Accurate resection of hilar cholangiocarcinoma using eOrganmap 3D reconstruction and full quantization technique
2023-10-21DaPengCuiShuangFanYingXueGuoQianWeiZhaoYueXinQiaoJianDongFei
Da-Peng Cui, Shuang Fan, Ying-Xue Guo, Qian-Wei Zhao, Yue-Xin Qiao, Jian-Dong Fei
Abstract
Key Words: eOrganmap; 3D reconstruction; Full quantification technology; Laparoscopic surgery; Hilar cholangiocarcinoma;Precise resection
INTRODUCTION
Hilar cholangiocarcinoma (HCCA) is a mucosal carcinoma of the common hepatic duct, the left and right hepatic ducts,or the confluence of the left and right hepatic ducts[1]. Although the global incidence of this disease is low, between 1 and 2 per 100000, it shows a significant increasing trend[2]. Radical surgery has made long-term survival and even cure possible for HCCA[3]. However, a variety of factors such as complex anatomical structures and high probability of variation of hilar bile ducts and blood vessels, invasion of tumor adjacent blood vessels, combined with obstructive jaundice, and impaired liver function limit the scope of resection and may lead to low resection rate and poor overall prognosis[4]. At present, there are modified liver dissection, associating liver partition and portal vein ligation for staged hepatectomy, and selective portal vein embolization, but a unified surgical selection strategy has not been formed. From the perspective of surgical anatomy, it is important to study the structure of hilum hepatis and find a reasonable surgical plan for treatment of HCCA. Accurate preoperative evaluation is an important basis for improving the surgical resection rate of HCCA and reducing the risk of surgical mortality[5]. At present, preoperative evaluation of HCCA mainly relies on traditional computed tomography (CT) and magnetic resonance imaging (MRI). Surgeons need to construct 3D models from 2D images in their minds according to clinical experience and anatomical knowledge, which is subjective and will affect the formulation of surgical plans. At the same time, this imaging evaluation method lacks an overall and 3D sense; therefore, it can lead to differences in image interpretation among surgeons[6]. In recent years, 3D visualization has been gradually applied in clinical practice with the advantages of stereoscopic and intuitive vision, and has shown high clinical value and application in preliminary studies on preoperative evaluation of HCCA[7]. However, its reliability still needs the support of more clinical data. In this study, we retrospectively analyzed the clinical data of 73 patients undergoing HCCA surgery in The First Affiliated Hospital of Hebei North University, to explore the application of laparoscopic precise resection of HCCA based on preoperative eOrganmap 3D reconstruction and full quantification.
MATERIALS AND METHODS
General data
We retrospectively analyzed clinical data of 73 patients undergoing HCCA surgery in The First Affiliated Hospital of Hebei North University from February 2019 to January 2022. All patients were assigned to two groups. The traditional group received 2D imaging planning before surgery (n= 35). The EOrganmap group underwent 3D reconstruction and full quantitative technical planning before surgery (n= 38). This study was approved by the Medical Ethics Committee of The First Affiliated Hospital of Hebei North University.
The inclusion criteria were as follows: (1) HCCA was diagnosed by preoperative imaging such as CT and MRI, and was diagnosed by postoperative pathology[8]; (2) clear indications for surgery, generally in good condition, with no major heart and lung diseases, and could tolerate anesthesia and surgery after adequate preoperative evaluation; (3) no history of major abdominal trauma or surgery; (4) preoperative liver function assessment was Child-Pugh grade A or B;and (5) pathological data were complete and the treatment compliance was good. The exclusion criteria were: (1) Imaging data showed that the patient had disease other than HCCA; (2) abnormal liver development; (3) extensive tumor metastasis or invasion of important blood vessels; (4) liver function was not significantly improved after the treatment of protecting liver and alleviating jaundice; (5) body mass index > 32 kg/m2; (6) switch to laparotomy; and (7) perioperative death or loss of follow-up. The clinicopathological characteristics of the two groups were similar (Table 1). The study protocol is shown in Figure 1.
Surgical methods
The eOrganmap group established preoperative eOrganmap 3D reconstruction and full quantification technology. In order to ensure the accuracy of the 3D reconstruction image, the procedure was carried out by experienced radiologists.The 320-slice spiral CT was used for scanning, the current and voltage were set to 110 mAs and 120kV, respectively, and the layer thickness was 5 mm. We input the original image data into the eOrganmap 3D reconstruction system. The system automatically sketched the contour of each layer of liver parenchyma according to its anatomical position and density, and it could be modified. 3D reconstruction of the tumor, blood vessels, bile ducts, and liver was conducted by a radiologist and a surgeon. Multislice spiral CT and 3D reconstruction image analysis were performed by a radiologist and a surgeon. The analysis included the tumor location and size, whether it invaded the bile duct at grade II or higher,whether it involved peripheral blood vessels, and whether it had lymph node or distant metastasis. The results were compared with the actual intraoperative situation and postoperative pathological results.
Surgical plan planning: Complete the surgical plan design on the computer. The surgical plan should meet the multidimensional R0 margin; the residual liver volume should be at least 30% greater than the standard. Ensure that the prereserved liver's blood flow and bile drainage are unobstructed. According to the preoperative evaluation, the surgical plan was formulated, and the patients were treated with hemihepatic resection, expanded hemicombined caudate lobectomy, extrahepatic bile duct resection, endoskeletonization of the hepatoduodenal ligament, and bile duct and jejunum Roux-en-Y anastomosis. A five-hole small incision was made to perform laparoscopic surgery, and abdominal exploration was performed for abdominal and distant lymph node metastasis. The hepatoduodenal ligament was dissected by skeletonization, and the liver on the side to be resected was fully dissociated. The pre-resection area was separated along the hepatic ischemia line and the whole specimen was resected after an appropriate blood flow blocking method was selected. Rapid cryoscopy was performed to determine whether the upper and lower resection margins reached the R0 resection level. After the trans-sectional bile duct was reconstructed, biliary anastomosis was performed with Roux-en-Y. All patients were followed up for 10 mo after surgery.
Observational indexes
For preoperative evaluation, HCCA tumor progression[9] was evaluated in two aspects: Longitudinal progression (tumor invasion along the bile duct) and vertical progression (tumor invasion through the bile duct into the adjacent portal vein and hepatic artery in the vertical direction). Bismuth-Corlette classification was used for longitudinal progression. For patients in the eOrganmap group, only the tumor and biliary system were displayed after removing other tissues in the 3D model. Longitudinal invasion information was obtained by rotating the field of vision, and the tumor and portal vein or hepatic artery were separately displayed in the 3D model to observe the relationship between the tumor and vasculature from multiple angles. For patients in the traditional group, surgeons judged the degree of longitudinal progression from the CT images, such as middle bile duct stenosis and intraluminal space occupation. The Baek classification[10] was used as the standard to evaluate the degree of hepatic artery and portal vein invasion. Grade 0 tumor invasion indicated no vascular invasion; Grade I indicated that the blood vessels were stained or moved (the tumor was adjacent to the blood vessels); Grade Ⅱ indicated that the tumor had fused to the blood vessel and completely enclosed it[11]. The Couniaud method was used for bile duct anatomical classification[12], the Cheng method for portal vein classification[13], and the Michels method for hepatic artery variation classification[14].
Indicators associated with surgery: Surgical margin pathology, number of lymph nodes dissected, operating time,amount of intraoperative blood loss, postoperative intestinal ventilation time, and postoperative hospital stay.Postoperative complications included bile leakage, abdominal fluid accumulation, lung infection, postoperative liver insufficiency, and stress ulcers. Evaluation of postoperative bile leakage was based on the diagnostic criteria of the International Liver Surgery Research Group[15]. Total bilirubin (TBIL) level in the abdominal drainage tube was three times higher than in the serum on postoperative day 3. Liver function was detected preoperatively (T1), and on postoperative day 1 (T2), postoperative day 3 (T3), and postoperative day 7 (T4), and the indexes included TBIL, alanine transaminase (ALT), aspartate aminotransferase (AST), and albumin (ALB).
Stress response indexes were: Interleukin (IL)-6, cortisol (Cor), and norepinephrine (NE). For detection, 5 mL venous blood was collected, centrifuged for 10 min at 3000 rpm (Heidelge Medical Equipment, Building 3, 229 Amber Road,Shanghai International Medical Park, Pudong New Area, Shanghai), and the serum was separated and stored at -20°C for testing. ELISA was used to detect the above indicators.
For patients in the eOrganmap group, the software module was used to automatically calculate the volume of each segment of the liver, and the volume of the excised liver was measured by the drainage method after surgery, and the volume of the virtual excised liver measured by 3D reconstruction before surgery was compared with the actual volumeof the excised liver specimen during surgery. For the drainage method[16], the excised liver and tumor specimens were wrapped in cling film and placed in a container filled with water. The overflow water was collected and the volume was measured in a measuring cup, which corresponded to the specimen volume.

Table 1 Clinicopathological characteristics
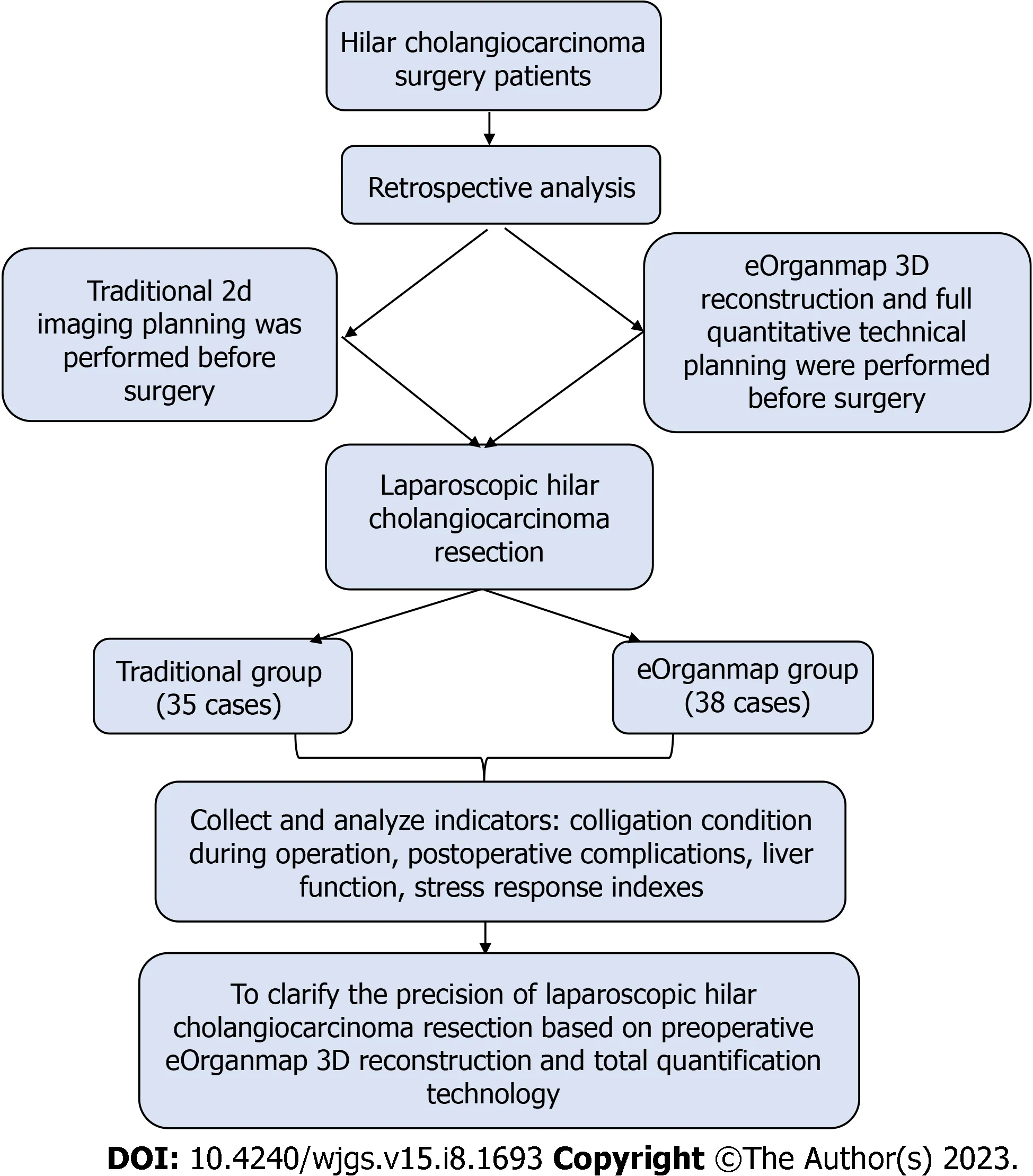
Figure 1 Study protocol.
Statistical analysis
SPSS 22.0 was used for statistical analysis. The measurement data in line with normal distribution were expressed as mean ± SD. Thettest was used for comparison of measurement data in the two groups, and repeated-measurement analysis of variance was used for comparison of data at different time points between groups. Numerical data were represented byn(%) andχ2test. Theχ2value was corrected when theoretical frequency was ≥ 1 and < 5, and calculated by exact probability method when theoretical frequency < 1. Pearson correlation test was used for correlation analysis.P< 0.05 was considered statistically significant.
RESULTS
Preoperative evaluation
The accuracy of longitudinal progression was 89.47% (34/38) in the eOrganmap group compared with 82.86% (29/35) in the conventional group (χ2= 0.231,P= 0.631). The accuracy of vertical progression in the eOrganmap group was compared with that in the traditional group: Portal vein invasion: 94.74% (36/38)vs91.43% (32/35) (χ2= 0.009,P= 0.924);hepatic artery invasion: 89.47% (34/38)vs85.71% (30/35) (χ2= 0.017,P= 0.895).
Anatomical classification of hepatic portal
In the eOrganmap group, seven cases of hepatic artery anatomical variation, three of portal vein variation, and four of bile duct anatomical variation were found preoperatively. All the results of preoperative evaluation were identical with the results of intraoperative exploration, and the classification accuracy was 100%. In the traditional group, four cases of hepatic artery anatomical variation, two of portal vein variation, and three of bile duct anatomical variation were found preoperatively. The typing accuracy was 64.29% in three cases of hepatic artery variation, one of portal vein variation and one of bile duct variation. Comparison of typing accuracy between the eOrganmap and traditional groups wasχ2= 8.027 andP= 0.005.
Indicators associated with surgery
Compared with the traditional group, the amount of intraoperative blood loss in the eOrganmap group was lower, the operating time and postoperative intestinal ventilation time were shorter, and R0 resection rate and lymph node dissection number were higher (P< 0.05) (Table 2).
Postoperative complications
The total complication rate in the eOrganmap group was 21.05%, compared with 25.71% in the traditional group (P>0.05) (Table 3). All patients with postoperative complications were cured after conservative treatment.
Liver function
The levels of TBIL, ALB, AST, and ALT in the eOrganmap group were significantly different from those in the traditional group (intergroup effect:F= 450.40, 79.120, 95.730, and 13.240, respectively; allP< 0.001). TBIL, AST, and ALT in both groups showed a decreasing trend with time (time effect:F= 30.270, 17.340, and 13.380, respectively; allP< 0.001), and there was an interaction effect between grouping and time (F= 3.072, 2.965, and 2.703, respectively;P= 0.0282, 0.032, and 0.046, respectively); ALB levels in both groups tended to increase with time (time effect:F= 22.490,P< 0.001), and there was an interaction effect between groups and time (interaction effect:F= 4.607,P= 0.004) (Table 4 and Table 5).
Stress response indexes
The levels of IL-6, Cor and NE in the eOrganmap group and traditional group were compared before and after surgery (P> 0.05) (Table 6 and Figure 2).
Liver volume
In the eOrganmap group, the preoperative virtual resection volume was 523.45 ± 112.03 mL, and the actual intraoperative resection volume was 558.43 ± 125.41 mL. There was a high correlation between the actual intraoperative resection volume and the preoperative virtual resection volume (t= 0.916,P< 0.001).
DISCUSSION
The prevalence of HCCA is increasing annually, and surgical resection is the main treatment. Accurate preoperative evaluation of HCCA and optimal surgical planning are important for good prognosis. In recent years, digital medical technology and the concept of 3D visualization precision diagnosis and treatment have been widely promoted and practiced. The 3D visualization and 3D printed models are more intuitive and accurate than traditional 2D CT and MRI images, making it possible to achieve accurate diagnosis, preoperative evaluation, and accurate surgery.
In this study, laparoscopic precise resection of HCCA was established based on preoperative eOrganmap 3D reconstruction and full quantification technology, which achieved good results. In the evaluation of degree of tumor invasion, we found that there was no difference in the accuracy of preoperative evaluation results and postoperative actual results between the two groups. Bismuth-Corlette classification is the most commonly used clinical classification method, which divides HCCA into types I-IV according to the extent of bile duct invasion by tumor. At present, the formulation of HCCA surgical plan is mainly based on this classification. However, there is no unified standard for surgical procedures established according to this classification method, and Bismuth-Corlette classification does not take into account tumor invasion of the portal vein and hepatic artery, which also affects the choice of surgical procedure. The eOrganmap 3D reconstruction and full quantification can make up for the deficiency of traditional preoperative evaluation methods.
Our results showed that the classification accuracy of patients based on preoperative eOrganmap 3D reconstruction and full quantification technology was significantly increased. The images based on eOrganmap 3D reconstruction and full quantification technology clearly showed the anatomical variation of the hilar liver. As a 3D visualization technology,eOrganmap 3D reconstruction and full quantification technology uses high-quality 2D-enhanced CT images or MR images to carry out 3D reconstruction, so as to display the lesions’ location, size, scope, and their anatomical relationship with the hepatic artery and portal vein in a 3D, comprehensive, and multiangular manner. According to this information,the tumor can be judged whether it has vascular invasion[17]. At the same time, it can evaluate the anatomical variation and spatial conformation of the hepatic artery, portal vein and bile duct, accurately measure the volume of each hepatic segment, and calculate the residual liver volume[18]. Accurate assessment of variation of the hepatic artery, portal vein and hepatic vein is important to surgical planning and avoidance of accidental injury during surgery[19]. In terms of vascular reconstruction, as long as CT image layer is thin enough, 3D software can automatically generate a vascular boundary without manual drawing, which reduces the secondary error caused by manual drawing. The 3D vascularimages can intuitively discover the variation in blood vessels and the relationship between tumors and blood vessels,which could improve the accuracy of resectable evaluation[20]. eOrganmap 3D reconstruction and full quantification technology is a new development of CT reconstruction. This technique can register and fuse images of different scanning periods, displaying liver, hepatic artery, portal vein, hepatic vein, and bile duct singly or in combination in the same 3D model, which can be observed by several physicians simultaneously[21,22]. Through the adjustment of transparency, the internal structure can be displayed, so that the spatial deformation of the important tubular structure in the liver can be observed, and an individualized surgical plan formulated. Couinaud’s anatomical segmentation can be carried out to achieve virtual surgery. Surgical information can be quantified through the built-in measurement tools of the software.The eOrganmap 3D reconstruction and full quantification technology has transformed the previous qualitative evaluation into quantitative evaluation, so as to accurately carry out individualized preoperative evaluation and surgical planning,which is consistent with the development of precision surgery.

Table 2 Colligation condition during operation
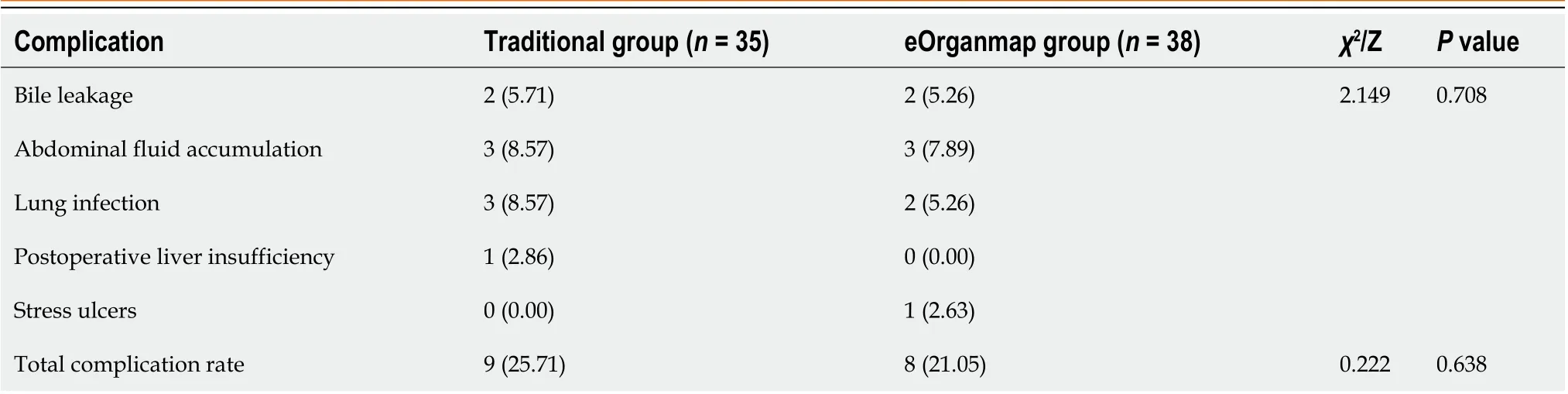
Table 3 Postoperative complications, n (%)

Table 4 Total bilirubin and albumin levels (mean SD)
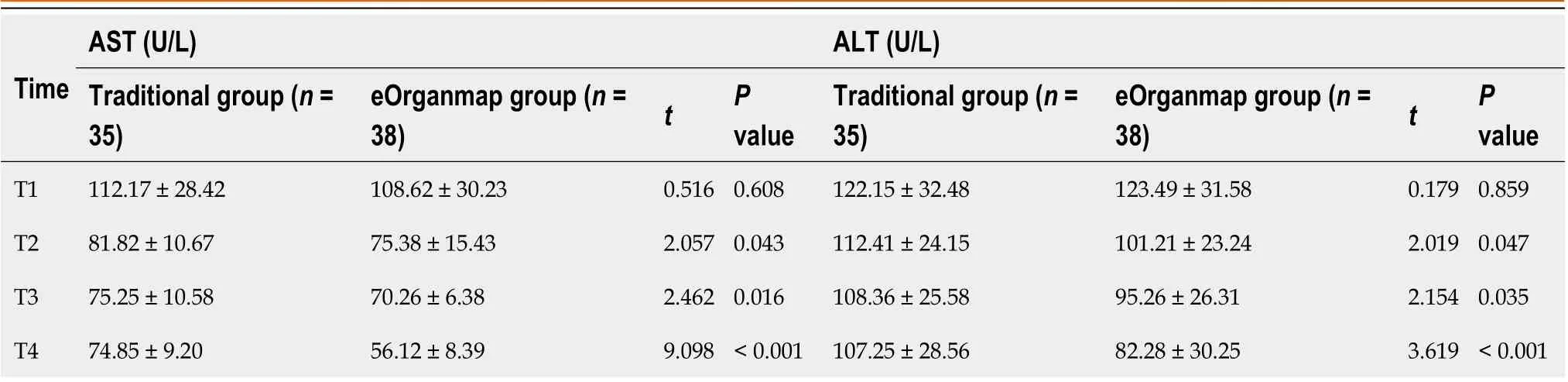
Table 5 Aspartate transaminase and alanine transaminase levels (mean SD)
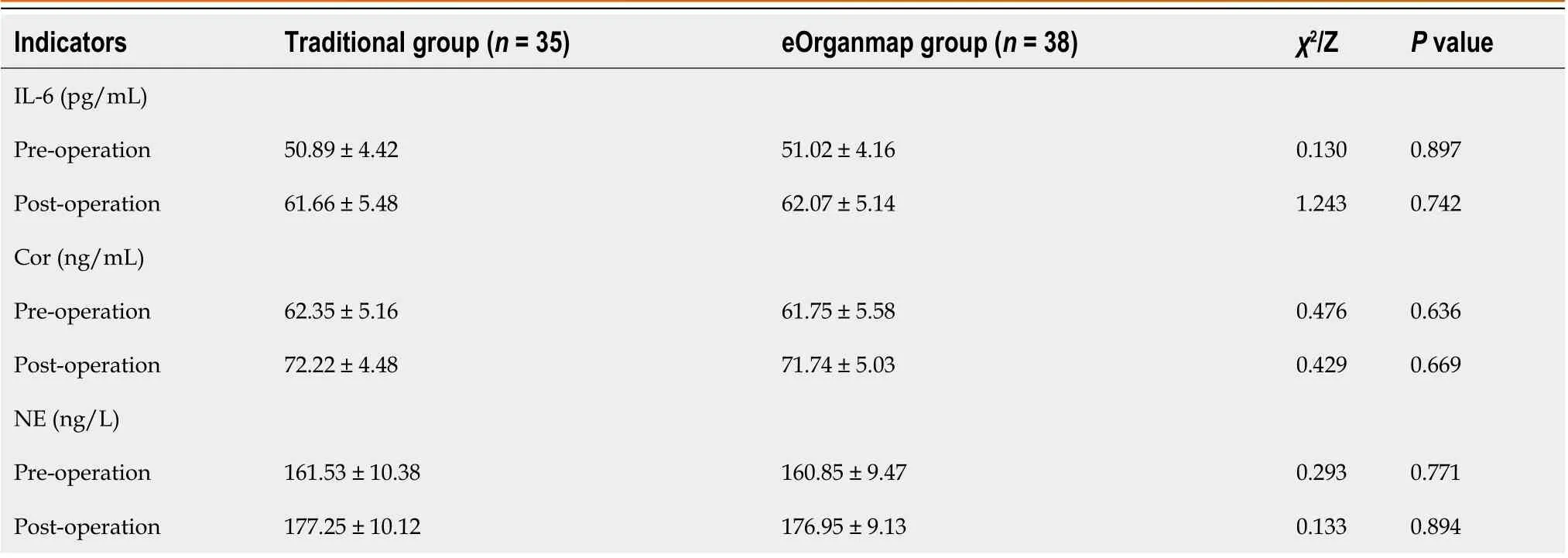
Table 6 Stress response indicators (mean SD)
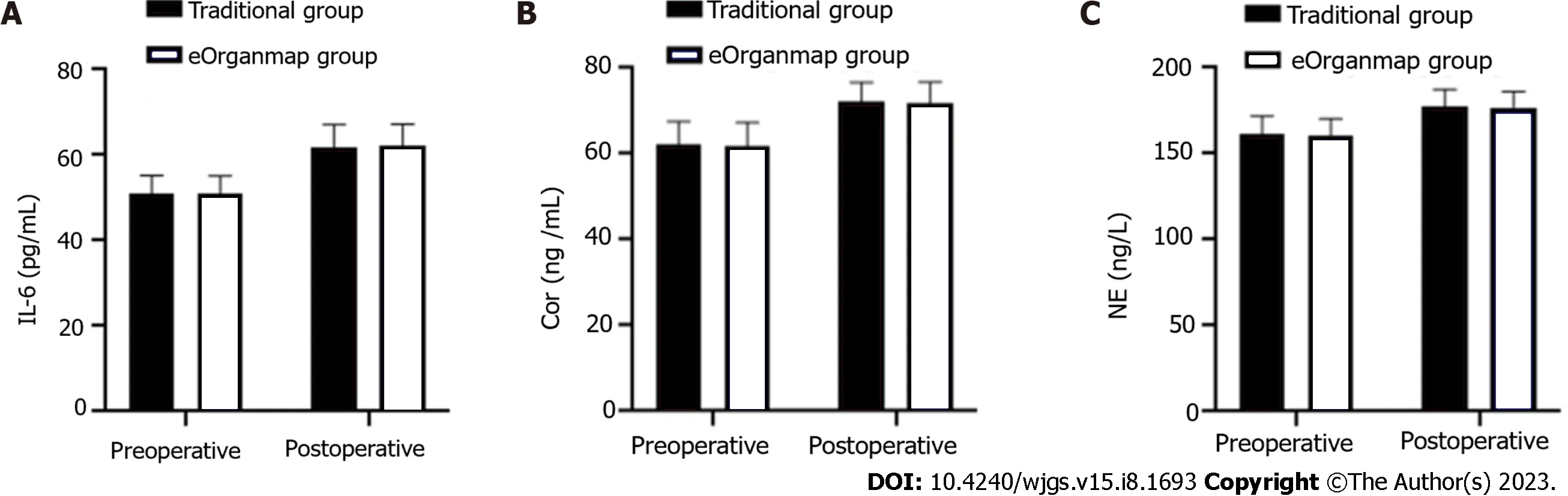
Figure 2 Interleukin-6, cortisol and norepinephrine levels. There were 35 cases in the traditional group and 38 in the eOrganmap group. A: Interleukin-6 Levels; B: Cortisol levels; C: Norepinephrine levels. Notes: IL-6: Interleukin-6; Cor: Cortisol; NE: Norepinephrine.
Preoperative reasonable planning of surgery, prior understanding of the anatomical variation of the perihilar bile duct and blood vessels, and possible intraoperative conditions could reduce the possibility of accidental intraoperative injury of the bile duct and blood vessels, thus avoiding the blindness of intraoperative exploration, and improving the safety of surgery and reducing liver dysfunction[23]. eOrganmap 3D reconstruction and full quantification technology can accurately measure liver volume, which prevents postoperative liver failure and ensures the safety of HCCA surgery. The results of this study showed that laparoscopic HCCA resection based on preoperative eOrganmap 3D reconstruction and full quantification technology reduced intraoperative blood loss, shortened operating time, and promoted postoperative intestinal ventilation, without increasing the incidence of complications and alleviating liver function damage. The results of this study also showed that the R0 resection rate and lymph node dissection number in the eOrganmap group were higher, indicating that 3D reconstruction and full quantification had some advantages in surgical margin treatment and lymph node dissection. Surgical margin and lymph node metastasis are both important factors affecting the prognosis of patients with hilar tumor[24]. Improving R0 resection rate and lymph node dissection are important measures to improve therapeutic efficacy and prognosis. Therefore, we hypothesized that laparoscopic HCCA resection based on preoperative eOrganmap 3D reconstruction and full quantification would promote efficacy and improve prognosis. There was a high correlation between the actual volume of intraoperative liver resection and the volume of preoperative virtual liver resection in the eOrganmap group. Accurate calculation of residual liver volume was important to ensure the safety of hepatectomy. Conventional CT or MRI integral measurement method was difficult to complete the measurement of segmental liver volume; however, preoperative eOrganmap 3D reconstruction and full quantification technology could accurately measure the volume of each segment of liver through a portal vein drainage algorithm, and it was consistent with the actual postoperative situation. In conclusion, accurate measurement of liver volume provides a reliable guarantee for the realization of accurate liver resection.
This study was the first to systematically carry out a new liver segmentation method based on three-dimensional reconstruction visualization and comprehensive quantification technology and the establishment of new indicators for liver reserve function evaluation and used a variety of mathematical models to analyze its feasibility and accuracy,providing a potential evaluation method for preoperative planning and postoperative risk prediction of hilar cholangiocarcinoma. On this basis, laparoscopic precise resection of HCCA was convenient for the application and promotion of this technology. However, this study also had some shortcomings. This study was retrospective and lacked prospective randomized controlled trials, so the reliability of the results could not be guaranteed. In addition, the number of cases included in this study was small, and the results may be biased, which needs to be confirmed by studies with a larger sample size.
CONCLUSION
Laparoscopic precise resection of HCCA based on preoperative eOrganmap 3D reconstruction and full quantification technology made preoperative assessment, hilar vasculature anatomical classification, and resected liver volume assessment more accurate. It improved the rate of R0 resection and the number of lymph nodes dissected. It also had a low impact on liver function and stress response. Therefore, the method is safe and worthy of wider application.
ARTICLE HIGHLIGHTS

FOOTNOTES
Author contributions:Cui DP conceived this study, collected clinical data, interpreted the results, wrote, and revised the manuscript; Fan S, Guo YX participated in collecting data and data statistics; Zhao QW, Qiao YX, Fei JD participated in the study design and revised the manuscript; and All authors read and approved the final manuscript.
Supported byKey R&D Program of Hebei Province, No. 223777101D.
Institutional review board statement:This study was approved by the Medical Ethics Committee of The First Affiliated Hospital of Hebei North University.
Informed consent statement:All patients have signed the previous consent form before the surgery. According to institutional policy,this retrospective study does not require informed consent.
Conflict-of-interest statement:The authors declare no conflicts of interest for this article.
Data sharing statement:The data set used for this study can be obtained from the corresponding author.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORCID number:Da-Peng Cui 0009-0001-7942-8207; Shuang Fan 0000-0001-7483-4081; Ying-Xue Guo 0009-0007-4953-2752; Qian-Wei Zhao 0009-0008-7707-6392; Yue-Xin Qiao 0009-0003-3225-382X; Jian-Dong Fei 0009-0008-4024-4831.
S-Editor:Wang JL
L-Editor:A
P-Editor:Ji MX
杂志排行
World Journal of Gastrointestinal Surgery的其它文章
- Initial suction drainage decreases severe postoperative complications after pancreatic trauma: A cohort study
- Vascular complications of chronic pancreatitis and its management
- Historical changes in surgical strategy and complication management for hepatic cystic echinococcosis
- Post-transplant biliary complications using liver grafts from deceased donors older than 70 years:Retrospective case-control study
- Goldilocks principle of minimally invasive surgery for gastric subepithelial tumors
- Prognosis after splenectomy plus pericardial devascularization vs transjugular intrahepatic portosystemic shunt for esophagogastric variceal bleeding
