Prevention and treatment of hepatic encephalopathy during the perioperative period of transjugular intrahepatic portosystemic shunt
2023-10-21LanJingWangXinYaoQiQiJianPingQin
Lan-Jing Wang, Xin Yao, Qi Qi, Jian-Ping Qin
Abstract Transjugular intrahepatic portosystemic shunt (TIPS) is an established procedure for treating the complications of portal hypertension in liver cirrhosis. While the pathogenesis of postoperative TIPS-related hepatic encephalopathy (HE) has yet to be fully understood, intraoperative portosystemic shunts may provide a pathological basis for the occurrence of postope-rative HE in patients with liver cirrhosis. Studies at home and abroad have expressed mixed opinions about TIPSrelated HE. This study presents a literature review on the risk factors for and prevention and treatment of perioperative TIPS-related HE in patients with liver cirrhosis, aiming to optimize the procedure and reduce the incidence of postoperative HE.
Key Words: Portosystemic shunt; Transjugular intrahepatic; Hepatic encephalopathy; Liver cirrhosis; Hypertension; Portal; Therapeutics
INTRODUCTION
Transjugular intrahepatic portosystemic shunt (TIPS) is a minimally invasive interventional procedure for treating the complications of portal hypertension in liver cirrhosis of various origins. By creating a shunt between the hepatic and portal veins, TIPS is widely used in clinical settings to alleviate variceal bleeding, refractory pleural effusion and ascites,and other complications associated with portal hypertension[1-3]. Hepatic encephalopathy (HE), one of the most common postoperative TIPS-related complications[4], often occurs within the first 1-3 mo after the procedure[5]. With an incidence rate of 25%-50%[6-9], HE has a profound impact on the prognosis and quality of life of patients with liver cirrhosis[10].According to the “2021 North American Practice-Based Recommendations for Trans-jugular Intrahepatic Portosystemic Shunts in Portal Hypertension”[3], intraoperative embolization of spontaneous portosystemic shunts and the narrowing of the stent diameter may reduce the risk of postoperative TIPS-related HE. The 2019 Clinical Practice Guidelines:Management of TIPS for Portal Hypertension by the Chinese College of Interventionalists (CCI)[11] recommend using stents particularly designed for TIPS to create a shunt from the left branch of the portal vein to reduce the risk of postoperative TIPS-related HE. “The 2023 French Recommendations for the Diagnosis and Management of Hepatic Encephalopathy”[12] viewed that the postoperative use of rifaximin can prevent postoperative TIPS-related HE.Although these recommendations have reached some levels of consensus, some have been debated. This study reviewed the risk factors for and the prevention and treatment of perioperative TIPS-related HE, thereby providing a reference to optimize TIPS and minimize the risk of postoperative HE.
DEFINITION AND CLASSIFICATION OF TIPS-RELATED HE
TIPS-related HE refers to a spectrum of central nervous system dysfunctions caused by metabolic disorders following the creation of a shunt between the portal vein and systemic circulation during TIPS after excluding other known brain diseases[13]. The 11th World Congress of Gastroenterology[14] classified HE into three types (A, B and C) based on etiological factors, with type C being the most common form of HE occurring in patients with chronic liver disease or cirrhosis and a portosystemic shunt. The Practice Parameters Committee of the American College of Gastroenterology[15]further graded HE into 0-4 according to the severity of symptoms based on the widely accepted West Haven Criteria.Seeing the difficulty in distinguishing between grades 0 and 1 in the West Haven Criteria, the International Society for HE and Nitrogen Metabolism[16] introduced an alternative grading system, called the spectrum of neurocognitive impairment in cirrhosis, to differentiate covert HE (CHE) from overt HE (OHE) based on the diagnosis of orientation disorders and asterixis. CHE is defined as a neuropsychiatric disorder without orientation disorders or asterixis in patients with liver cirrhosis. The pathogenesis of postoperative TIPS-related HE is not fully understood. Most patients undergoing TIPS due to portal hypertension have varying degrees of liver dysfunction. This undermines the liver’s ability to detoxify ammonia produced by the intestines. Toxins enter the central nervous system through the blood-brain barrier, interfering with brain energy metabolism and eventually causing HE. Natural and artificial shunts play an important role in HE development as they allow toxins in the visceral blood to bypass the liver and enter systemic circulation. Moreover, these shunts can reduce liver perfusion and further exacerbate liver dysfunction. Studies[17-19]have revealed the close associations between the diameter of an intraoperative portosystemic shunt and HE incidence.This may explain the occurrence of postoperative HE in patients with portal hypertension[13]. Nevertheless, HE has other contributory causes, such as liver cell dysfunction, natural collateral circulation, increased production of intestinal neurotoxins, and increased blood-brain barrier permeability[20].
RISK FACTORS FOR POSTOPERATIVE TIPS-RELATED HE
Numerous risk factors are closely associated with the development of HE, which should be carefully considered to optimize preoperative patient selection. A meta-analysis[21] has shown that prior HE is one of the key independent predictors of postoperative TIPS-related HE [odds ratio (OR): 3.07, 95% confidence interval (CI): 1.75-5.40]. The 2020 TIPS guidelines jointly developed by the British Society of Gastroenterology, the British Society of Interventional Radiology,and the British Association for the Study of the Liver (BASL)[22] highlighted that TIPS can aggravate or induce HE; thus,patients should undergo preoperative screening for CHE and OHE before nonemergency TIPS. The latest guidelines for preventing and treating HE[12] and the European Association for the Study of the Liver (EASL) guidelines[10] have suggested that a single episode of OHE is not an absolute contraindication for nonemergency TIPS. Most studies[23-26]have not excluded patients with a history of HE or have merely excluded those with a history of recurrent OHE. As a result, the reported incidence of postoperative TIPS-related HE was not higher than the HE incidence following standard modalities, such as endoscopic treatment plus drug therapy and large-volume paracentesis plus albumin infusion. Many studies[7,27,28] have addressed the relationship between CHE and an increased risk of postoperative TIPS-related HE.However, no preoperative medication (e.g., lactulose or rifaximin) is recommended for patients diagnosed with CHE and scheduled for TIPS. Therefore, the 2021 North American Practice-Based Recommendations[3] considered whether preoperative medication for CHE and cirrhosis can minimize the risk of OHE after TIPS an emerging research interest. A recent study[29] has indicated that HE is not a contraindication for early TIPS for acute variceal bleeding. Several studies[25,30,31] have suggested that early TIPS does not necessarily entail an increased risk of postoperative HE. Therefore, the 2023 French recommendations[12] state that prior HE is not a contraindication for early TIPS.
The severity of liver disease is closely associated with the occurrence of postoperative TIPS-related HE[21,23,32,33]. The aforementioned meta-analysis[21] has suggested that Child-Turcotte-Pugh class C liver function is the most robust independent predictor of postoperative TIPS-related HE (OR: 4.0, 95%CI: 1.4-11.1). A single-center retrospective study[33] has shown that according to the Model for End-Stage Liver Disease scoring system, > 18 is independently associated with the occurrence of postoperative TIPS-related HE (58%vs37%,P= 0.009). Advanced age[33,34], elevated creatinine levels[34], and hyponatremia[35,36] have also been reported to be closely related to the occurrence of postoperative TIPSrelated HE. Nutritional status is potentially associated with postoperative TIPS-related HE. The BASL[22] has suggested that cachexia is associated with more postoperative TIPS-related HE. In two recent prospective studies[35,36], sarcopenia has been reported to have a strong association with the occurrence of HE. Nardelliet al[36] suggested that sarcopenia is independently associated with the development of postoperative TIPS-related HE (subdistribution hazard ratio: 31.3,95%CI: 4.5-218.07;P< 0.001). They reported that the relationship between sarcopenia and HE contributed to decreased ammonia clearance and highlighted the importance of considering sarcopenia during patient selection for TIPS. Two prospective studies by Tapperet al[37,38] have demonstrated a close association between the development of OHE and frailty in patients with cirrhosis, as evaluated by the chair stand, grip strength, and walking speed tests. Neyet al[39] have found that based on the Montreal Cognitive Assessment (MoCA) and the Clinical Frailty Scale (CFS), the complex MoCA-CFS scoring system has a predictive value for readmission due to HE within 6 mo postoperatively in liver cirrhosis cases without prior HE. These findings show that in patients with liver cirrhosis, frailty is a risk factor for HE.However, no research has discussed the effect of frailty on the occurrence of postoperative TIPS-related HE. Even if patients were carefully selected by considering all aforementioned factors, there is no guarantee that these patients will not have postoperative TIPS-related HE. The 2021 North American Practice-Based Recommendations[3] consider TIPS unsuitable for patients with cognitive impairment and inadequate family or social support because they are at a higher risk of developing HE after the procedure. In nonspecific cases, informing patients and their families that postoperative HE may unavoidably occur and that they should be aware of possible signs and symptoms to identify the condition as early as possible is necessary for the surgeon and attending physician. According to the risk of postoperative TIPS-related HE, the relative contraindications for nonemergency TIPS are shown in Table 1.
EFFECTS OF PORTAL VEIN BRANCHES ON HE
According to the 2019 CCI Clinical Practice Guidelines[11], establishing a shunt from the left portal vein branch can reduce the incidence of postoperative TIPS-related HE. A study[40] has proposed that the reflux from the splenic and superior mesenteric veins reaches the left and right portal vein branches, respectively, before being thoroughly mixed.Specifically, the blood from the superior mesenteric vein mainly goes to the right branch, whereas that from the splenic vein enters the left branch. Therefore, the blood ammonia level in the systemic circulation after puncturing the left portal vein branch is lower than that of the right branch. In another study[41], based on CO2venography, an iodinated contrast was used to replace traditional imaging methods to treat patients with chronic liver disease who underwent percutaneous transhepatic puncture of the portal vein and splenic vein catheterization. The study has reported a difference in the imaging of the blood flow in the left and right portal vein branches by injecting 30 mL of the contrast at a rate of 5 mL/sviaa mechanical injection system. In 2009, a randomized controlled study[42] assigned 72 patients to two groups and found that the incidence rates of HE and newly occurring HE were both lower when the shunt was established from the left portal vein branch than when the shunt was established from the right branch (P= 0.036 and 0.012, respectively). A similar conclusion was reached in a 2014 retrospective study[43]. Based on these findings, some interventional physicians in China now prefer the left portal vein branch when creating a shunt by puncture. However, before the Viatorr stent,particularly designed for TIPS, was released in the Chinese market in October 2015, Fluency covered stents were the most frequently used stents for TIPS. Characterized by strong support and robust axial elastic tension, these stents can malfunction due to occlusion as they are released at a position too low in the hepatic vein, or the portion in the portal vein is too short. With the shunt receiving blood from the main portal vein, whether postoperative HE is associated with the choice of puncture site (at either the left or right portal vein branch) is debatable. Whether there is blood flow into the left and right portal vein branches following the convergence of the splenic and mesenteric blood into the main portal vein in patients with liver-cirrhosis-induced portal hypertension remains controversial[44]. In China, a 2020 study[45] collected data from 120 patients with liver-cirrhosis-induced portal hypertension who underwent TIPS with the Viatorr stent. The study results have shown that in 52 patients, the shunt was created at the left portal vein branch, whereas the remaining 68 patients had a shunt established at the right branch, and the two groups had no significant difference in the incidence of postoperative HE (χ² = 0.159,P= 0.69). Another domestic study in 2020[46] included 15 patients with hepatitis-Brelated cirrhosis and upper gastrointestinal bleeding who underwent TIPS. Blood samples were collected from the left and right branches and the main trunk of the portal vein during the procedure and were found to have similar plasma ammonia levels (left branch: 96.4 ± 17.6 μmol/L, right branch: 113.5 ± 18.4 μmol/L, main trunk: 106.9 ± 38.7 μmol/L; allP> 0.05). A recent retrospective cohort study[47] has reported that the incidence of postoperative TIPS-related HE did notdiffer between patients with a shunt created at the left portal vein branch and those with a shunt created at the right branch (13%vs24%) (P= 0.177). More multicenter prospective randomized controlled studies are needed to clarify whether the occurrence of postoperative TIPS-related HE is related to the choice of shunting branch.
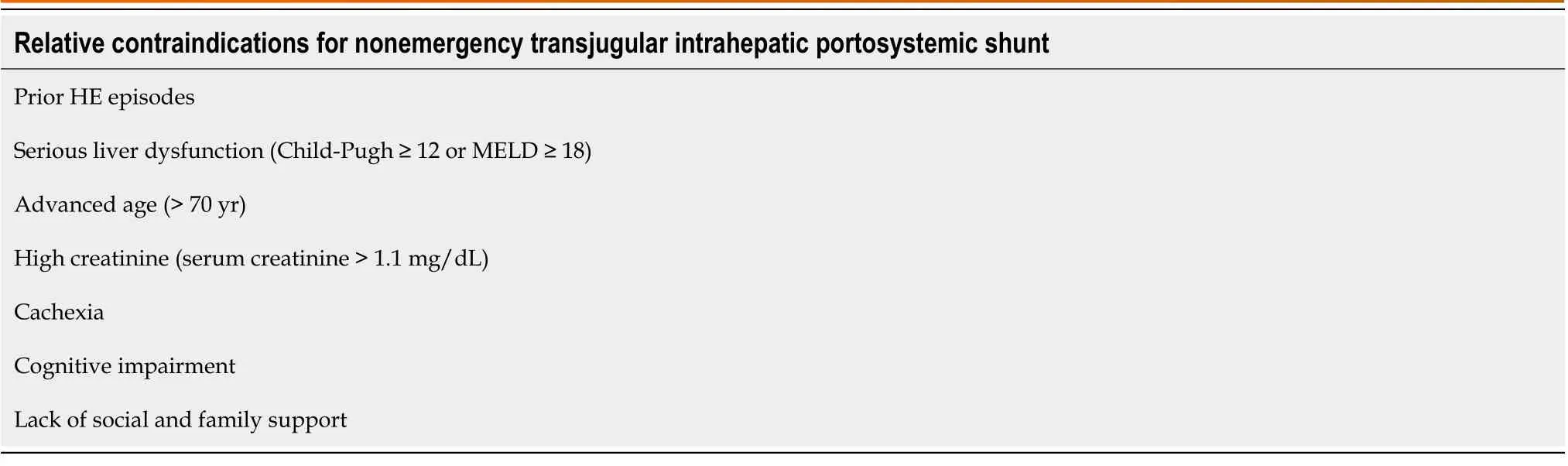
Table 1 Relative contraindications for nonemergency transjugular intrahepatic portosystemic shunt (based on the risk of postoperative-transjugular intrahepatic portosystemic shunt related hepatic encephalopathy)
EFFECTS OF SPONTANEOUS PORTOSYSTEMIC SHUNTS ON HE
As the resistance to the blood flow in the portal vein increases, a compensatory network of blood vessels will take form between the portal vein and systemic circulation to achieve partial diversion of the portal venous flow. As portal hypertension deteriorates, some branches gradually become enlarged, leading to the formation of vascular shunts, termed spontaneous portosystemic shunts (SPSS)[48]. Theoretically, the formation of SPSS and the post-TIPS shunting increase the risk of HE by further diversion of portal blood from the liver. A multicenter study by Lalemanet al[49] has demonstrated that SPSS occurs in 46%-70% of patients with refractory HE. A large-scale multicenter retrospective study[50] has found that an SPSS with a diameter > 8 mm is associated with an increased risk of OHE and death in patients with liver cirrhosis. Likewise, a recent study[51] has reported that an SPSS with a total cross-sectional area > 83 mm²implies an increased risk of OHE and death in patients with liver cirrhosis. In a comparative analysis of unembolized and embolized SPSS by Heet al[52], patients with unembolized SPSS are at a higher risk of developing postoperative TIPSrelated OHE. In contrast, a meta-analysis[53] has shown that SPSS embolization plus TIPS can reduce the risk of recurrent bleeding compared with TIPS alone, and yet, the two surgical approaches lack significant differences in the incidence of HE. A retrospective study[54] has compared TIPS with and without SPSS embolization and reported no significant difference in the incidence of postoperative TIPS-related HE. The 2021 North American Practice-Based Recommendations[3] has suggested that in patients with refractory pleural effusion and ascites, the embolization of SPSS with a diameter >6 mm during TIPS can be considered to reduce the risk of postoperative HE. The 2020 TIPS guidelines[22] have recommended the embolization of SPSS when a patient is at a high risk of postoperative TIPS-related HE. However,large-scale, multicenter prospective studies are needed to address whether SPSS should be embolized during TIPS, a much debated question.
EFFECTS OF THE PORTAL VENOUS PRESSURE GRADIENT ON HE AFTER SHUNT PLACEMENT
Studies have shown that a greater decrease in portal venous pressure (PVPG) after TIPS is associated with better outcomes and an increased risk of postoperative TIPS-related HE in patients with liver cirrhosis and complications of portal hypertension[55]. At present, it is believed that PVPG should be reduced to at least 12 mmHg or 50% of the initial baseline level to effectively prevent or treat the complications of portal hypertension[19]. In contrast, some researchers[56]have argued that a decrease in PVPG to below 10 mmHg can significantly increase the incidence of postoperative TIPSrelated HE. The 2020 TIPS guidelines[22] have suggested that patients with variceal bleeding are at a higher risk when PVPG is reduced to < 12 mmHg and that a safer option is maintaining PVPG at 20% below baseline. In the face of refractory ascites and other indications, the PVPG level should be managed through individualized assessment of therapeutic effects and the risk of HE. In our recent study[44], a Viatorr stent with 8 mm inner diameter was used for shunting. With all included patients having consistent baseline characteristics, PVPG was reduced to 12 mmHg or below 50% of the initial baseline level. The study results have shown that the incidence of HE differed among patients with liver cirrhosis of different origins, with the incidence of postoperative HE in patients with hepatitis-B-related liver cirrhosis lower than in those with alcoholic or primary biliary cirrhosis. It is believed that the development of HE is somehow associated with post-TIPS liver reserve function, which can be explained by subsequent etiological treatment and patient management in addition to perioperative treatment. There is no clear consensus over the treatment protocol for PVPG in TIPS. Therefore, the appropriate PVPG level should be determined based on individual conditions, such as the cause of liver cirrhosis and symptoms of portal hypertension, which proposes a direction for future studies.
EFFECTS OF THE CHOICE OF STENT ON HE
A randomized controlled trial[57] has revealed that using a covered stent for TIPS can significantly reduce the risk of malfunction, despite the negligible benefits for HE development and survival. The aforementioned meta-analysis[9] has shown that compared with bare stents, covered stents can improve stent patency and survival; however, these devices have no obvious advantage for minimizing the risk of HE. In a direct comparison between bare and covered stents[8], the study results have shown a lack of significant difference in the incidence of OHE. Therefore, the 2021 North American Practice-Based Recommendations[3] have considered discussing the difference in the incidence of OHE between bare and covered stents. Despite their history, bare stents are virtually obsolete in TIPS because of low survival rates and limited therapeutic effects.
PVPG plays a significant role in the development of postoperative TIPS-related HE, and according to Poiseuille’s law (r= 8L × η/r 4), stent diameter is essentially associated with PVPG[19]. Both the North American and British TIPS guidelines[3,22] have proposed that a smaller stent diameter can reduce the risk of HE but has little effect on reducing PVPG. A prospective, nonrandomized controlled study by Schepiset al[58] has shown that as far as covered stents are concerned, a smaller diameter (6-7 mm) can significantly reduce the incidence of HE at 1 year postoperative compared with standard diameters (≥ 8 mm). Several studies[17-19] have reported that the incidence of OHE was significantly lower in patients using an 8-mm-diameter stent than in those using a 10-mm-diameter stent. In the study using a 6-mmdiameter covered stent[59], the efficacy profile was satisfactory, with propranolol compensating for the limited decrease in PVPG and reducing the risk of HE. On this basis, researchers[19] have introduced the use of TIPS-dedicated stents with smaller diameters (6-8 mm) and medication or other treatment methods as combined modalities can effectively treat and prevent the complications of portal hypertension while reducing the risk of HE. Notably, a prospective study on expandable stents[60] has introduced a promising modality of placing an expandable stent into the body during the procedure and adjusting the stent diameter between 8 and 10 mm according to the response to the treatment until satisfactory outcomes are achieved. The study results have shown effective control of variceal bleeding and a reduced risk of HE in patients with portal hypertension following the placement of the expandable stent.
PREVENTION AND TREATMENT OF POSTOPERATIVE TIPS-RELATED HE
While lactulose and rifaximin are recommended medications for HE, their benefits for HE prevention after TIPS remain a controversial and much disputed subject. Two randomized controlled trials[61,62] on secondary prevention of OHE have shown that nonabsorbable disaccharides can significantly reduce the risk of developing recurrent HE. The French guidelines[12] have recommended using lactulose or lactitol as a first-line treatment to prevent recurrent OHE. A randomized controlled trial on bare stents[63] has reported a lack of significant difference in the incidence of postoperative TIPS-related OHE between patients using lactulose, rifaximin and placebo. A large-scale randomized controlled trial[64] in 2021 highlighted that the experimental group using prophylactic rifaximin before TIPS had a lower incidence of postoperative TIPS-related HE than the placebo control group (34%vs53%; OR: 0.48, 95%CI: 0.27-0.87).Based on this study, the latest HE prevention and treatment guidelines[12] have recommended using rifaximin to prevent postoperative TIPS-related HE. Despite the notable findings, this trial has not considered prior OHE because lactulose was merely administered to prevent postoperative TIPS-related HE instead of as preoperative prophylaxis. The latest EASL HE guidelines[10] and the Baveno VII consensus[65] have only recommended the prophylactic use of rifaximin before TIPS in patients with prior HE. A recent prospective study[66] has shown that postoperative infusion of human albumin has no clear association with the incidence of postoperative TIPS-related HE.
Although careful patient selection and perioperative measures can lower the risk of TIPS-related HE, achieving effective management of postoperative TIPS-related HE remains challenging. In addition to standard management and pertinent medication therapy, interventional stent restriction is required in the case of refractory HE. As described by the BASL[22], standard HE management is to correct and restore biochemical parameters to normal levels, withdraw nocturnal sedation, stop using proton pump inhibitors (PPIs), and ensure regular bowel movements using lactulose. The 2021 North American Practice-Based Recommendations[3] have recommended administering lactulose for the first episode of OHE after TIPS and lactulose plus rifaximin for recurrent HE. Several studies[67,68] have shown that decreased tissue zinc levels are observed in patients with liver cirrhosis and are related to the pathogenesis of HE. In contrast, these findings are the opposite of the reported effects of zinc supplementation on HE[69-71]. EASL[10] does not recommend HE management through zinc supplementation. The incidence of refractory HE after TIPS is 3%-8%[72-75].Although there is no standard definition for refractory HE, the 2021 North American Practice-Based Recommendations[3]have recommended shunt flow restriction if patients with persistent HE do not respond to medication therapy or need readmission due to at least three episodes of HE in the past 3 mo. Most patients show improvement in symptoms through shunt flow restriction[76]; however, they are at risk of the recurrence of complications associated with portal hypertension. In a retrospective study[77], 20 patients with postoperative TIPS-related HE underwent shunt flow restriction (n= 18) or TIPS occlusion (n= 2). Of these patients, HE was responsive in 11 (55%) cases and unresponsive in nine (45%), and the responsive cases showed an improvement in HE grades and did not have refractory ascites or recurrent variceal bleeding. This provides supportive evidence for the safety of shunt flow restriction in treatingpostoperative TIPS-related HE, particularly in patients with OHE. However, no response to shunt flow restriction usually indicates a poor prognosis[78]. Finally, liver transplantation remains the last resort for patients with portal hypertension and liver cirrhosis. Modalities for preventing and treating postoperative TIPS-related HE are listed in Table 2.
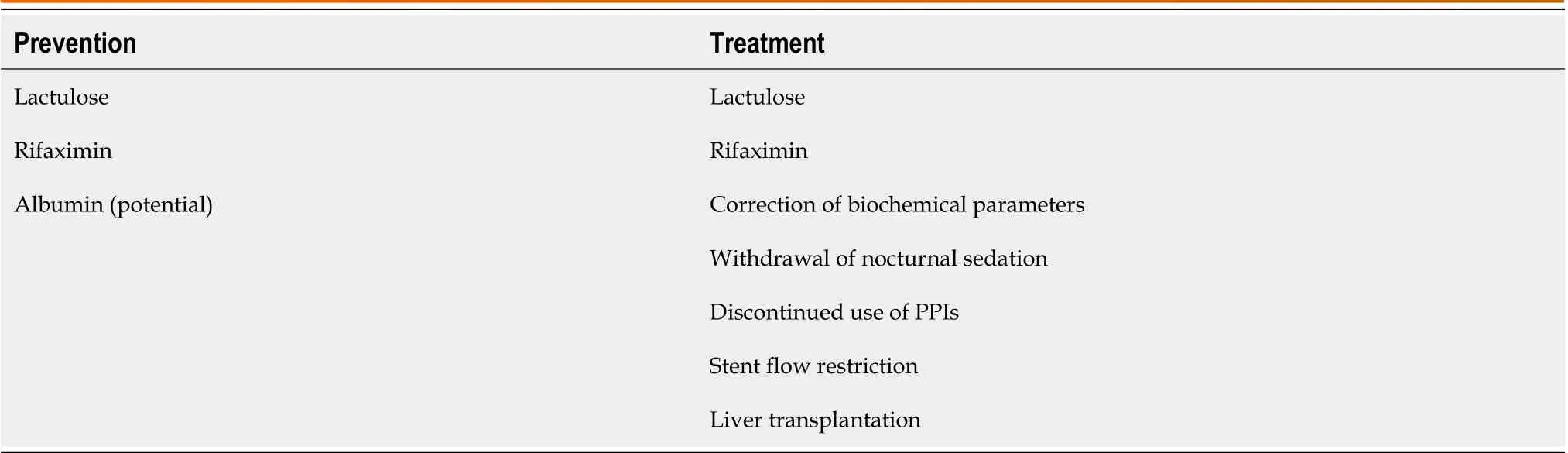
Table 2 Modalities for preventing and treating postoperative transjugular intrahepatic portosystemic shunt-related hepatic encephalopathy
CONCLUSION
As the TIPS technique and related materials, particularly stents, continue to evolve, the incidence of postoperative TIPSrelated HE and related symptoms is expected to decrease. At present, whether the occurrence of postoperative TIPSrelated HE is associated with the choice of portal vein branch shunting remains unclear. Given the lack of evidence, the invariable use of the left portal vein branch as the puncture site not only makes the procedure even more challenging but hinders the promotion and application of this technique. Patients with liver cirrhosis largely have widened liver fissures,which could expose the bifurcation of the left portal vein branch and carry an increased risk of intraperitoneal hemorrhage during intraoperative puncture of the left portal vein branch. Currently, it is believed that in patients with liver cirrhosis and portal hypertension, using an 8-mm-inner-diameter stent is associated with satisfactory shunting performance and a significantly reduced incidence of postoperative HE compared with using a 10-mm-inner-diameter product. However, further studies are needed to determine the ideal size of a stent and evaluate the clinical use of the emerging expandable stents with a controllable inner diameter in treating elderly patients with liver cirrhosis and those with poor liver reserve function. Considering the wide spectrum of etiologies causing liver cirrhosis in different countries and regions, it is an intriguing research interest to tailor post-TIPS PVPG management for the Chinese population and develop individualized treatment plans for patients with liver cirrhosis. Proactive etiological treatment can reduce the risk of postoperative TIPS-related HE. In China, post-TIPS etiological treatment has produced favorable outcomes in patients with liver cirrhosis and portal hypertension caused by the most commonly occurring hepatitis B virus[79]. Based on etiological treatment, future studies can hopefully benefit more patients with hepatitis-B-induced liver cirrhosis by achieving recompensated cirrhosis, as defined by the Baveno VII consensus[65]. Considering the complex pathogenesis of HE associated with clinical operations, postoperative patient management, and many other factors, future research requires larger sample sizes and should adopt a multicenter randomized controlled study design.
FOOTNOTES
Author contributions:Qin JP and Yao X contributed to the conception and design of the article and revised the manuscript critically;Wang LJ and Qi Q contributed to literature search and wrote the manuscript; all authors have read and approve the final manuscript.
Conflict-of-interest statement:There is no conflict of interest associated with any of the senior author or other coauthors contributed their efforts in this manuscript.
Data sharing statement:Not available.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORCID number:Lan-Jing Wang 0000-0003-0804-4828; Xin Yao 0000-0002-9977-6153; Qi Qi 0000-0001-6526-4427; Jian-Ping Qin 0000-0001-7834-8830.
S-Editor:Yan JP
L-Editor:Kerr C
P-Editor:Cai YX
杂志排行
World Journal of Gastrointestinal Surgery的其它文章
- Initial suction drainage decreases severe postoperative complications after pancreatic trauma: A cohort study
- Vascular complications of chronic pancreatitis and its management
- Historical changes in surgical strategy and complication management for hepatic cystic echinococcosis
- Post-transplant biliary complications using liver grafts from deceased donors older than 70 years:Retrospective case-control study
- Goldilocks principle of minimally invasive surgery for gastric subepithelial tumors
- Prognosis after splenectomy plus pericardial devascularization vs transjugular intrahepatic portosystemic shunt for esophagogastric variceal bleeding
