Effects of ultrasound monitoring of gastric residual volume on feeding complications, caloric intake and prognosis of patients with severe mechanical ventilation
2023-10-21XiaoYanXuHuiPingXueMingJunYuanYouRongJinChunXiaHuang
Xiao-Yan Xu, Hui-Ping Xue, Ming-Jun Yuan, You-Rong Jin, Chun-Xia Huang
Abstract
Key Words: Gastric residual monitoring; Mechanical ventilation; Vomit; Caloric intake; Prognosis
INTRODUCTION
Patients with invasive mechanical ventilation in intensive care units (ICU) are in a high catabolic state and are prone to malnutrition, resulting in intestinal ischemia and reperfusion injury and affecting intestinal immune functions[1]. As one of the important therapeutic nutritional support interventions for severe patients, enteral nutrition can maintain the normal physiological functions of the gastrointestinal tract, prevent intestinal villus atrophy, and guarantee intestinal barrier functions[2]. The nutrition guidelines recommend that if there is no contraindication, enteral nutrition support can be started at 24-48 h after ICU admission[3]. To reduce the mortality rates, infection incidences, as well as hospitalization time and improve the prognostic outcomes of patients, early implementation of enteral nutrition should conform to the physiological needs of the gastrointestinal tract of patients[4]. However, for ICU patients, their gastrointestinal functions are impaired, and there are feeding intolerance (FI) risks during enteral nutrition implementation. There is no unified standard definition for FI. Currently, the definitions proposed by the European Society of Intensive Care Medicine in 2012[5] are widely used, including gastrointestinal adverse reactions, low rate of energy requirements and termination of enteral nutrition. Incidence of FI during early enteral nutrition have been reported to be between 30.5%-67.5%. Therefore,timely and accurate evaluation of gastrointestinal functions is particularly important. Monitoring of gastric residual is an important approach for evaluating gastric emptying of patients with mechanical ventilation. By monitoring gastric contents, the enteral nutrition scheme can be adjusted in time to ensure feeding safety[6,7]. Various methods for monitoring gastric residual volume (GRV) in clinics have been proposed. The most traditional and common method is aspiration, which involves using a syringe to extract gastric contents through the gastric tube. Even though this method is simple to operate, its measurement results are affected by many factors, such as position of the tip of the gastric tube and suction force degree. The extracted gastric contents are exposed to the air and are easily contaminated[8]. Moreover,when the gastric contents are discarded, it is easy to lose the nutrient solution and the digestive fluid in the stomach, and when target feeding amount cannot be attained, it increases the malnutrition risk in patients. Gastric ultrasound can provide information about the nature and volume of gastric contents at the bedside[9]. The accuracy and repeatability of gastric ultrasound has been reported in previous studies. Although it cannot fully assess the gastric functions and state(such as pH value), it can provide important and useful information, such as volume and nature of gastric contents(transparent liquid, solid or not)[9-11]. The accuracy of ultrasonic monitoring of GRV is also high, and there is no need to withdraw gastric contents, which reduces body fluid exposure risks[12]. However, the correlation between gastric residual and poor prognostic outcomes, such as aspiration, ventilator-related pneumonia and FI has not been fully elucidated[13-15]. The guidelines[16] issued by the critical illness Association and the American Association for parenteral and enteral nutrition in 2016 do not recommend monitoring of gastric residual amounts in clinical routine or assessing the feeding tolerance of patients by only relying on gastric residual amounts. However, a previous survey[6,17-19] revealed that 97.1% of nurses judge whether patients have FI by monitoring gastric residual amounts because the monitoring method is simple and convenient.
The aim of this study was to investigate the effects of ultrasound monitoring on incidence of feeding complications,daily caloric intake and clinical prognosis of patients with severe mechanical ventilation. Moreover, we analyzed its clinical significance to provide a theoretical basis for guiding clinical practice.
MATERIALS AND METHODS
Study participants
Patients admitted to the department of emergency medicine of the Affiliated Hospital of Nantong University from January 2018 to June 2022, and who received invasive mechanical ventilation and continuous enteral nutrition support within 24-48 h after admission were enrolled in this study. Medical records of the patients within 7 d of hospitalization were retrospectively analyzed to compare incidences of feeding complications, daily caloric intake and clinical prognosis between patients with gastric residual ≥ 250 mL and those with < 250 mL, as monitored by ultrasound on the third day of admission.
Patient data were retrospectively collected from the electronic medical records system of the intensive care units.Screening of study participants and data collation were performed as shown in Figure 1.
The inclusion criteria were: (1) No previous gastrointestinal dysfunction and enteral nutrition for 3 d; (2) Aged ≥ 18 years; and (3) Patients or family members who agreed to sign the informed consent form.
The exclusion criteria were: (1) Presence of aspiration pneumonia, diarrhea or diabetes before admission to intensive care units; (2) Shock, gastrointestinal bleeding, gastrointestinal surgery, severe intestinal obstruction, severe abdominal distension and diarrhea; (3) Abdominal space syndrome; (4) Enteral nutrition treatmentviajejunum feeding or gastroenterostomy; and (5) Patients with incomplete case data records.
General observation index
The general data and clinical characteristics of study participants, including age, sex, body mass index (BMI), acute physiology and chronic health evaluation II (APACHE II), sequential organ failure assessment (SOFA), and disease diagnosis among others were collected.
Feeding complications
Vomiting: Stomach contents flow out of the mouth and nose through the esophagus. Diarrhea: The number of daily defecations is more than 3 times, feces are thin, the water content is high, and the daily defecation volume is more than 200 g. Abdominal distension: Discomfort caused by abdominal swelling or fullness.
Prognostic indicators
Data on time of mechanical ventilation, daily caloric intake from day 3 to day 7 after hospitalization in the ICU, the time to reach the feeding target, ICU hospitalization days and mortality were collected. The time to reach the feeding target:the number of days to reach 25 kcal/kg/D in gastrointestinal nutrition.
Daily caloric intake: Obtained by multiplying the volume of nutrient solution (mL) taken by the patient every day by the energy density of the nutrient solution (kcal/mL) divided by body weight.
Ultrasonic monitoring of gastric remnants
The monitoring frequency of gastric remnants was once every 4 h. Briefly, patients were placed in supine positions (the head of the bed was raised by 30°-45°), the portable color ultrasound diagnostic instrument was selected, the probe frequency was set at 2-5 mhz, and the single section of the antrum selected, that is, the ultrasound probe was placed under the xiphoid process of the patient and perpendicular to the abdomen angle. The antrum, the superior mesenteric artery, the left lobe of the liver and the abdominal aorta were examined to locate the position of the antrum, and ultrasound used to determine the size of the antrum. The area of the antrum was calculated by measuring the transverse and anterior posterior diameters of the antrum, after which the gastric residual was obtained by comparing the area of the antrum with age. When residual amount of the stomach exceeded 250 mL, enteral nutrition was stopped and further monitoring performed after 2-4 h. If < 250 mL, enteral nutrition was continued. If the gastric residual was still high, the jejunal nutrition tube or drug treatment was reserved according to patient's conditions, and if necessary, it was changed to parenteral nutrition support. Since some patients were hospitalized for 24-48 h, continuous enteral nutrition was not given until the condition was relatively stable. The GRV of patients was collected on the third day of ICU hospitalization,and the patients were assigned into ≥ 250 mL and < 250 mL groups.

Figure 1 Study flowchart.
Statistical analysis
The results for each scale were input into the computer for score conversion. The SPSS 24.0 software (IBM Corp., Armonk,NY, United States) was used for statistical analyses. Measurement data are expressed as means ± SD, while the counting data are expressed as frequencies and percentages.t-tests, analysis of variance, and chi square tests were used for intergroup statistical analyses. Logistic regression models were established for multivariate analyses. BilateralP< 0.05 was set as the threshold for statistical significance.
RESULTS
Baseline data
A total of 513 patients (451 in the < 250 mL group and 62 in the ≥ 250 mL group) were enrolled in this study. There were 267 (59.2%) males in the 250 mL group, with age (53.04 ± 3.9 years), BMI (20.39 ± 2.5), APACHE II scores (6.39 ± 2.44), and SOFA (3.51 ± 0.53). There were 33 (53.2%) males in the ≥ 250 mL group, with age (53.92 ± 4.29 years), BMI (20.87 ± 2.49),APACHE II scores (16.71 ± 2.41), and SOFA (3.47 ± 0.5). Differences in general data between the groups were insignificant(Table 1).
Comparisons of medication and complications between the groups
Results showed that 29.9% and 25.1% of patients in the < 250 mL group used sedatives or sedatives, compared to 48.4%and 38.7% in the ≥ 250 mL group (P< 0.05). The probabilities of abdominal distension, diarrhea and vomiting in the < 250 mL group were 18.4%, 23.9% and 4.0%, compared with 21.0%, 32.3% and 6.5% in the ≥ 250 mL group (P> 0.05; Table 2).
Comparisons of prognostic outcomes between groups
The time to reach the feeding target was significantly shorter for the ≥ 250 mL group, compared to that of the < 250 mL group (P< 0.05). Differences in mechanical ventilation time, ICU hospitalization days and mortality rates between the two groups were not significant (P> 0.05). Caloric intake (22.0, 23.6, 24.8, 25.3 kcal/kg/d) for patients in the < 250 mL group was lower compared with that of patients in the < 250 mL group (23.2, 24.8, 25.7, 25.8 kcal/kg/d). Caloric intakes on the 4thday (Z= 4.324,P= 0.013), 5thday (Z= 3.376,P= 0.033) and 6thday (Z= 3.098,P= 0.04) were significant (Figure 2 and Table 3).
Effects of each variable on prognosis
When residual gastric volume > 250 mL, sedative drugs, analgesics, vomiting, and time to reach the feeding target were taken as independent variables and respectively introduced into the logistic regression model for analysis, it was found that the time to reach the target feeding was an independent risk factor influencing the prognosis and extension of ICU stay. However, GRV > 250 mL had no significant effects on patient death and ICU stay outcomes (Tables 4 and 5).
DISCUSSION
The 2016 guidelines of the American Society of critical care medicine and the society of enteral and parenteral nutrition recommend monitoring of tolerance of enteral tube feeding (ETF) for critically ill patients in combination withradiological images, physical examination, flatulence and defecation[20]. The ETF intolerance is mainly manifested by nasal feeding tube withdrawal, abnormal imaging, vomiting, abdominal distension or diarrhea, which can occur in up to one third of hospitalized patients. The TF intolerance is associated with poor prognostic outcomes[21]. The 2021 international guidelines for management of sepsis and gastric shock recommend that GRV should be routinely measured for patients with FI or high risk of aspiration[22]. Currently, the definition of GRV has not been standardized. A metaanalysis[23] involving 72 articles showed that the definition of FI includes one or all of the three aspects: large gastricresidues (average 250 mL), gastrointestinal symptoms, and insufficient intake of calories. A previous study[24] revealed that the degree of influence of FI on poor prognostic outcomes is associated with definition of FI, and that the definition of high GRV (more than 500 mL for 24 h) and gastrointestinal symptoms is strongly correlated with 90-day mortality. The 2017 European Society of critical care clinical practice guidelines recommend delayed gastrointestinal nutrition for critically ill patients with GRV > 500 mL/6 h[25]. In 2021, expert consensus recommendation in China reported that residual gastric residue ≥ 250 mL suggest FI, and intervention treatments should be started as soon as possible [26]. This is why 250 mL was selected as the grouping standard in this study. Studies[23,27-30] have confirmed that FI increases mortality outcomes and prolongs the ICU hospitalization as well as mechanical ventilation times. Currently, there is no unified definition standard for FI. Abdominal distension, diarrhea and vomiting are regarded as the signs of FI and increased aspiration risk. In this study, it was found that when gastric residues of patients > 250 mL, clinical interventions did not significantly increase the incidences of abdominal distension, diarrhea and vomiting. Regarding the relationship between gastric residual allowance and enteral nutrition complications, studies[13-15] have confirmed that occurrences of vomiting, diarrhea, aspiration, pneumonia and other complications in ICU patients are not directly related to setting of critical values of gastric residual allowance, and that increasing the critical value of gastric residual allowance has no significant impact on enteral nutrition complications. In 2016, the Association for critical illness and the American Association for parenteral and enteral nutrition proposed the nutrition treatment guidelines[16]: They recommend monitoring gastric residual allowance in an irregular manner in clinical practice. For ICU patients, when the gastric residual allowance is less than 500 mL and if the patient has no abdominal symptoms such as vomiting and diarrhea,enteral nutrition should not be stopped. Therefore, we do not recommend clinical interventions to prevent vomiting when the patient's gastric residue exceeds 250 mL, unless the patient has abdominal symptoms or the gastric residue exceeds 500 mL. We found that > 250 mL gastric remnants for ICU patients had no significant effects on mortality outcomes and ICU hospitalization time. Therefore, we postulate that gastric residue is only one of the signs of FI, and it cannot predict whether the patient has FI, thus, it will not have a significant impact on prognostic outcomes. Assessment of feeding tolerance or estimating its impact on prognostic outcomes should not be based on gastric residues only.
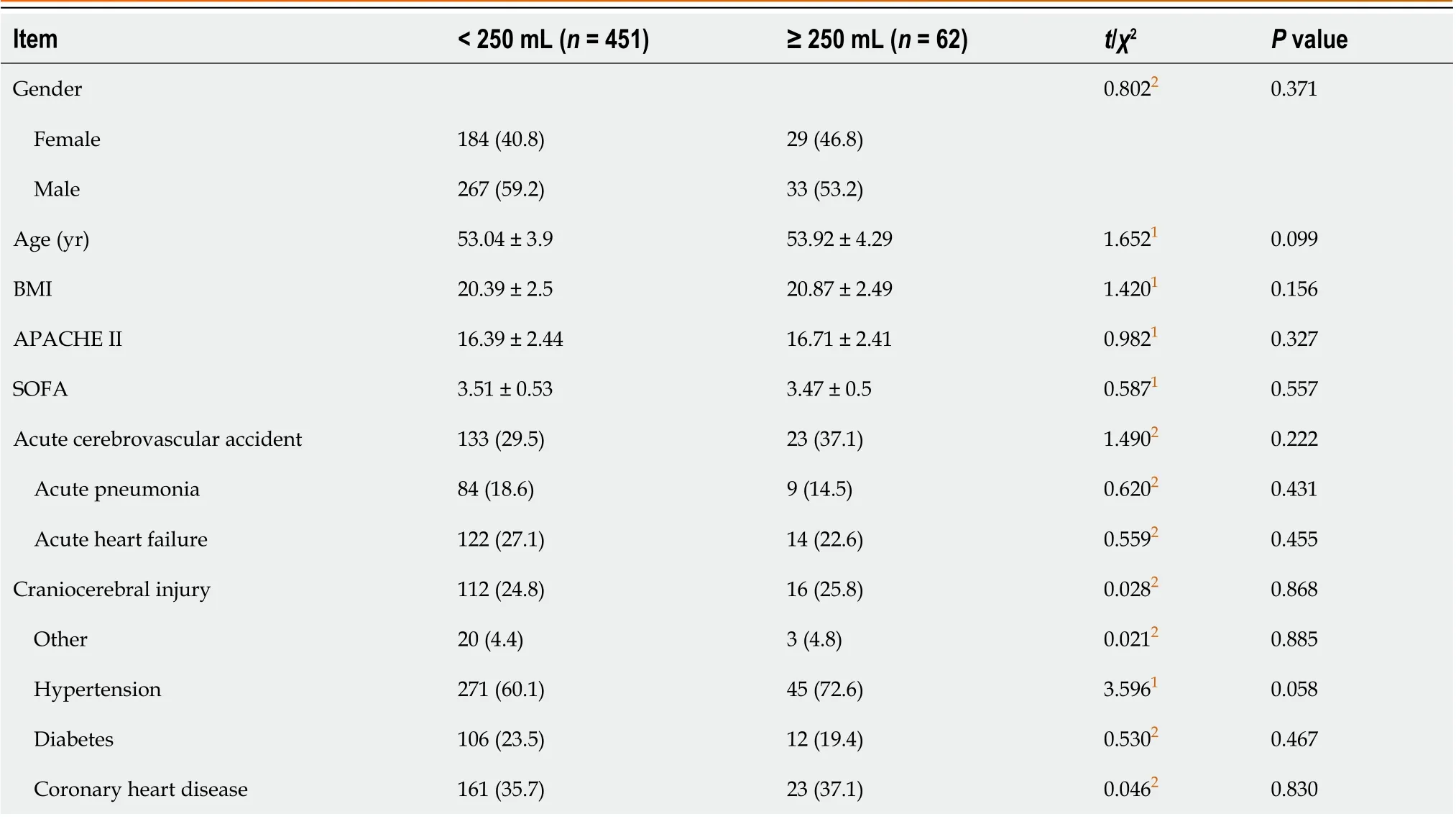
Table 1 Baseline characteristics of participants: Comparisons of the 2 groups, n (%)
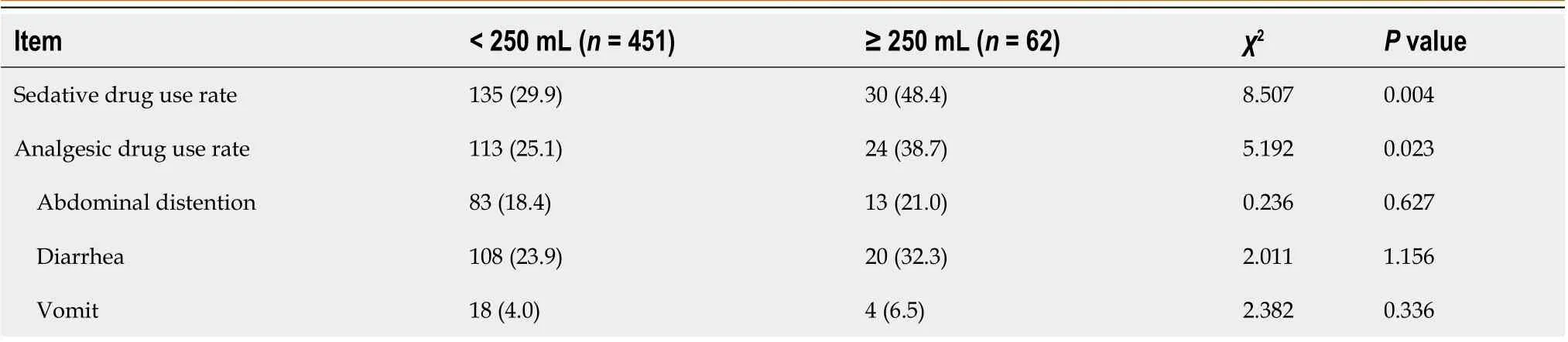
Table 2 Comparisons of medication and complications between the groups, n (%)

Table 3 Comparisons of prognostic outcomes between the groups, n (%)

Table 4 Logistic regression analysis of risk factors for death
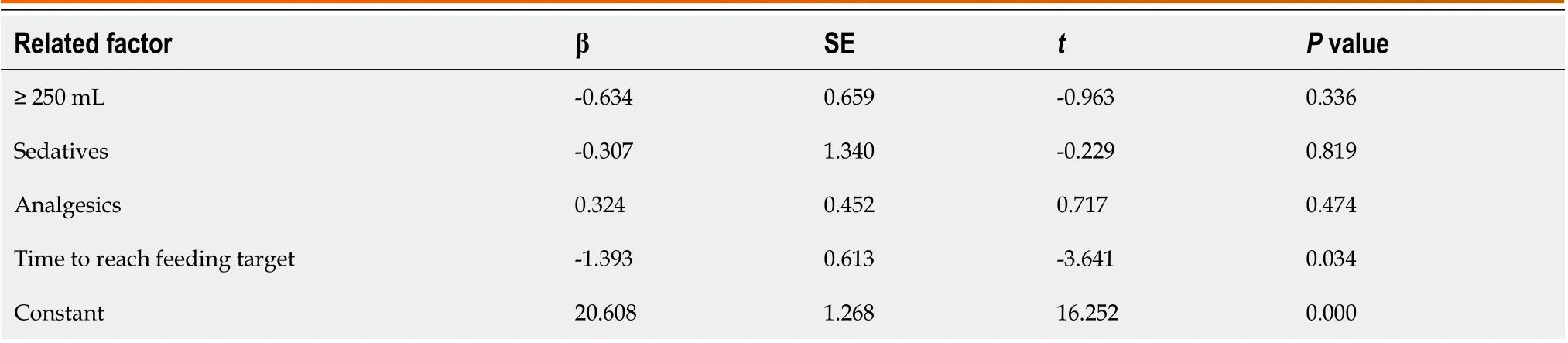
Table 5 Linear regression analysis of risk factors for length of stay in the intensive care unit

Figure 2 Daily caloric intake for the two groups.
We also found that food intake for ICU patients with gastric residual > 250 mL from the 4thto the 7thday was lower than that of patients with gastric residual < 250 mL, and that differences between the groups from the 4thto the 6thday were significant. This may have been because enteral nutrition was stopped for 2-4 h when the GRV exceeded 250 mL.The higher the number of times the patient suspends enteral nutrition, the less calories he consumes on that day. If the GRV cannot accurately reflect the gastrointestinal movement, it causes unnecessary interruption of nutrition supply and increases the mortality as well as complication rates for patients, which is attributed to insufficient energy supply. When monitoring the gastric residual amount, interruption or cessation of enteral nutrition due to high gastric residual amounts leads to insufficient feeding of the patient, which affects the patient's caloric intake, and ultimately increases the mortality outcomes[31,32]. The monitoring frequency of GRV also has an impact on daily caloric intake for patients. A multicenter study involving a large sample size by Reignieret al[33] reported that the proportion of patients who did not routinely monitor GRV and reached the target feeding volume was significantly higher than that of the routine monitoring group.It was 1.77 times that of the routine monitoring group. Wieseet al[15] found that 84.5% of patients who did not routinely monitor gastric residual amounts had their actual enteral nutrition feeding amounts reaching more than 90% of the target feeding amount within 24 h, and that 83.3% of patients had their actual enteral nutrition feeding amount being more than 90% of the target feeding amount during ICU hospitalization, which were significantly higher than those in the routine monitoring group (46.4% in 24 h and 61.9% in ICU hospitalization).
CONCLUSION
Ultrasound monitoring of gastric residual and clinical interventions when the monitoring value exceeds 250 mL have no significant impacts on complication rates and clinical prognosis of ICU patients, but significantly reduces the intake of calories during ICU hospitalization, prolongs the time to reach the feeding target, increases the risk of insufficient nutrition of patients, and affects the prognostic outcomes of patients. When the gastric residual exceeds 250 mL, clinical interventions that increase the nutritional intake are not recommended. This study has some limitations. As a retrospective single center study, there may be some information bias, therefore, our findings should be further confirmed by prospective and large sample studies.
ARTICLE HIGHLIGHTS

FOOTNOTES
Author contributions:Xu XY designed research; Xue HP performed research; Yuan MJ contributed new reagents or analytic tools; Jin YR analyzed data; Huang CX and Xu XY wrote the paper.
Institutional review board statement:The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Affiliated Hospital of Nantong University (Approval No. 2022015).
Informed consent statement:All study participants or their legal guardian provided informed written consent about personal and medical data collection prior to study.
Conflict-of-interest statement:The authors declare no conflicts of interest for this article.
Data sharing statement:Data sharing statement: Technical appendix, statistical code, and dataset available from the corresponding author at 1289811956@qq.com. Participants gave informed consent for data sharing.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORCID number:Xiao-Yan Xu 0009-0009-1930-4219; Hui-Ping Xue 0000-0001-9215-5728; Chun-Xia Huang 0009-0006-6692-1939.
S-Editor:Yan JP
L-Editor:A
P-Editor:Wu RR
杂志排行
World Journal of Gastrointestinal Surgery的其它文章
- Initial suction drainage decreases severe postoperative complications after pancreatic trauma: A cohort study
- Vascular complications of chronic pancreatitis and its management
- Historical changes in surgical strategy and complication management for hepatic cystic echinococcosis
- Post-transplant biliary complications using liver grafts from deceased donors older than 70 years:Retrospective case-control study
- Goldilocks principle of minimally invasive surgery for gastric subepithelial tumors
- Prognosis after splenectomy plus pericardial devascularization vs transjugular intrahepatic portosystemic shunt for esophagogastric variceal bleeding
