二维镍配位聚合物(Ni-CP)和Ag@Ni-CP肖特基结的制备及其光催化降解阳离子染料
2023-10-19马志虎任宜霞王智香张美丽王记江
马志虎 任宜霞 王智香 张美丽 王记江
(陕西省化学反应工程重点实验室,新能源新功能材料实验室,延安大学化学与化工学院,延安 716000)
Metal-organic coordination polymers (M-CPs)have attracted wide attention from researchers due to their regulatory structural features and wide application prospects,such as photocatalytic degradation,fluorescent probes,electronic materials,gas adsorption and storage,and chemical sensing[1-3].With the development of industry,especially the dyeing and printing of the textile industry,more than 8000 organic dyes have been used to form the basis of environmental pollution.In recent years,more and more attention has been paid to contaminant-degraded materials[4-7].It has been found that semiconductors play an important role in environmental treatment.As a new type of semiconductor,M-CPs are favored for their large specific surface area,adjustable structure,highly ordered porosity,and uniform metal sites[8-11].The photocatalytic activity of semiconductors depends largely on three factors:adsorption behavior,photo-response region,and separation efficiency of electron-hole pairs[12-14].So it is very important to study the three factors.Because M-CPs have larger porosity and specific surface area,but the separation efficiency of electron and hole and the oxidation efficiency of photo-generated carriers are not particularly remarkable,the semiconductor and metal can form a Schottky barrier at the interface by doping non-metal or transition metal[15-19].Under the excitation of light,the efficiency of charge carrier separation and transition can be enhanced leading to the decrease of carrier recombination rate and the improvement of photocatalysis performance.Using precious metals such as silver,platinum,gold,and palladium as electron acceptors,the photoinduced hole/electron pair separation facilitates the interfacial charge transfer process[14,20-21].In general,AgNO3is commonly used as an electronic collector for semiconductor materials,enabling photoelectrons to jump from the n-type M-CPs conduction band (CB),which in turn returns to the Ag[22-24].Because of its Schottky barrier,the separation efficiency of electrons and holes is increased,and the oxidative nature of the holes can either directly degrade the dye or produce ·OH to degrade the dye,the design of a composite Ag@M-CP structure is exciting,thus facilitating interface electron transfer,reducing the composition of carriers on the semiconductor surface and improving the photocatalytic efficiency of M-CPs[25-31].
In this study,a new Ni-CP and its monodisperse silver nanoparticles loaded product Ag@Ni-CP were prepared by a simple photoreduction method.With methylene blue (MB),basic fuchsin (BF),and rhodamine B (RhB) as the target pollutants,the photocatalytic degradation of three dyes was carried out.In addition,the relationship between the photocatalytic activity and morphology,band structure,and silver content was also discussed.The results show that Ag@Ni-CP has good photocatalytic activity,high stability,and easy recovery.
1 Experimental
1.1 Materials and methods
All chemicals were purchased for direct use without further purification.Scanning electron microscopy(SEM) and energy dispersive spectroscopy (EDS) were performed by the German ZEISS Sigma 300 instrument.X-ray photoelectron spectroscopy (XPS) was carried out with a Thermo Scientific K-Alpha+instrument.Thermal stability was measured on a Hitachi TG/DTA7200 thermogravimetric analyzer.The fluorescence spectrum was determined by an F-7100 fluorescence spectrophotometer at room temperature.UV absorption was studied by a Shimadzu UV-2550 spectrophotometer.Powder X-ray diffraction (PXRD) patterns were obtained using an XRD - 7000 Advance X-ray powder diffractometer,working voltage: 40 kV,working current: 40 mA,source of radiation: CuKα,wavelength: 0.154 nm.Scan range: 5°-60°.The photocatalytic degradation experiments were carried out using an XP A-7 photocatalytic reactor.Mott-Schottky measurements were carried out at a CHI660E Electrochemical station.FTIR was carried out with a Thermo Scientific Nicolet iS50.
1.2 Synthesis of [Ni(DDB)0.5(2,2′-bipy)(H2O)]·H2O(Ni-CP)
A mixture of Ni(Ac)2·4H2O (0.024 g),H4DDB(0.022 g),and 2,2′-bipy (0.032 g) was added into a mixed solvent of 2 mL water,and 0.5 mL isopropanol,then stirred for 30 min at room temperature and placed in a Teflon-lined autoclave (25 mL) for 72 h at 160 ℃.After filtration and drying in the air,some green block crystals were obtained (Yield: 44% based on Ni).Elemental analysis Calcd.for C21H17N2NiO7(% ): C 53.89,H 3.66,N 5.99.Found(%): C 53.67,H 3.38,N 5.72.IR (KBr,cm-1): 3 667(w),3 597(w),3 390(s),1 611(s),1 493(s),1 441(s),1 352(s),1 199(s),975(s),763(s).
1.3 Synthesis of Ag@Ni-CP
Ag@Ni-CP was prepared by the photo-reduction method.By magnetic stirring,the powder of Ni-CP was distributed in the water,and the right amount of AgNO3was added to obtain a suspension.Then the suspension was irradiated for 3 h using a 500 W xenon arc lamp at ambient temperature,and light below 450 nm was cut off using a cut-off filter.The Ag@Ni-CP powder was washed with water to remove NO3-and dried in a vacuum at 60 ℃for 12 h and in the air for 2 h.To study the effect of silver content on the photocatalytic activity of Ni-CP,the content of silver added during photoreduction was 10%-150% based on the molar amount of Ni-CP (Fig.S1,Supporting information).When the reference percentage of Ag reached 60%,the optimal degradation rate was achieved,so we selected Ag60%@Ni-CP as the aim Ag-loaded product,named Ag@Ni-CP.The other two representatives (Ag30%@Ni-CP and Ag40%@Ni-CP)were used for comparison.
1.4 Crystal structure determination
Single crystals with suitable sizes of Ni-CP were synthesized hydrothermally and selected.Single crystal diffraction data were performed on a Bruker SMART APEX CCD diffractometer equipped with graphite monochromatic MoKαradiation (λ=0.071 073 nm).All data were corrected for LP factors and empirical absorption,and these structures were solved by the direct method of SHELXS,and the non-hydrogen atomic coordinates and each anisotropic temperature factor were refined by the full matrix least squares methodF2.The hydrogen atoms were set at the calculation position and the crystal structure was plotted with Diamond 3.1 software.The crystallographic information of Ni-CP is provided in Table 1.The selected bond lengths and angles are listed in Table S1.
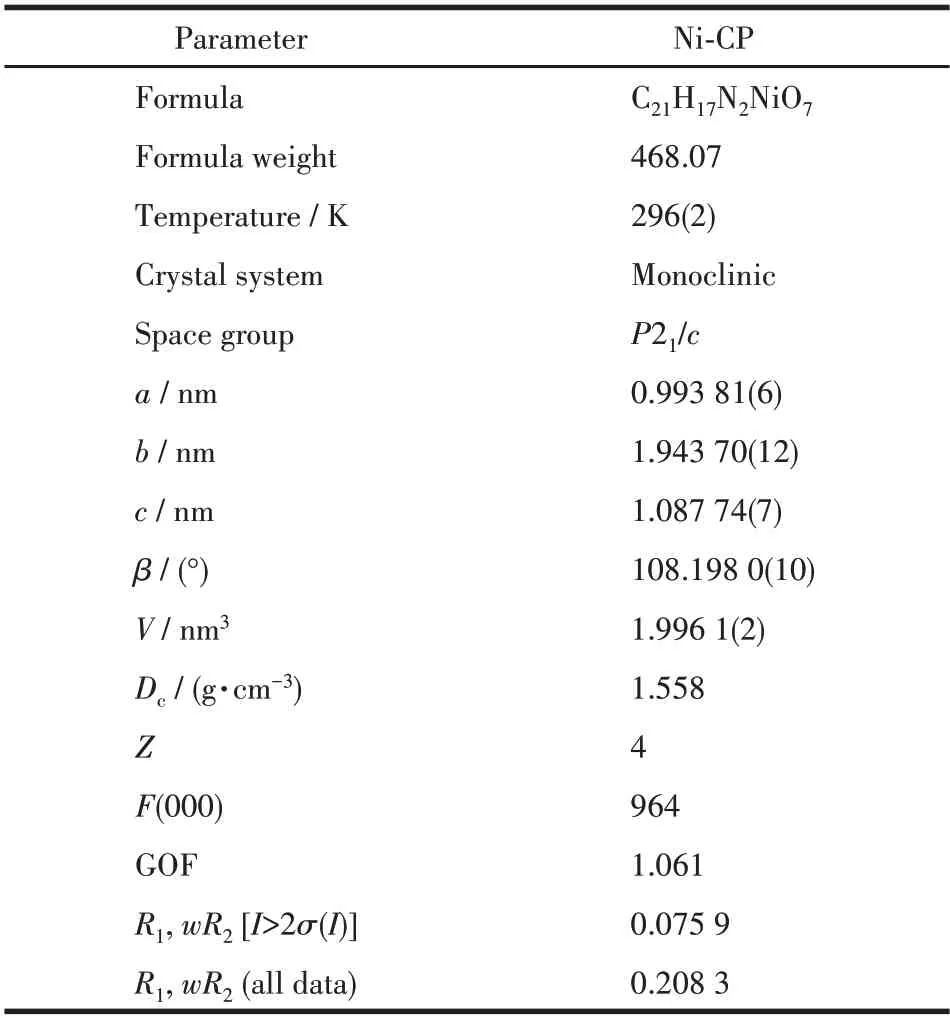
Table 1 Crystallographic data of Ni-CP
CCDC:2201522,Ni-CP.
1.5 Photocatalytic experiments of Ni - CP and Ag@Ni-CP
The photocatalytic experiment was carried out in the photocatalytic reactor,using a xenon lamp (500 W)as the light source,and the organic pollutants were MB,BF,and RhB,and then the xenon lamp of the photocatalytic reactor was turned on,rotated,and stirred.After an interval of 30 min,centrifugation was carried out in the centrifuge for 5 min,and the upper solution was put into the UV-Vis spectrometer to determine and analyze MB (λ=664 nm),BF (λ=543 nm),RhB (λ=554 nm) concentrations.The measurements were repeated until the pollutant degradation rate reached close to 100%.
2 Results and discussion
2.1 Crystal structure of Ni-CP
Single crystal X-ray diffraction analysis exhibits Ni-CP possesses a 2D wavy brick-wall network.The central nickel ion is situated in a six-coordinated octahedral geometry encircled by three oxygen atoms(O1A,O3,and O4) from two DDB4-ligands,two nitrogen atoms (N1 and N2) from one chelated 2,2′-bipy molecule,and one coordinated water molecule (O5),in which O5 and N2 atoms act as the axis atoms,and the other four atoms form the plane of the quadrilateral(Fig.1a).The V-type meta-carboxylate from one benzene ring of DDB4-ligands inμ2-bridged and chelated mode link the adjacent Ni2+ions into the 1D wavy chain (Fig.1b).By theμ4coordination mode (Fig.S1),the DDB4-ligands connect the 1D wavy chains to the 2D wavy brick-wall network(Fig.1c).
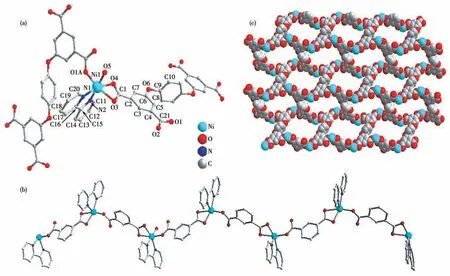
Fig.1 (a)Coordination environment of Ni2+ion in Ni-CP;(b)1D wavy chain;(c)2D network structure
Then the H-bonds among water molecules and the carboxyl oxygen atoms (O5…O2:0.273 3 nm,O5…O7:0.272 0 nm,O7…O2: 0.295 4 nm,O7…O4: 0.278 0 nm)andπ-stacking interactions between the near 2,2′-bipy molecules (the face-to-face distance is 0.353 3 nm)build 3D supramolecular structure in common.
2.2 Thermal stability of Ni-CP
To study the thermal stability of Ni-CP,the thermogravimetry curve was measured in air (Fig S2).From the curve,we find that the first weight loss occurred from 75 to 167 ℃with a weight loss rate of 7.68%,which could be attributed to the loss of all coordination and lattice water molecules (Calcd.7.70 %).After a small platform,the second weight loss process was from 331 to 391℃due to the collapse of the Ni-CP skeleton.
2.3 PXRD analysis
After measuring the single crystal diffraction to get crystal structure,the PXRD patterns were tested at room temperature to detect the purity of the Ni-CP powder.As shown in Fig.S3,the measured patterns of Ni-CP were compared with the simulated ones from the crystal structure data.Their positions of the diffraction peaks were almost coincident,indicating the powder of high purity and good crystallinity.After the photocatalytic oxidation of the dye,the powder was filtered and collected for PXRD and FTIR,as shown in Fig.S3 and S4.The position of the diffraction peak did not change,indicating that the Ni-CP has good stability during photocatalytic degradation.
2.4 XPS analysis
The chemical composition characterization of Schottky structures of Ag@Ni-CP was identified by XPS.The full XPS spectrum of Ag@Ni-CP is shown in Fig.2a,calibrating the corresponding binding energy according to the C1s(284.8eV) component signal (wrt)(Fig.2b).In the XPS spectrum of the sample,in addition to the typical N and O signals,the bimodal signals at 374.2 and 367.7 eV can be attributed to Ag3d,and by comparing it with the XPS electron binding energy comparison table,the valence state of Ag was 0,which means that there is an Ag primitive material on top of Ni-CP through XPS (Fig.2c).The peak at 855.4 eV can be attributed to Ni2p(Fig.2d) by comparing Ni to a valence state of +2,which is consistent with CCD testing.
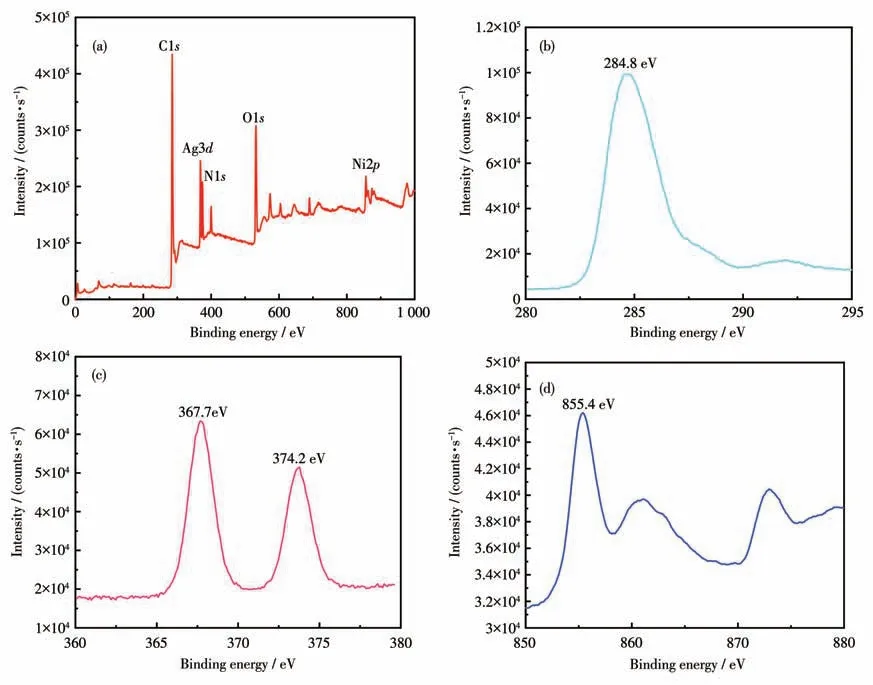
Fig.2 (a)Survey,(b)C1s,(c)Ag3d,and(d)Ni2p XPS spectra of Ag@Ni-CP
2.5 SEM of Ni-CP and Ag@Ni-CP
To study the morphology of the samples,the SEM images of Ni - CP (Fig.3a and 3b) and Ag@Ni-CP(Fig.3c and 3d) have been obtained,showing their same stick-like morphology.Some small prominent particles of about 200 nm were observed on the surface of the Ni-CP stick as shown in Fig.3c and 3d.It indicates that the silver nanoparticles are highly dispersed on the Ni-CP surface,which is consistent with the XPS results.To further confirm the successful deposition of silver on the Ni-CP surface,the electron image,the elemental mapping image,and the EDS layered image were analyzed (Fig.3e-3i).As we can see from the diagram,the silver elements are well-distributed(Fig.3i),and the mapping shows that the elements are evenly distributed.Finally,an EDS layered image displays the location relationship between elements,further confirming that Ag particles are deposited on Ni-CP(Fig.S4).
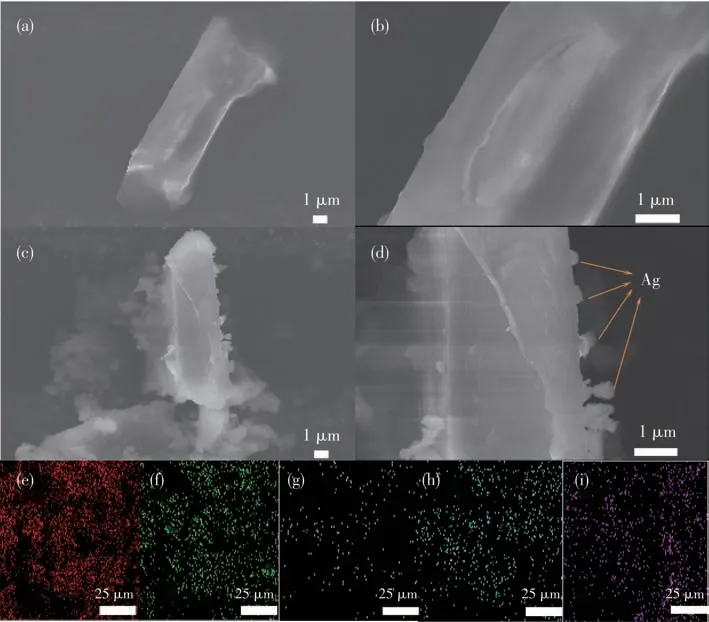
Fig.3 (a,b)SEM images of Ni-CP;(c,d)SEM images of Ag@Ni-CP;(e-i)EDS element mappings of Ag@Ni-CP
2.6 Photocatalytic activities
To study the photocatalytic activities of Ni-CP and Ag@Ni-CP,the luminous intensities were measured at room temperature first.Inhibition of the e-/h+recombination can prolong carrier life and thus improve interfacial charge transfer efficiency.For Ag@Ni- CP,Ag nanoparticles act as traps for photoelectrons in Ni-CP Schottky junction assemblies,which means that the Ag@Ni-CP Schottky junction can enhance photocatalytic activity.Photochemical measurements were made to study the activity differences among the catalysts.The photoluminescence (PL) spectra showed the lowest luminescence intensity of Ag@Ni-CP (Fig.4a),and the lower intensity usually indicates a lower photoluminescence carrier recombination rate.As shown in Fig.4b,Ag@Ni-CP had the highest photocurrent effect,which means an increase in the separation rate of electrons excited from the valence band to the conduction band.From the Nyquist plots of different electrodes (Fig.4c),Ag@Ni-CP had the shortest diameter and the highest slope line,which reflects the ion diffusion resistance caused by the porous structure in the active material.The cross-validation of the above three examples discovers that the best photocatalyst is Ag@Ni-CP.The degradation of BF with the three materials also indicates that the most effective degradation catalyst is Ag@Ni-CP (Fig.4d).Thus,it is concluded that photogenerated electrons can be efficiently transported to the Schottky junction interface,and it is expected to show the highest efficiency in photocatalytic applications.
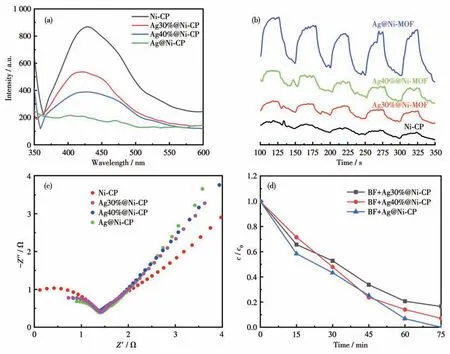
Fig.4 (a)PL spectra,(b)transient photocurrent response,and(c)Nyquist plots of different materials;(d)Photocatalytic degradation of BF by Schottky junction formed with Ag@Ni-CP in different Ag proportions
In photocatalysis,the photoinduced reaction usually plays a dominant role near the catalyst′s surface.To evaluate the photocatalytic effect of the synthesized Ag@Ni-CP Schottky structure under visible light,three dyes (MB,RhB,and BF) were selected as typical cationic organic pollutant models frequently released from the textile manufacturing industry.The photocatalytic activity of Ag@Ni-CP of the Schottky structure and its kinetics for the degradation of dyes in visible light is shown in Fig.5.In this study,self-degradation (dark degradation) of dyes was found to be negligible in the absence or presence of a catalyst in the dark.BF,MB,and RhB aqueous solutions were catalyzed and degraded by Ag@Ni-CP as photocatalysts,respectively (Fig.5a-5c).It can be observed that after photocatalytic degradation at different intervals under visible light,the maximum absorption characteristic peaks of 547 nm(BF),553 nm (RhB),and 664 nm (MB) gradually decreased with time,and the degradation rates of MB,RhB,and BF reached 99%,99%,and 96% after 60 min of degradation,respectively,which shows that Ag@Ni-CP has a good photocatalytic degradation effect on MB,BF,and RhB systems in visible light.

Fig.5 UV-Vis absorption spectra of(a)BF,(b)RhB,and(c)MB systems after visible illumination with Ag@Ni-CP photocatalyst;(d)Linear fitting diagram of photocatalytic degradation kinetics for various catalysts;(e)Photoreaction rate constants(k)of BF,RhB,and MB in the presence of various catalysts
The experimental data were accorded with a firstorder model with the following formula: -ln(c/c0)=kt(c0is the initial concentration of dye at the time of irradiation,cis the residual concentration of pigment in solution after irradiation time,kandtare the photocatalytic degradation rate constants and light duration,respectively).By converting the photocatalytic degradation data,the linear relation between -ln(c/c0) andtcould be obtained,and the slope of the linear fitting curve wask(Fig.5d).Ag@Ni-CP showed a higher photocatalytic reaction rate for MB,BF,and RhB,and the rate constants for MB,BF,and RhB were 0.012,0.006,and 0.001 8 min-1,respectively (Fig.5f).It is shown that the Schottky system with Ag@Ni-CP has good photocatalytic properties for MB,BF,and RhB for Agdeposition on the surface of Ni-CP to form Schottky junction.The comparative analysis of the degradation of different dyes by different materials is shown in Table 2.Compared with other CPs-based degradation materials,our material can quickly achieve a high degradation rate,which is a very effective photocatalytic degradation material.
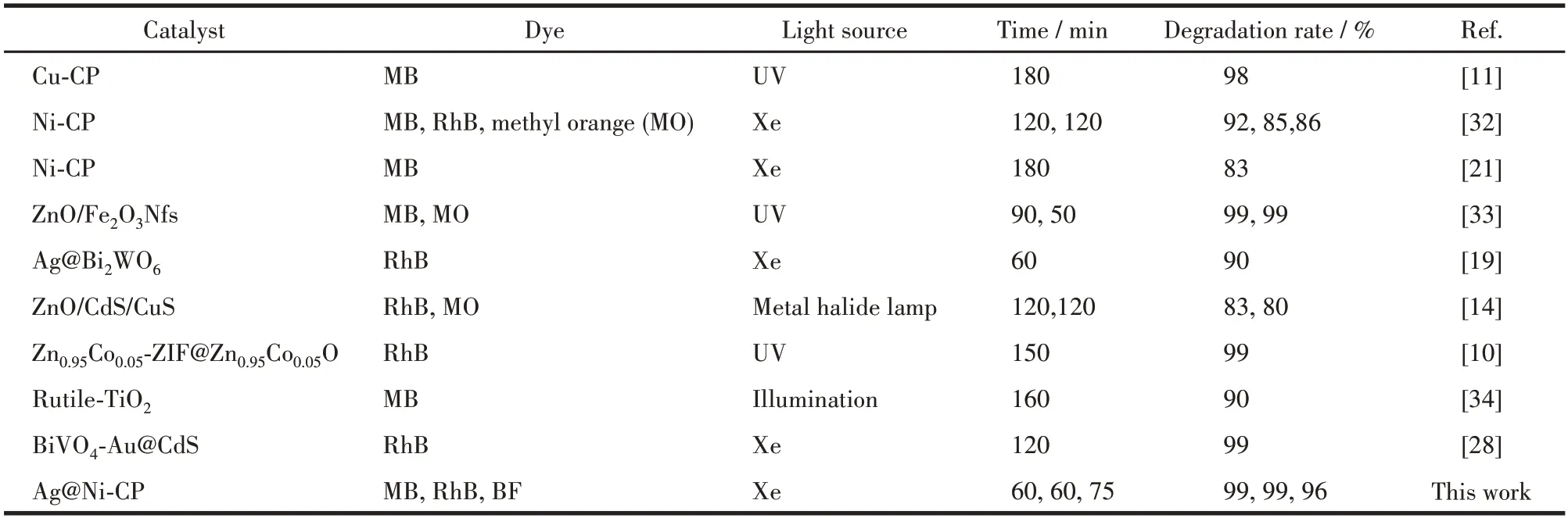
Table 2 Comparative analysis of dye degradation by different photocatalysts
2.7 Photocatalytic mechanism
To analyze the photocatalytic degradation mechanism of Ni-CP and Ag@Ni-CP,we measured the UVVis diffuse reflectance spectra at room temperature.As shown in Fig.6a,the band gap energy was calculated from the Kubelka-Munk equation:αhν=(hν-Eg)1/2.The linear portion of the absorption line extrapolates that the energy band gap (Eg) of Ni-CP,Ag30%@Ni-CP,Ag40%@Ni-CP,and Ag@Ni-CP were 2.90,2.94,2.97,and 3.17 eV,respectively,showing their potential semiconductor properties.
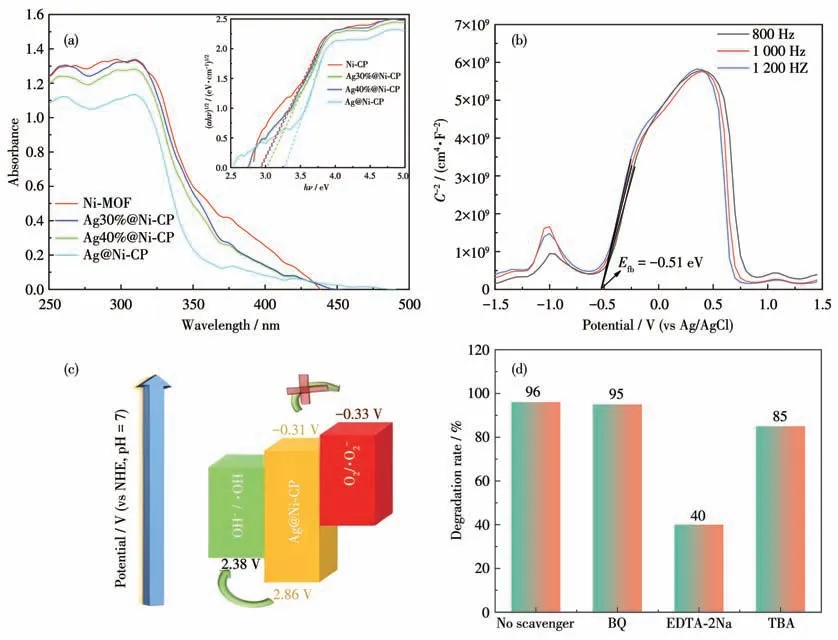
Fig.6 (a)UV-Vis diffuse reflectance spectra of Ni-CP and Ag-loaded Ni-CPs;(b)Mott-Schottky curve of Ag@Ni-CP;(c)Mechanism diagram;(d)Effect of scavenger on dye degradation photocatalyzed by Ag@Ni-CP
Mott-Schottky curves were obtained at 800,1 000,and 1 200 Hz in 0.5 mol·L-1Na2SO4electrolyte for Ag@Ni-CP.The flat band potential of the semiconductor was calculated by the Mott-Schottky curve.Fig.6b shows the positive slope at three different frequencies,indicating that the semiconductor is a typical n-type semiconductor.Based on the following equation:ENHE=EAg/AgCl+0.059pH-0.197,(EAg/AgCl=0.197 V at 25 ℃)[14],theEfbvalue relative to the standard hydrogen electrode (NHE) was deduced from the tangent line by a potential of -0.51 eV,and theECBwas 0.2 eV lower than theEfb[15-16],soECBis -0.31 eV.The valence band(EVB) of Ag@Ni-CP could be calculated as 2.86 eV according to the formula ofEg=EVB-ECB.
After the above analysis,the photocatalytic mechanism of Ag@Ni-CP of the activation products could be proposed (Fig.6c).Under simulated illumination,the activated valence band electrons of Ag@Ni-CP are excited to the conduction band to form a photoelectronhole pair.The valence bandEVBis 2.86 eV,producing·OH.The photo-generated holes in the valence band have an affinity for the adsorbed dye molecules,which can directly oxidize the dye molecules and eventually degrade to small intermediates or final products.Active substances can effectively degrade organic pollutants.These active substances include superoxide radicals (·O2-),hole (h+),and hydroxyl (·OH) radicals.Because different photocatalysts have different band structures and phase compositions,the degraded active substances may change.Thus,to elucidate the mechanism of photocatalytic activity of Ag@Ni-CP and assess the contribution of active substances,free radical trapping experiments for active substances were carried out usingp-benzoquinone (BQ),EDTA-2Na andtert-butyl alcohol (TBA) as superoxide radicals ·O2-,h+and ·OH radicals scavengers,respectively,and BF as the dye(Fig.6d).The results showed that the addition of different scavengers inhibited the degradation of BF in various degrees.Adding EDTA-2Na,the degradation rate dropped from 96% to 40%.The degradation rate of BF decreased from 96%to 85%after adding TBA.By adding BQ,the degradation rate did not change significantly.The above results show that the h+plays a crucial role in the photocatalytic degradation of BF,and ·OH can affect the degradation process,thanking for the formation of Schottky junctions that prolong the lifetime of photocarriers.
Based on these experimental results,a possible mechanism was proposed to obtain superior photocatalytic activity for composite Ag@Ni-CP.XPS results suggest that Schottky junctions may form at the interface between Ni-CP and Ag,where electrons are excited from the VB to the CB of Ni-CP by simulated sunlight,leaving holes in the VB.The photoelectrons in Ni-CP can be further trapped by the Schottky junction and then transferred to Ag,where the Schottky junction effectively prevents the photoelectrons from returning to the holes in the VB of Ni-CP and further prevents the re-assembly of the photoelectrons and the holes.Subsequently,OH-reacted with h+to form ·OH.h+with strong oxidation can also respond with dyes to degrade dyes to inorganic molecules or CO2and H2O.The mechanism is summarized as follows:
e-+Ag+→Ag
Ni-CP+hν→Ni-CP(h+)+Ni-CP(e-)
H2O →H++OH
OH-+h+→·OH
·OH+dye →CO2+H2O
h++dye →CO2+H2O
3 Conclusions
We have successfully prepared a 2D waved network nickel(Ⅱ)coordination polymer (Ni-CP) based on 1,4-di(3,5-dicarboylphenoxy) benzene and 2,2′-bipyridine and its Ag-loaded product (Ag@Ni-CP) by light reduction method.Photocatalytic degradation investigation discovers the Ag@Ni-CP possessed excellent degradation ability for cationic dyes MB,BF,and RhB with a degradation rate of 99%,96%,and 99% in 60 min,respectively.The photocatalytic mechanism shows that h+plays a key role,and ·OH can have a slight effect on the degradation process of Ag@Ni-CP for MB,BF,and RhB.Mechanism analysis evaluates that the electrons in Ni-CP are trapped by the Schottky junction and transferred to Ag,which prevents the photoelectrons from flowing back to Ni-CP.This study provides a new idea for photo-reduction of coordination polymers to prepare composite catalysts and photocatalytic degradation.
Conflicts of interest:There are no conflicts to declare.
Supporting information is available at http://www.wjhxxb.cn
