organoids., 7(9):4128-4135. https://doi.org/10.1021/acsbiomaterials.0c01801
Lee J, Shin D, Roh JL, 2018. Development of an in vitro cell-sheet cancer model for chemotherapeutic screening., 8(14):3964-3973. https://doi.org/10.7150/thno.26439
Lee TJ, Bhang SH, La WG, et al., 2011. Spinner-flask culture induces redifferentiation of de-differentiated chondrocytes., 33(4):829-836. https://doi.org/10.1007/s10529-010-0488-1
Lei KF, Lin BY, Tsang NM, 2017. Real-time and label-free impedimetric analysis of the formation and drug testing of tumor spheroids formed via the liquid overlay technique., 7(23):13939-13946. https://doi.org/10.1039/c7ra00209b
Li M, Song X, Jin S, et al., 2021. 3D tumor model biofabrication., 4:526-540. https://doi.org/10.1007/s42242-021-00134-7
Liu XM, Liu H, Wu BC, et al., 2006. Suspended aggregates as an immobilization mode for high-density perfusion culture of HEK 293 cells in a stirred tank bioreactor., 72(6):1144-1151. https://doi.org/10.1007/s00253-006-0409-3
Metzger W, Sossong D, Bächle A, et al., 2011. The liquid overlay technique is the key to formation of co-culture spheroids consisting of primary osteoblasts, fibroblasts and endothelial cells., 13(8):1000-1012. https://doi.org/10.3109/14653249.2011.583233
Mueller M, Rasoulinejad S, Garg S, et al., 2020. The importance of cell-cell interaction dynamics in bottom-up tissue engineering: concepts of colloidal self-assembly in the fabrication of multicellular architectures., 20(4):2257-2263. https://doi.org/10.1021/acs.nanolett.9b04160
Nunes AS, Barros AS, Costa EC, et al., 2019. 3D tumor spheroids as in vitro models to mimic in vivo human solid tumors resistance to therapeutic drugs., 116(1):206-226. https://doi.org/10.1002/bit.26845
Quail DF, Joyce JA, 2013. Microenvironmental regulation of tumor progression and metastasis., 19(11):1423-1437. https://doi.org/10.1038/nm.3394
Rourou S, van der Ark A, van der Velden T, et al., 2007. A microcarrier cell culture process for propagating rabies virus in Vero cells grown in a stirred bioreactor under fully animal component free conditions., 25(19):3879-3889. https://doi.org/10.1016/j.vaccine.2007.01.086
Ryan HE, McNulty W, Elson D, et al., 2000. Hypoxia-inducible factor-1α is a positive factor in solid tumor growth., 60(15):4010-4015.
Sarkar S, Horn G, Moulton K, et al., 2013. Cancer development, progression, and therapy: an epigenetic overview., 14(10):21087-21113. https://doi.org/10.3390/ijms141021087
Shri M, Agrawal H, Rani P, et al., 2017. Hanging drop, a best three-dimensional (3D) culture method for primary buffalo and sheep hepatocytes., 7(1):1203. https://doi.org/10.1038/s41598-017-01355-6
Sodek KL, Ringuette MJ, Brown TJ, 2009. Compact spheroid formation by ovarian cancer cells is associated with contractile behavior and an invasive phenotype., 124(9):2060-2070. https://doi.org/10.1002/ijc.24188
Tang R, Murray CW, Linde IL, et al., 2020. A versatile system to record cell-cell interactions., 9:e61080. https://doi.org/10.7554/eLife.61080
Tung YC, Hsiao AY, Allen SG, et al., 2011. High-throughput 3D spheroid culture and drug testing using a 384 hanging drop array., 136(3):473-478. https://doi.org/10.1039/c0an00609b
Wang J, Miao Y, Huang Y, et al., 2018. Bottom-up nanoencapsulation from single cells to tunable and scalable cellular spheroids for hair follicle regeneration., 7(3):170047. https://doi.org/10.1002/adhm.201700447
Yang YJ, Wu HC, Jia JB, et al., 2019. Scaffold-based 3-D cell culture imaging using a miniature electrical impedance tomography sensor., 19(20):9071-9080. https://doi.org/10.1109/jsen.2019.2924154
使用移液器吸头培养三维肿瘤球
潘茸1,杨晓艳1,武士铭1,谢媛媛1,陈凤1,宁珂1,孙伟2,余玲1
1西南大学,材料与能源学院,清洁能源与先进材料研究所,发光分析与分子传感教育部重点实验室,中国重庆,400715;2海南师范大学,化学化工学院,中国海口,571158
三维细胞培养相较于常规的二维培养在模拟肿瘤微环境上具备很大的优势。本文旨在将移液器吸头(商品化且实验室通用的一种耗材)作为独特的细胞培养容器进行细胞培养,以实现高通量、简单、省时且经济高效的三维细胞球培养。1. 将移液器吸头作为细胞培养容器,其实验过程未涉及任何机械加工和化学处理,因此极大地简化了肿瘤球培养实验过程,为实验条件有限的实验室提供了三维细胞培养的替代方案;2. 该培养平台集稳定、便捷、省时、低成本和高通量于一体;3. 该平台在培养过程中可进行原位观测;4. 除了用于细胞球的培养外,该培养方式还展示出在肿瘤球融合和药物筛选等方面的应用潜力。1. 将细胞悬液吸到移液器吸头中,并将吸头置于吸头盒内,然后放入细胞培养箱里进行常规细胞培养。2. 培养24 h后,在显微镜下观察细胞团聚形成三维细胞球;可直接推动移液器按钮进行细胞球的转移和后续分析。3. 将两个或多个在吸头内形成的细胞球转移到同一个吸头,实现多个肿瘤球的配对和融合。4. 待细胞成球后,在移液器吸头内加入药物,评价药物的细胞毒性。1. 将移液器吸头作为三维细胞培养平台,通过简单地抽吸和培养,即可在短时间内高通量地获得细胞球。2. 成功构建了两种肿瘤细胞(DU145)与血管内皮细胞(HUVEC)的共培养模型。3. 两个或多个细胞球可通过配对和融合组装成更大的细胞聚集体,显示了该平台在微组织工程领域中的应用前景。4. 成功在移液器吸头内完成了原位药物筛选;不同浓度的阿霉素会对移液器吸头中的细胞球造成不同程度的损伤。
移液器吸头;三维细胞培养;肿瘤球;共培养;原位观测
27, 2022;
https://doi.org/10.1631/jzus.A22D0235
https://doi.org/10.1631/jzus.A22D0235
Revision accepted Mar. 28, 2023;
Crosschecked Sept. 14, 2023
© Zhejiang University Press 2023
猜你喜欢
杂志排行
Journal of Zhejiang University-Science A(Applied Physics & Engineering)的其它文章
- Micro-Newton scale variable thrust control technique and its noise problem for drag-free satellite platforms: a review
- Effect of CO2-mixing dose and prolonged mixing time on fresh and hardened properties of cement pastes
- Geo-environmental properties and microstructural characteristics of sustainable limestone calcined clay cement (LC3) binder treated Zn-contaminated soils
- Design and comparative analysis of self-propelling drill bit applied to deep-sea stratum drilling robot
- Experimental investigation of the thermal insulation performance of Ce/Si/Ti oxide heat-reflective coating
- Microfluidic fuel cells integrating slanted groove micro-mixers to terminate growth of depletion boundary layer thickness
