Prediction model of stress ulcer after laparoscopic surgery for colorectal cancer established by machine learning algorithm
2023-10-21DongMeiYuChunXiaoWuJunYiSunHuiXueZheYuwenJiangXueFeng
Dong-Mei Yu, Chun-Xiao Wu, Jun-Yi Sun, Hui Xue, Zhe Yuwen, Jiang-Xue Feng
Abstract
Key Words: Colorectal cancer; Laparoscope; Stress ulcer; Risk factors; Nomogram
INTRODUCTION
Colorectal cancer (CRC) is a common malignant tumor with an increasing incidence, which seriously threatens people's health[1]. It is easily overlooked by patients as the early symptoms are not obvious. As the cancer progresses, patients may experience abdominal pain, constipation and diarrhea, and in the advanced stage, they may experience general symptoms such as anemia and weight loss[2]. CRC kills over 900000 individuals worldwide each year, which makes it the fourth most lethal cancer[3]. Due to the advantages of low trauma, aesthetic criteria, and rapid postoperative recovery, laparoscopic radical dissection of CRC has emerged as the primary treatment for this disease[4,5]. However, CO2pneumoperitoneum, hemodynamic changes, local immune dysfunction and other reasons may lead to changes in neuroendocrine metabolism in patients, causing a significant stress response[6,7]. Stress ulcer (SU) is one of the more typical postoperative complications in patients with CRC. The main clinical manifestations are acute mucosal erosion and ulceration, which eventually progress to hemorrhage and intestinal perforation, leading to a major physiological and psychological impact on the patient[8]. Severe bleeding from a SU can result in longer hospital stays and an elevated risk of death. Therefore, analyzing and preventing SU risk factors in CRC patients can effectively reduce mortality, accurately forecast CRC predisposition, and provide targeted treatment for high-risk groups. In the present study, we examined the clinical information of 135 CRC patients admitted to Hebei Traditional Chinese Medicine Hospital, examined the risk factors for the growth of SU after CRC surgery, and built a nomogram model to provide a foundation for the early prevention and risk reduction of SU after CRC surgery.
MATERIALS AND METHODS
Data source and inclusion criteria
All 135 patients with CRC who underwent laparoscopic surgery at the Hebei Traditional Chinese Medicine Hospital between November 2021 and June 2022 were chosen for this retrospective study. The patients consisted of 80 males and 55 females, ranging in age from 22 to 85 years, with a mean age of (55.16 ± 13.18) years. Inclusion criteria were: (1) Meet the diagnostic criteria for CRC in the “Chinese protocol of diagnosis and treatment of colorectal cancer (2020 edition)”[9] and confirmed by pathology; (2) All patients underwent laparoscopic surgery; and (3) Complete clinical data. Exclusion criteria were: (1) Presence of hematological diseases; (2) Recent peptic ulcer; (3) Recent gastrointestinal surgery or invasive gastrointestinal examination; and (4) Other trauma, bleeding,etc. leading to blood in the digestive tract.
Research methods
The patients were divided into the SU (23 cases) and non-SU (112 cases) group according to whether they developed a SU after surgery, with an SU incidence of 17.04%. SU was diagnosed if the patient met one of the following parameters: (1) Spitting or gastric extract showed visible bright red or coffee colored liquid; (2) Tar colored stool, black stool or fecal occult blood test was positive; and (3) Microscopic examination revealed that the patient's gastric mucosa showed patchy or punctate bloody lesions, ulceration or erosion[10].
Observed indices
Clinical data collected included gender, age, smoking history, drinking history, liver and kidney function, lymph node status,etc. Laboratory examinations included routine blood and biochemical blood indicators including C-reactive protein, tumor necrosis factor α, interleukin (IL)-6, IL-8, heat shock protein (HSP) 70, HSP90, gastrin (GAS), hemoglobin, albumin, fasting blood glucose at admission, serum potassium,etc.
Statistical analysis
SPSS26.0 statistical software was used for data processing and analysis. Theχ2test was applied to compare the two groups. Data on counts are presented as [n(%)]. The Shapiro-Wilk test was used to determine normalcy. The two independent samplest-test was used to analyze measurement data with a normal distribution. The non-normal distribution measurement data were reported as the median and interquartile range [M (P25, P75)], and the Mann-WhitneyUtest was employed. The risk factors for SU after CRC surgery were evaluated using univariate and multivariate logistic regression analysis, and the risk factors were imported into R software to develop a nomogram model to predict the risk of SU following CRC surgery. To assess the model's discrimination, a receiver operating characteristic (ROC) curve was created and the area under the curve (AUC) was determined. An AUC ≥ 90% was excellent, 70%-89% was good, 50%-69% was moderate, and 0.05 indicated good stability.P≤ 0.05 was regarded as statistically significant.
RESULTS
Clinical data of the SU group and non-SU group
The differences in age, lymph node metastases, HSP70, HSP90, and GAS levels between the two groups were statistically significant, while the other indicators were not. The SU group had higher levels of HSP70, HSP90, and GAS than the non-SU group (Table 1).
Multivariate logistic regression analysis
Univariate analysis was employed to identify statistically significant variables among the 17 factors related to CRC, including gender, age, drinking history, smoking history, abnormal liver and kidney function, lymph node metastasis, postoperative albumin decline and other laboratory indicators, to assign them (Age: < 65 years = 1, ≥ 65 years = 2; lymph node metastasis: yes = 1, no = 0), and regression analysis was carried out using a multivariate model. The results indicated that age ≥ 65 years, lymph node metastasis, and increased levels of HSP70, HSP90 and GAS were all independent risk factors for the development of SU in CRC patients after surgery (P< 0.05) (Table 2).
Construction and validation of the nomogram
Based on the independent risk factors for postoperative SU in CRC patients screened out in multivariate analysis, the risk prediction model of postoperative SU in patients was established by R statistical software. The individual scores for each risk factor were obtained from the scale at the top of the nomogram for that factor, and the scores for all risk factors were added together to obtain a total score to obtain the incidence of SU in the corresponding patient. A higher total score indicated a greater likelihood of developing a SU (Figure 1). The AUC of the ROC was 0.988 (95%CI: 0.971-1.0), indicating that this nomogram model discriminated well (Figure 2). When the Youden index was 0.908, the related sensitivity and specificity were 93.3% and 97.5%, respectively. The training and validation set calibration curves suggested that the simulated and actual curves essentially followed the same trend (Figure 3), suggesting that prediction of the probability of postoperative SU in patients with CRC obtained by the nomogram model had good consistency with the actual probability. The Hosmer-Lemeshow goodness of fit test revealed no statistically significant change (χ2= 0.753,P= 0.999), indicating that the model was well calibrated and stable. The decision curve analysis (DCA) is based on continuous potential risk thresholds, and the net benefit of risk-stratifying patients illustrates the model's clinical value. The prediction model's decision curves revealed that the model trended away from extreme curves with a high net benefit and clinical practicability (Figure 4).
DISCUSSION
SU is one of the postoperative complications of CRC. It occurs when the human body is subjected to various major injuries or psychological diseases, causing acute gastrointestinal mucosal erosion, ulcer and other lesions. Severe cases can be complicated by gastrointestinal bleeding or even perforation, leading to aggravation and deterioration of the original disease and increased mortality[11]. Analyzing the risk factors for SU in patients with CRC is of great significance for the prevention and prognosis of SU after CRC surgery.
Serum HSP70 and HSP90 are highly conserved stress proteins in the heat shock protein family. They have anti-inflammatory and anti-oxidation effects and can affect cell stability and the stress response[12]. Studies have found that HSP70 and HSP90 Levels are closely linked to SU risk[13,14]. When gastric mucosal cells are stimulated by trauma factors, the cell protein configuration changes, which can induce cell inflammatory response and activate HSP70 and HSP90. With the increase in HSP70 and HSP90 content, this promotes the synthesis and folding of proteins in gastric mucosal cells, thereby reducing the damage caused by the stress response to mucosal cells and exerting a role in gastric mucosal protection[15]. According to our findings, patients in the SU group had greater serum levels of HSP70 and HSP90 than patients in the non-SU group. It is suggested that the higher the levels of HSP70 and HSP90, the more severe the postoperative stress response in patients with CRC, the greater the compensatory increase in HSP70 and HSP90, the more serious the damage caused by stress response to gastric mucosal cells, and the higher the risk of SU. Therefore, serum levels of HSP70 and HSP90 are indicators of the likelihood of developing a SU in individuals who undergo laparoscopic surgery for CRC.
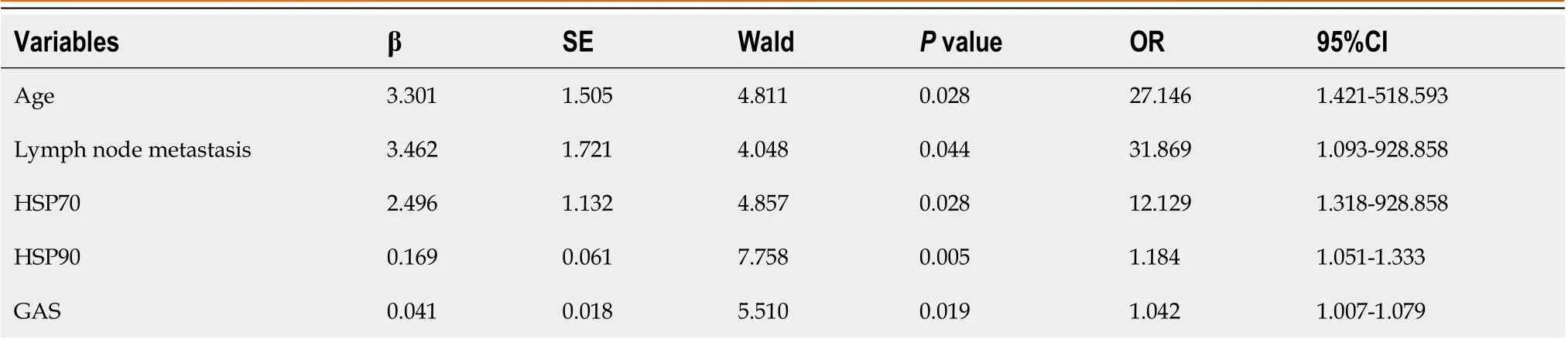
Table 2 Multivariate logistic regression analysis of factors affecting postoperative stress ulcer in patients with colorectal cancer

Figure 1 Nomogram for predicting postoperative stress ulcer in colorectal cancer patients. HSP70: Heat shock protein 70; HSP90: Heat shock protein 90; GAS: Gastrin.
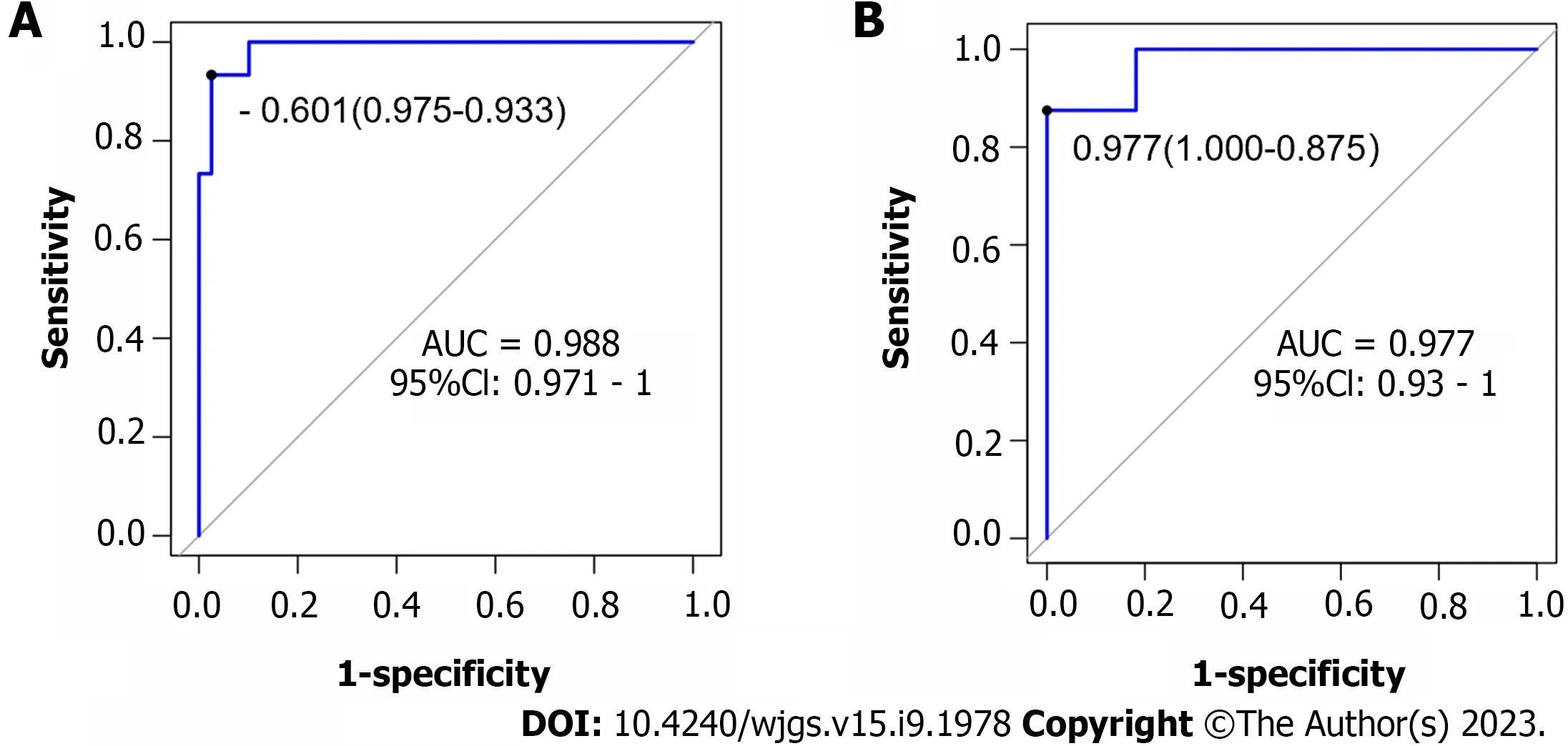
Figure 2 Validation of the nomogram by the receiver operating characteristic curve. A: The training cohort; B: The validation cohort. AUC: Area under the curve.
GAS is a potent hormone that promotes gastric acid secretion. The increase in GAS level may be related to sympathetic nerve excitation caused by trauma, continuous contraction of gastric mucosal blood vessels, vagus nerve choline fiber excitation caused by increased intracranial pressure, use of a strong dose of dehydrating agent and catabolic disorder[16]. Studies have found that when the level of serum GAS in critically ill patients increases, it will increase gastric acid secretion, resulting in transient small intestinal dysfunction and decreased gastric emptying capacity and gastric pyloric sphincter tension. Food reflux from the small intestine stimulates GAS secretion[17]. According to the study findings, patients with SUs had higher serum GAS levels than patients without SUs. The results of regression analysis showed that GAS level was a risk factor for SU, which was consistent with the results reported in the literature[12]. In this study, it was concluded that the increase in serum GAS level correlated with disease severity, and that the severity of the patient's condition was correlated with the intensity of the stress response and stomach mucosal damage, which could be utilized as a predictor of the likelihood of developing a SU.
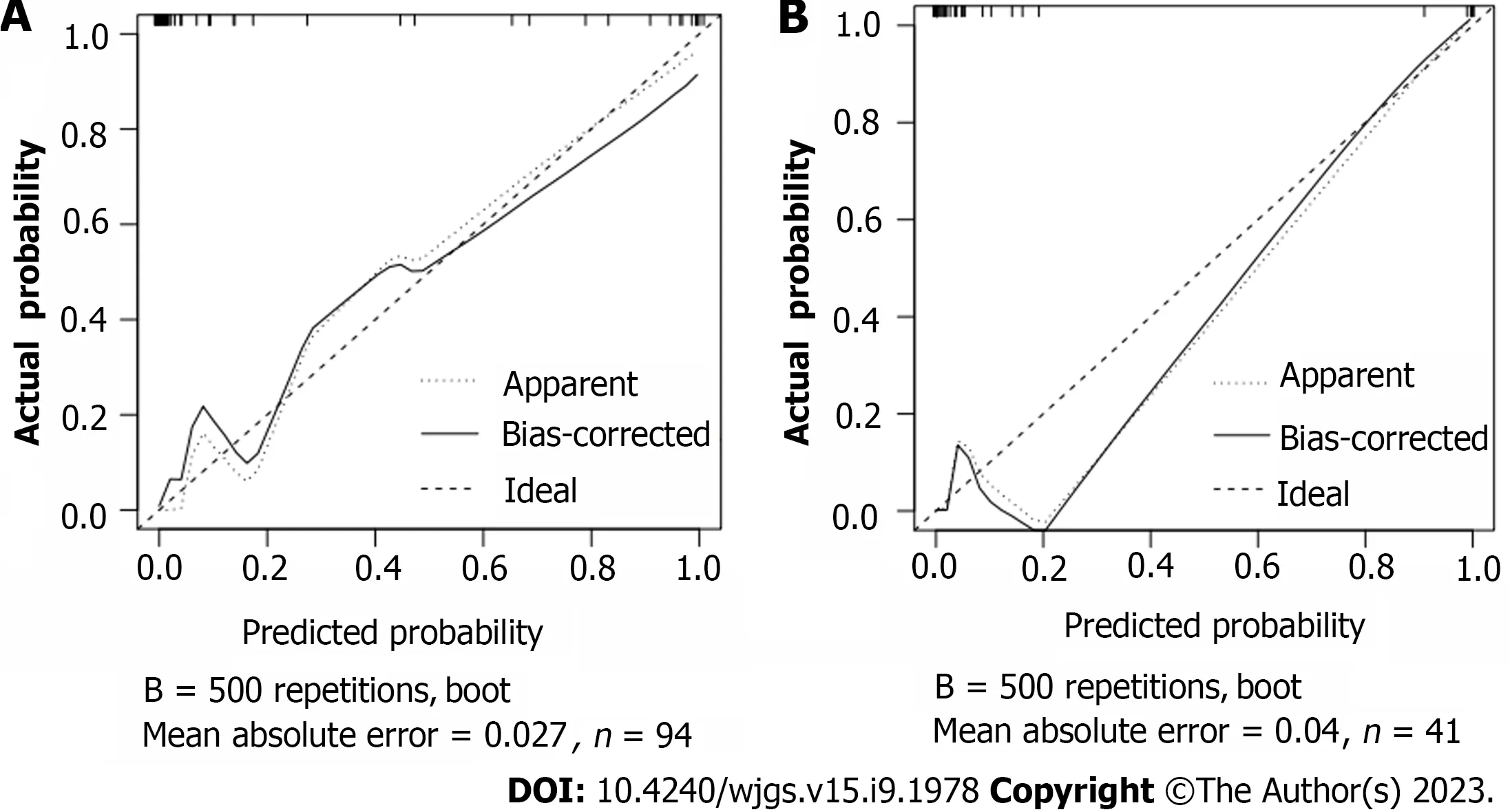
Figure 3 Calibration plot of the nomogram for the probability of metastasis. A: The training cohort; B: The validation cohort.
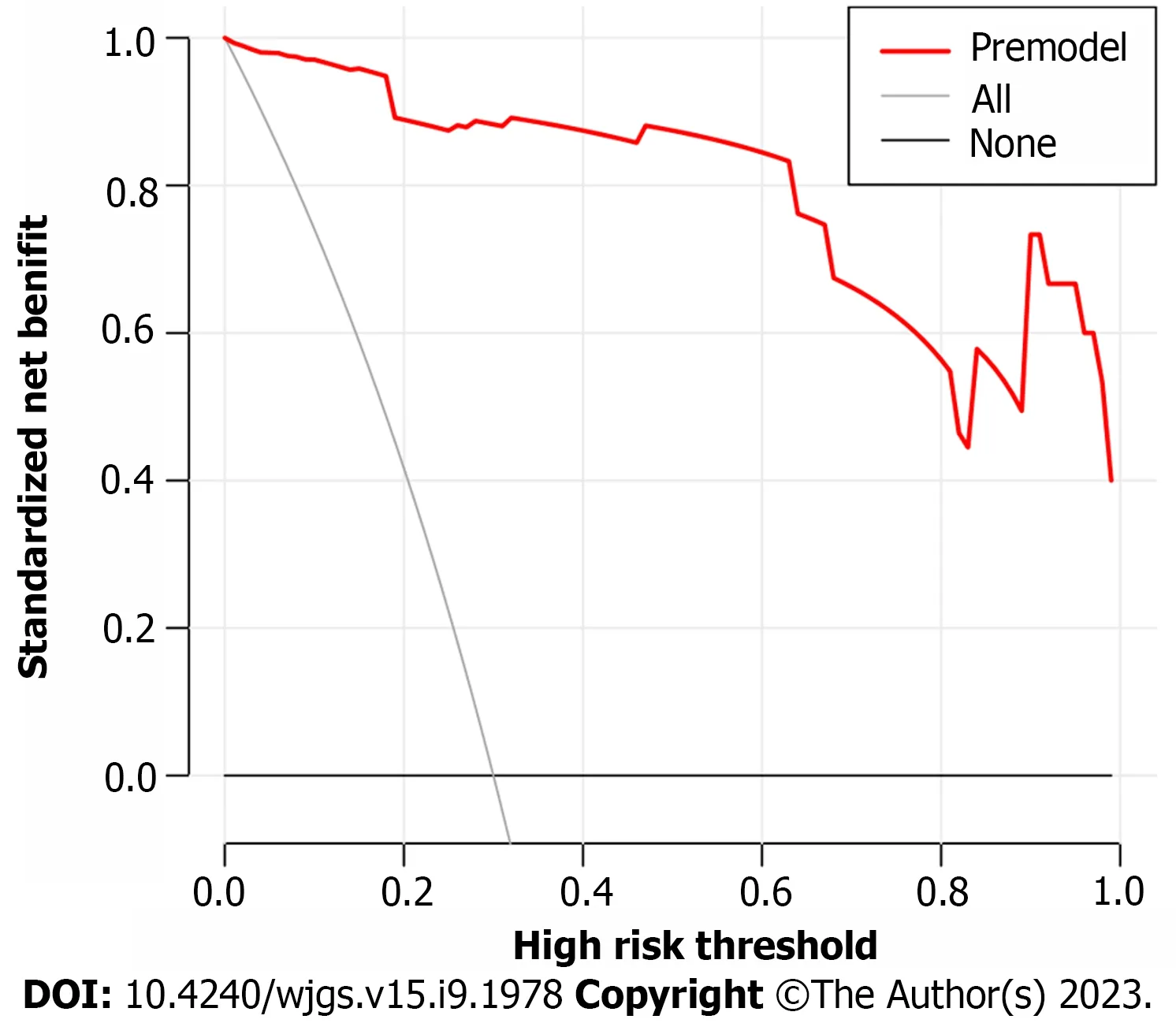
Figure 4 Prediction model decision curve analysis diagram.
The results of this study suggest that age ≥ 65 years and lymph node metastasis are risk factors for the development of SU following CRC surgery. This may be the reason why elderly patients are prone to SU, and may be related to stress changes such as relatively low physical resistance and decreased postoperative self-regulation ability. Lymphatic metastasis is a common feature of advanced cancer. Surgical treatment of advanced rectal cancer often involves a long operation time and complicated procedures. Lymph node dissection leads to increased surgical trauma, stress response, acid-base imbalance and further acidosis. At the same time, the increase in oxygen free radicals increases the risk of SU bleeding[18].
In this study, 17 factors that may affect postoperative SU in patients with CRC were investigated by univariate analysis combined with multivariate logistic regression analysis. To construct a nomogram model, five independent risk factors identified in the logistic analysis were entered into R software. The nomogram model performed well in terms of discrimination, as indicated by the AUC of 0.988 (95%CI: 0.971-1.0), and this performance was further confirmed in the validation set. In addition, the calibration curves also demonstrated the good consistency of the nomogram model. No significant difference was found by the Hosmer-Lemeshow goodness of fit test (χ2= 0.753,P= 0.999), indicating a stable and wellcalibrated model. However, these results do not fully explain whether the nomogram model can be applied in clinical practice. Therefore, further analysis by DCA was carried out and the results of the prediction model DCA showed that the model was far from the extreme curve and the net benefit rate was high, showing that the nomogram had good clinical applicability. In this retrospective study with a small number of affecting factors, the findings may be biased as it was not a multi-center, large-sample epidemiological survey. In the future, a more reasonable and larger-sample prospective randomized controlled clinical trial will be designed to further improve the model's predictive value.
CONCLUSION
Age ≥ 65 years, lymph node metastasis, and elevated HSP70, HSP90, and GAS are independent risk factors for postoperative SU in patients with CRC. The nomogram model constructed accordingly had high clinical application value, calibration, and stability. It is helpful for clinicians to take targeted measures to reduce the incidence of postoperative SU in patients with CRC.
ARTICLE HIGHLIGHTS

FOOTNOTES
Author contributions:Yu DM designed and performed the research and wrote the paper; Feng JX designed the research and supervised the report; Yu DM and Feng JX designed the research and contributed to the analysis; Sun JY, Wu CX, Xue H and Yuwen Z provided clinical advice; Sun JY, Wu CX, Xue H and Yuwen Z supervised the report; all authors have read and approved the final version to be published.
Institutional review board statement:The study was reviewed and approved by the Hebei Traditional Chinese Medicine Hospital.
Informed consent statement:All study participants or their legal guardian provided informed written consent regarding personal and medical data collection prior to study enrolment.
Conflict-of-interest statement:The authors declare no conflicts of interest for this article.
三是无法剥离型财产。关联性企业中往往存在着交叉型财产,所谓交叉型财产是在企业合法运营程序和非法运营本质的共同作用下产生的财产。这部分财产不具有典型的涉黑财产本质,但在财产获取或者流向上存在部分非法的状态。由于财产具有不可分性或者财产非法成分具有不可估价性,或许会导致合法财产无法从中予以剥离的情形。为了从根本上打击关联性企业,应当将此部分财产作为单纯性涉黑财产来进行整体性处置,但是存在善意相对人的情况应当保留其对财产的相关诉讼权利,以做到公平公正、不伤及无辜。
Data sharing statement:The data set for this study can be obtained from the corresponding author.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORCID number:Dong-Mei Yu 0009-0008-2990-7358; Chun-Xiao Wu 0000-0002-9883-2141; Jun-Yi Sun 0009-0008-5390-6478; Hui Xue 0009-0007-6296-5245; Zhe Yuwen 0009-0001-4459-6833; Jiang-Xue Feng 0009-0007-0826-2995.
S-Editor:Yan JP
L-Editor:A
P-Editor:Cai YX
猜你喜欢
杂志排行
World Journal of Gastrointestinal Surgery的其它文章
- Preoperative and postoperative complications as risk factors for delayed gastric emptying following pancreaticoduodenectomy: A single-center retrospective study
- Comparative detection of syndecan-2 methylation in preoperative and postoperative stool DNA in patients with colorectal cancer
- Preoperative prediction of microvascular invasion in hepatocellular carcinoma using ultrasound features including elasticity
- Surgical management of gallstone ileus after one anastomosis gastric bypass: A case report
- Hepatic ischemia-reperfusion syndrome and its effect on the cardiovascular system: The role of treprostinil, a synthetic prostacyclin analog
- Advances and challenges of gastrostomy insertion in children
