Simple Modulation of Side-chains of Near-infrared Absorbing Non-fullerene Acceptor for Higher Short-circuit Current Density
2023-10-10WANGJiachengCAIGuilongZHANGYajingWANGJiayuLUXinhuiZHANXiaoweiCHENXingguo
WANG Jiacheng, CAI Guilong, ZHANG Yajing, WANG Jiayu, LU Xinhui, ZHAN Xiaowei*, CHEN Xingguo*
Simple Modulation of Side-chains of Near-infrared Absorbing Non-fullerene Acceptor for Higher Short-circuit Current Density
WANGJiacheng1, CAIGuilong3, ZHANGYajing1, WANGJiayu2, LUXinhui3, ZHANXiaowei2*, CHENXingguo1*
(,,,430072,;,,100871,;,,999077,)
A long flexible side-chain of-octyl group has been simultaneously introduced at indacenodithieno[3,2-b]thiophene(IDT) central core and (1,1-dicyanomethylene)rhodanine end-group to build a new “A--D--A” type non-fullerene acceptor(NFA)(JC11) with thiophene-fused benzothiadiazole(BTT) unit as a-bridge, which has been proposed to investigate the influence of different side-chains at different sites of the same conjugated backbone on the properties. Compared with the previous reported acceptors A2 and JC1, the introduction of a suitable long alkyl side-chain of octyl groups at central core and terminal group not only endows JC11 to show more favorable intermolecular stacking, thus exhibiting more red-shifted absorption and better improved crystallinity than A1 with a more rigid and steric hexylphenyl group at central core, but also ensures its better solubility to avoid the poor phase-separation morphology in the blend film with PTB7-Th than JC1 with a short ethyl side-chain at end-group. Moreover, JC11 owns a higher molar extinction coefficient of up to 1.14×105L∙mol-1∙cm-1and a higher electron mobility of 1.01×10-3cm2∙V-1∙s-1as well as more balanced electron/hole mobilities(e/h=2.72)in the D/A blend film than A2 and JC1. Therefore, the optimized PTB7-Th∶JC11 based device achieves a more promising power conversion efficiency(PCE) of 10.09% with a much higher short circuit current density(SC) value of 24.20 mA/cm2, which greatly surpass the PCE of 8.20% and 8.16% with relatively lowSCvalues of less than 20 mA/cm2for the optimized devices based on PTB7-Th∶A2 and PTB7-Th∶JC1, respectively. This work indicates that the regulation of side-chains is a simple but very effective strategy to improve the absorption and crystallization properties of the NFAs, thus resulting in higherSCand PCE for bulk heterojunction organic solar cells.
A--D--A structure; Non-fullerene acceptors(NFAs); Side-chain engineering; Organic solar cell
1 Introduction
Solution-processed bulk heterojunction organic solar cells(BHJ-OSCs) have drawn great attention in the past two decades. Due to the inherent advantages, such as low cost, mechanical flexibility, light weight and short energy payback time, BHJ-OSCs have been intensively studied[1—9]. Electron donor materials and acceptor materials are the main constituents that form the photoactive layer in OSCs devices[10—12]. For electron acceptor materials, fullerenes and their derivatives were once favored based on their excellent electron transport properties, and the power conversion efficiencies(PCEs) of fullerene-based devices are 11%—12%[13—18]. However, inherent drawbacks of fullerene-based acceptors(such as weak absorption in the visible region, difficult structural modification, and morphological instability) constrain the further improvement of device efficiency[19]. In 2015, Zhan and coworkers[4]creatively designed a new fused-ring electron acceptor(FREA), ITIC that achieved a promising PCE of 6.8% at that time. Since then, the FREAs have attracted a great attention for OSCs. Generally, The FREAs adopt an A-D-A type structure, in which a fused-ring electron-donating unit(D) acts as the central core to connect two strong electron-withdrawing end groups as the electron-accepting unit(A)[20—24]. Compared to fullerene derivatives, these FREAs own strong absorption in the visible and even near-infrared region, and tunable energy levels to well match with various high-performance donor materials[25—33]. Up to now, the FREAs-based single-junction OSCs and tandem OSCs have exhibited excellent device performances with PCEs over 17% and even up to 20%[34—42].
For A-D-A type FREAs, the electron-donating cores and terminal electron-withdrawing groups have been widely explored and thoroughly studied. At the same time, some aromatic units have been chosen as the “-bridge” to build another kind of FREAs, A--D--A type structures[43—46], in which the multiple components of conjugated backbones can synergistically regulate the frontier orbital levels, absorption property and crystallinity of the FREAs and ultimately the performance of the devices[47—52]. Moreover, the introduction of the suitable side-chain substituents at the conjugated backbone has also exerted great influence on the properties of FREAs[53,54]. As known, side-chains can ensure good solubility and hinder the formation of large aggregates of the FREAs, which promote the formation of an appropriate nanoscale phase separation in photoactive layers[55]. Since the stable triaryl cations are conducive to the synthesis of the fused-ring electron cores under Friedel-Crafts reaction conditions, some groups such as alkylphenyl and alkylthienyl groups are commonly introduced onto the ladder type backbone as side-chains to regulate the solubility and the intermolecular aggregation[55,56]. However, the steric effect of rigid alkylphenyl side-chains always hinders the intermolecular⁃stacking that is unfavorable to the charge transport. To overcome this drawback, some flexible side-chains have been introduced onto the ladder type backbone of NFAs to strengthen the intermolecularinteraction for improving the charge transport[57]. In the reported literatures, long linear alkyl-chain-based FREAs usually exhibited better photovoltaic performances than the alkylphenyl-based ones[58]. Therefore, systematic investigation on the side-chains is necessary to realize high-efficiency FREAs.
Recently, a series of “A--D--A” type FREAs has been reported by our group[59—64]and it has been found that some thiophene-fused aromatic rings such as thiophene-fused benzothiadiazole(BTT) and thiophene-fused benzotriazole(BTAZT) units as-bridge can strengthen the intramolecular charge transfer(ICT) of the conjugated backbone and further broaden the absorption spectrum to improve the short circuit current density(SC). For example, the acceptor A2[59]and JC1[60]with BTT unit showed more red-shifted absorption than that of the non-fused benzothiadiazole-based ones. Both of them extended their absorption to the near-infrared(. 950 nm) region and obtained a competitiveSCvalue when blend with the corresponding donor materials to fabricate the OSC devices, indicating that the BTT-based FREAs are the promising FREAs for OSCs. As can be seen from Fig.1, A2 and JC1 showed the same conjugated skeleton, but different side-chains have been introduced onto conjugated backbone, which may affect the photophysical, electrochemical and crystallization properties of the BTT-based A--D--A type FREAs. However, we did not pay much attention to systematic comparison of substituent effect. Obviously, it deserves to be investigated for deeply understanding the structure-property correlation. Herein, we designed another new BTT-based non-fullerene acceptor(namely, JC11), in which a flexible-octyl group as the side-chain has been simultaneously introduced at indacenodithiophene(IDT) central core and (1,1-dicyanomethylene)rhodamine end group. It can be seen in Fig.1 that JC11 shows the same conjugated skeleton as A2 and JC1, so we can conveniently investigate the influence of different side-chains at different sites on their properties by comparison with each other. In such a design, the long flexible alkyl side-chains introduced at central core and end-groups can not only improve the crystallinity and intermolecular packing, but also ensure good solubility of the acceptor to avoid poor morphology in the donor/acceptor(D/A) blend films. The experimental results show that linear alkyl-substituted acceptors JC1 and JC11 exhibit more red-shifted absorption than that of alkylphenyl-substituted acceptor A2. Moreover, JC11 shows a higher molar extinction coefficient of up to 1.14×105L∙mol-1∙cm-1and a higher electron mobility of 1.01×10-3cm2∙V-1∙s-1than A2 and JC1. Considering that all of them show the strong and broad absorption extending to the near-infrared region, the classical low-bandgap polymer PTB7-Th is selected as the donor material to form photoactive layers. As expected, the optimized PTB7-Th∶JC11 based device achieves a promising PCE of 10.09% with a much higherSCvalue of 24.20 mA/cm2, while the PCE of the optimized devices based on PTB7-Th∶A2 and PTB7-Th∶JC1 are only 8.20% and 8.16% with relatively lowSCvalues of less than 20 mA/cm2.
2 Experimental
2.1 Reagents
Unless stated otherwise, solvents and chemicals were obtained commercially and used without further purification. M1 was prepared according to our previous work[59,60].
2.2 Synthesis of JC11
M1(75 mg, 0.058 mmol), 3-octyl-2-(1,1-dicyanomethylene)rhodanine(160 mg, 0.58 mmol) and ammonium acetate(90 mg, 1.16 mmol) were dissolved in CH3COOH/CHCl3(volume ratio 1∶4, 20 mL), which was refluxed for 24 h under argon atmosphere. After cooling to room temperature, the mixture was poured into methanol. After filtration, the residue was washed with methanol for several times. And then it was further purified by column chromatography over silica gel with petroleum/CHCl3mixtures as the eluent to give JC11 as a green solid(80 mg, 76%).1H NMR(400 MHz, CDCl3),: 8.80(s, 2H), 8.38(s, 2H), 8.01(s, 2H), 7.53(s, 2H), 4.51(q,=8 Hz, 4H), 4.29(t,=8 Hz, 4H), 2.17(m, 4H), 2.01(m, 4H), 1.82(m, 4H), 1.48—1.00(m, 74H), 0.92—0.7(m, 18H).13C NMR(100 MHz, CDCl3),: 166.94, 166.91, 161.48, 157.04, 154.63, 151.52, 150.73, 150.10, 148.66, 138.43, 137.04, 136.54, 136.32, 128.56, 128.28, 127.58, 121.06, 115.93, 114.50, 113.30, 112.55, 62.55, 55.49, 54.58, 45.51, 39.17, 31.82, 31.76, 30.12, 29.41, 29.30, 29.15, 29.10, 28.87, 26.07, 24.59, 22.66, 22.63, 14.35, 14.13, 14.10. MS(MALDI-TOF),/calculated for C100H120N10O6S8: 1813.72; found: 1813.73. Elemental anal.(%) calcd. for C100H120N10O6S8: C 66.19, H 6.67, N 7.72; found: C 66.84, H 6.55, N 7.72.
3 Results and Discussion
3.1 Synthesis
The synthetic route of JC11 is presented in Scheme 1. The starting material M1 that contains-octyl- substituted IDT core and BTT-bridge was prepared according to our previous work[59,60]. Then, the Knoevenagel condensation of M1 with 3-octyl-2-(1,1-dicyanomethylene)rhodanine produced JC11 in a yield of 76%. The structure of JC11 was verified by1H NMR,13C NMR, TOF-MS, and elemental analysis. Certainly, JC11 shows better solubility in common organic solvents such as chloroform(CF) and-dichlorobenzene(-DCB) at room temperature than JC1, which will prevent JC11 to form large self-aggregation. Thermogravimetric analysis(TGA) was performed to investigate the thermal stability of the acceptors(Fig.S1, see the Supporting Information of the paper). It is found that JC11 exhibits the decomposition temperature(5% mass loss) up to 372 ℃, while A2 and JC1 show the decomposition temperature of 374 ℃ and 347 ℃, respectively. Obviously, despite having different side-chain types and lengths, all of them exhibit excellent thermal stability.

Scheme 1Synthetic route of small molecular acceptor JC11
3.2 Optical and Electrochemical Properties
The UV-Vis absorption spectra of JC11, A2 and JC1 as well as PTB7-Th in dilute CF solution are shown in Fig.2(A). By comparison, JC11 and JC1 with linear alkyl-substituted groups as side-chains exhibit more red-shifted absorption with maximum at 723 nm than that of alkylphenyl-substituted acceptor A2(max=708 nm) in solution, indicating that less steric effect of the flexible alkyl group at central core in JC1 and JC11 than-hexylphenyl group in A2 does some favor for the interchain-stacking for aggregation to be responsible for their red-shifted absorption. Additionally, JC11 owns improved light-harvesting ability with higher molar extinction coefficient() of up to 1.14×105L∙mol-1∙cm-1compared to those of JC1(=0.88×105L∙mol-1∙cm-1) and A2(=0.85×105L∙mol-1∙cm-1) in Fig.S2(see the Supporting Information of this paper). The higher ε for JC11 indicates that the long flexible alkyl side-chains at the IDT core and end group can enhance the absorption capacity of the FREAs. In addition, in the neat films, JC11, A2 and JC1 show the similar absorption tendency to the solution, but the neat films of JC11, A2 and JC1 exhibit a broader and more significantly red-shifted absorption than those in solution with the absorption peaks at 831, 771 and 828 nm, respectively, as shown in Fig.2(B). From solution to film, the larger red-shift for JC11(108 nm) and JC1 (105 nm) than that of A2(63 nm) indicates that linear alkyl-substituted acceptors own stronger intermolecular-interaction and closer intermolecular stacking in the aggregation state. Remarkably, JC11, A2 and JC1 extended their absorption range to the near-infrared region with optical bandgap of 1.33, 1.37 and 1.30 eV, respectively, calculated from the absorption edges[65], suggesting that they show good complementary absorption with PTB7-Th donor material. The optical data are summarized in Table 1.

Table 1 Optical and electrochemical properties of JC1 and JC2
The electrochemical properties of FREAs were studied by the cyclic voltammetry(CV) method with Ag/Ag+as reference electrode. Fig.2(C) shows the CV curves of JC11 as well as A2 and JC1 for comparison. Based on the reductive potentials, the lowest unoccupied molecular orbital(LUMO) levels have been estimated from the equationLUMO=-[red-1/2(c/c+)+4.8](eV) and summarized in Table 1. The LUMO energy levels of alkyl-substituted acceptors JC1 and JC11 are slightly lower than that of phenylalkyl-substituted acceptor A2, indicating potentially lower open-circuit voltage(OC) values for JC11-based and JC1-based devices.

Fig.2 Normalized UV⁃Vis absorption spectra of acceptors in CF solution(A), acceptors and donor material PTB7⁃Th in thin film(B), CV curves of acceptors in CH3CN/0.1 mol/L Bu4NPF6 at 100 mV/s(C) and the inverted device fabrication structure(D)
3.3 Photovoltaic Performances
The OSC devices were fabricated with the inverted configuration[shown in Fig.2(D)] to investigate the photovoltaic performances of the three FREAs, in which the active layer was casted onto the ZnO by spin- coating process. To optimize the performance of PTB7-Th∶FREA based OSCs, different solvents, D/A mass ratios and additives were explored and the optimization process is described in the Supporting Information. The as-cast and optimized photovoltaic parameters are summarized in Table 2. The as-cast OSC devices based on PTB7-Th∶A2 and PTB7-Th∶JC1 exhibit PCE values of 7.02%(OCof 0.722 V,SCof 15.85 mA/cm2and FF of 61.3%) and 6.42%(OCof 0.662 V,SCof 15.48 mA/cm2and FF of 62.7%), respectively. The as-cast JC11 based device shows a relatively high PCE value of 7.53% withOCof 0.688 V,SCof 19.71 mA/cm2and FF of 55.5%. After addition of solvent additives chloronaphthalene(CN) or 1,8-diiodooctane(DIO), allSCvalues of the processed device are significantly improved, while allOCvalues are slightly reduced. The optimized PTB7-Th∶A2 and PTB7-Th∶JC1 based devices yield the best PCE values of 8.20%(OCof 0.701 V,SCof 19.47 mA/cm2, and FF of 60.1%) and 8.16%(OCof 0.656 V,SCof 19.12 mA/cm2, and FF of 65.0%), respectively. However, the optimized JC11-based device achieves a more promising PCE of up to 10.09% withOCof 0.674 V, FF of 61.9%, and a much higherSCvalue of 24.20 mA/cm2than A2 and JC1, which can be attributed to the higher absorptivity and better absorption complementarity with PTB7-Th for JC11 because its long flexible alkyl side-chains endows JC11 to show more favorable intermolecular stacking with more red-shifted absorption and better improved crystallinity as well as its better solubility to avoid the poor phase-separation morphology in the D/A blend. Furthermore, the addition of additives has a significant positive effect on the FF of alkyl-substituted acceptors JC1-based and JC11-based devices, but little improvement on alkylphenyl-substituted acceptor A2-based ones. This result means that the active layers containing the alkyl-substituted acceptors are more sensitive to the additive, which would facilitate the regulation of the morphology to form better phase separation.

Table 2 Photovoltaic performance of the devices based on the PTB7-Th:acceptor under the illumination of AM 1.5G, 100 mW/cm2
. Volume fraction;. the average value with standard deviation was obtained from 10 devices
Consistent with the absorption properties of acceptors and donor, all the external quantum efficiency(EQE) spectra of the optimized A2-, JC1-, and JC11-based devices exhibit wide photoresponse ranges as illustrated in Fig.3(B). The EQE maxima of the optimized PTB7-Th∶A2, PTB7-Th∶JC1, and PTB7-Th∶JC11 blends are 74%, 72%, and 83%, respectively; the higher EQE response of JC11-based devices could be attributed to its stronger absorption. In addition, the PTB7-Th∶JC11 device shows EQE over 70% in the range of 570—850 nm, which is far superior to that of A2 and JC1, indicating a more efficient photoelectric conversion process and eventually obtaining higherSCvalue in the JC11-based device. The calculatedSCvalues from integration of EQE spectra of all as-cast and optimized devices match well with thecurves[Fig.3(A)] within the error range of <5%, as summarized in Table 2.

Fig.3 J⁃V characteristics(A), and EQE curves(B), Jph⁃Veff curves and JSC⁃light intensity plots(D) of the optimized OSCs devices
To further investigate and compare the charge collection capacity, we measured the photocurrent density (ph)the effective voltage(eff) of optimized devices[66], as shown in Fig.3(C).phcan be defined asph=L-D, whereLandDrepresent theSCvalues under illumination and dark condition, respectively.efffollows the formulaeff=0-a, whereais the applied bias voltage and0is the voltage whenphequals to 0. Generally, photogenerated excitons among the active layer would all be dissociated into free charge carriers and thephreaches saturated condition(sat) at higheff. Therefore, thesc/satratio can characterize the ability of charge extraction under short-circuit condition. Both the optimized A2-based and JC1-based devices show lowsc/satratios of 93% and 91%, respectively, while the ratio climbs to 99% for optimized JC11-based device. This result indicates more effective charge extraction in JC11-based device, which is beneficial to higherSC. In addition, we measured theSCvalues at different incident light intensities() to investigate the degree of bimolecular recombination in OSC devices, and the relation between theandSCfollows the formulaSCP[67]. When all free carriers are collected by electrodes completely, recombination phenomenon is nonexistent and the exponentis equal to 1.00. If<1, it means a certain degree of bimole-cular recombination. As shown in Fig.3(D), thevalues of the optimized PTB7-Th∶A2, PTB7-Th∶JC1, and PTB7-Th∶JC11 devices are calculated to be 0.95, 0.96, and 0.99, respectively, indicating that there are negligible bimolecular recombination phenomena among these devices.
At the same time, exploring the effect of carrier transport on the photovoltaic performance is essential. As shown in Fig.S2, the space-charge limited current(SCLC) method was performed to measure the hole mobility (h) and electron mobility(e) of neat films and optimized blend films[68]. The electron mobilities of A2, JC1, and JC11 neat films are 7.46×10-4, 5.00×10-4, and 1.01×10-3cm2∙V-1∙s-1, respectively. Thanks to the superiority of the BTT-based structure, all the FREAs exhibit relatively high mobility. Moreover, the electron mobility of JC11 is one order of magnitude higher than that of A2 and JC1, indicating that alkyl side-chain in the IDT core and suitable side-chain length in end group could synergistically enhance the intermolecular interaction and stacking. For blend films, the optimized PTB7-Th∶A2 blend film shows thehof 1.27×10-3cm2∙V-1∙s-1andeof 2.27×10-4cm2∙V-1∙s-1withh/eratio of 5.59, and the optimized PTB7-Th∶JC1 blend film owns an exaggeratedh/eratio of up to 66.67 with thehof 1.50×10-3cm2∙V-1∙s-1andeof 2.25×10-5cm2∙V-1∙s-1, which may be related to its poor phase morphology. By contrast, the optimized PTB7-Th∶JC11 blend film shows a much smallerh/eratio of 2.72 with thehof 1.66×10-3cm2∙V-1∙s-1andeof 6.10×10-4cm2∙V-1∙s-1. Apparently, the more balanced charge mobility and higher electron mobility do some favor for improving theSC.
3.4 Morphology Characterization
To further investigate the influence of side-chains on the morphology, optimized FREAs-based blend films were characterized by atomic force microscopy(AFM) and transmission electron microscopy(TEM). As shown in Fig.4, all the optimized PTB7-Th∶A2, PTB7-Th∶JC1, and PTB7-Th∶JC11 blend films exhibit smooth and uniform surfaces with the root mean square(RMS) roughness of 2.11, 1.25, and 1.19 nm, respectively. Although no valid information is obtained from the AFM height images, the phase distribution can be clearly distinguished from the TEM images. As for optimized PTB7-Th∶A2 blend film, a relatively homogeneous feature is observed[Fig.4(D)]. However, optimized PTB7-Th∶JC1 blend film exhibits large domains and obvious phase separation, as shown in Fig.4(E), which could be attributed to the strong crystallinity of JC1 with short alkyl side-chain at the end group. In comparison, the optimized PTB7-Th∶JC11 blend film shows well-proportioned aggregation domains with suitable phase separation[Fig.4(F)] due to the good crystallinity of JC11 with long flexible alkyl side-chains at the end group, which can effectively prevent the excessive self-aggregation. These results indicate that the optimized JC11-based blend film has more efficient exciton separation and charge transfer capability, therefore, realizing higherSCvalue for corresponding device.

Fig.4 AFM height images(A—C) and TEM images(D—F) of the optimized PTB7⁃Th∶A2(A, D), PTB7⁃Th∶JC1(B, E), and PTB7⁃Th∶JC11(C, F) blend films
Moreover, the grazing-incidence wide-angle X-ray scattering(GIWAXS) was also used to investigate the influence of side chains replacement on the film morphologies[69]. Fig.5 and Fig.S5(see the Supporting Information of this paper) show the two-dimensional(2D) patterns and the corresponding intensity profiles of the pure and blend films, respectively. The polymer donor PTB7-Th film shows strong lamellar peak atr=0.273 Å-1(=23.0 Å)(1 Å=0.1 nm) and broad-peak atz=1.66 Å-1(=3.78 Å) in in-plane(IP) and out-of-plane(OOP) direction, respectively. Pure A2 film displays a lamellar peak atr=0.327 Å-1(=19.2 Å), while JC11 exhibits obvious lamellar peak atr=0.337 Å-1(=18.6 Å) and-peak atq= 1.85 Å-1(=3.39 Å). In addition to intense-peak atq=1.87 Å-1(=3.36 Å), JC1 shows obvious lamellar peaks atr=0.308/0.371 Å-1(=20.4/16.9 Å) andq=0.531 Å-1(=11.8 Å) in both IP and OOP direction. Compared to A2 and JC1, JC11 exhibits higher crystallinity and more predominant face-on orientation, which is consistent with the higher electron mobility. Optimized PTB7-Th∶A2 blend film exhibits very strong lamellar peak atq=0.327 Å-1(=19.2 Å) but weak lamellar and-peak atr/q=0.273/1.67 Å-1, indicating preferred edge-on orientation. The JC11-based blend film presents the similar scattering features as the JC1-based film in terms of molecular packing orientation and crystallinity. However, JC1-based film displays two lamellar peaks atq=0.408/0.527 Å-1(=15.4/11.9 Å) in OOP direction relative to PTB7-Th∶JC11 film, which can hinder the regular packing of molecules. Therefore, appropriate extension of alkyl side chains in end groups is an effective strategy for the regulation of molecular packing in the active layer.
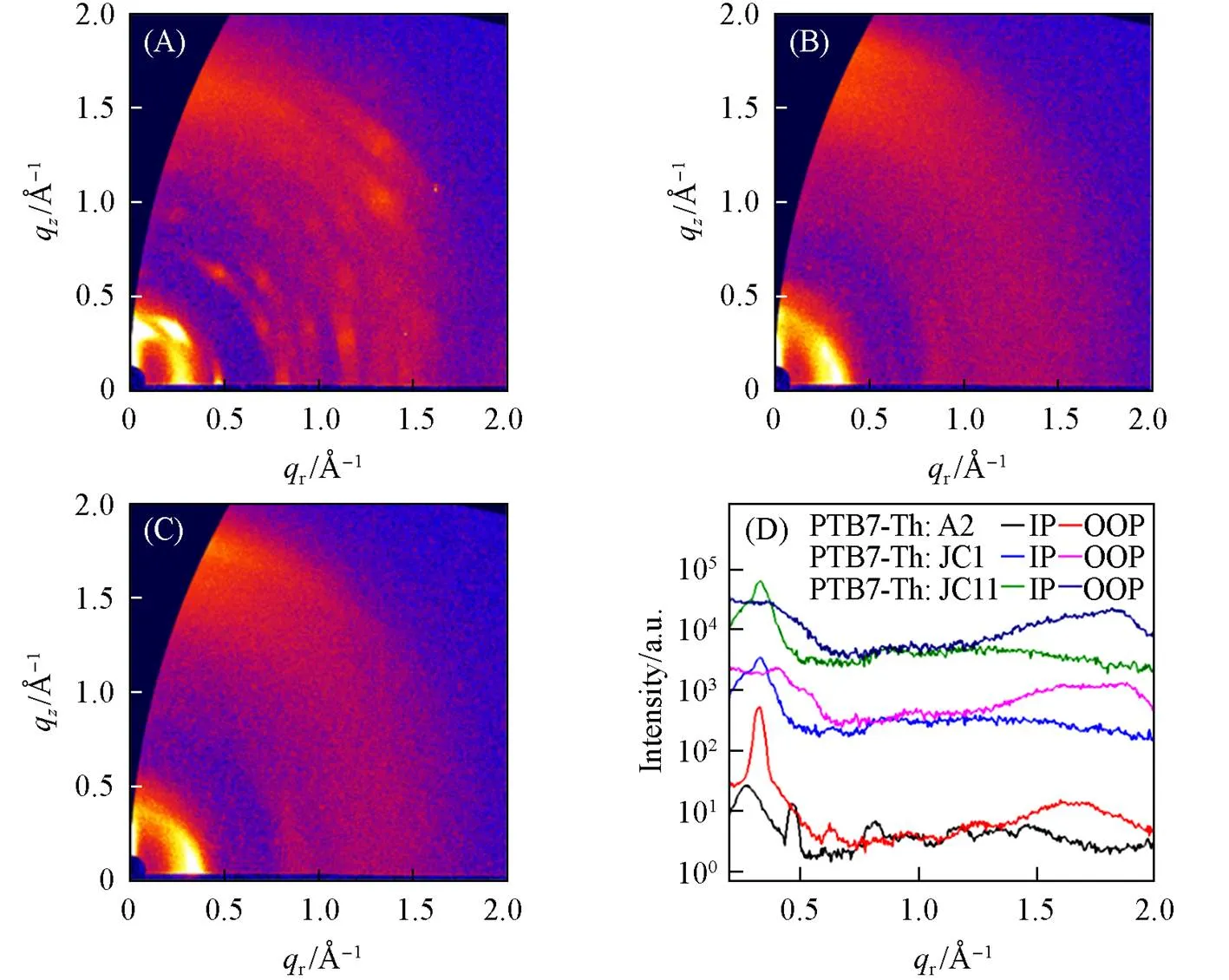
Fig.5 2D GIWAXS patterns of PTB7⁃Th∶A2(A), PTB7⁃Th∶JC1(B) and PTB7⁃Th∶JC11(C) optimized films and the corresponding intensity profiles along the in⁃plane and out⁃of⁃plane directions(D)
4 Conclusions
In summary, a new BTT-based non-fullerene acceptor JC11 withoctyl group as the side-chains introduced simultaneously at IDT core and rhodanine end group was designed and synthesized. The influence of different side-chains at different sites of the same conjugated skeleton on the properties was investigated and compared with each other. It is found that a suitable flexible alkyl side-chain simultaneously introduced at central core and terminal group for JC11 is favorable for its intermolecular stacking and solubility that endows it to show more red-shifted absorption and better improved crystallinity than A1 as well as better phase-separation morphologies mixed with PTB7-Th than JC1. Moreover, JC11 owns higher molar extinction coefficient of up to 1.14×105L∙mol-1∙cm-1and higher electron mobility of 1.01×10-3cm2∙V-1∙s-1. For photovoltaic performances, the optimized PTB7-Th∶JC11 based device achieves a promising PCE of 10.09% with a much higherSCvalue of 24.20 mA/cm2, while the PCE of the optimized devices based on PTB7-Th∶A2 and PTB7-Th∶JC1 are only 8.20% and 8.16% with relatively lowSCvalues of less than 20 mA/cm2. Overall, this work demonstrates that the regulation of side-chains is a simple but very effective strategy to improve the absorption and crystallization properties of the FREAs, thus resulting in higherSCand PCE for BHJ-OSCs.
The supporting information of this paper see http://www.cjcu.jlu.edu.cn/CN/10.7503/cjcu20230163.
[1] Li G., Zhu R., Yang Y.,, 2012,, 153—161
[2] Lu L., Zheng T., Wu Q., Schneider A. M., Zhao D., Yu L.,,2015,, 12666—12731
[3] Lin Y., Zhan X.,.,2016,, 175—183
[4] Lin Y., Wang J., Zhang Z. G., Bai H., Li Y., Zhu D., Zhan X.,,2015,, 1170—1174
[5] Dai S., Zhao F., Zhang Q., Lau T. K., Li T., Liu K., Ling Q., Wang C., Lu X., You W., Zhan X.,.,2017,, 1336—1343
[6] Li Y.,,2012,, 723—733
[7] Wang J., Xue P., Jiang Y., Huo Y., Zhan X.,,2022,, 614—634
[8] Xue P., Cheng P., Han R. P. S., Zhan X.,.,2022,, 194—219
[9] Yan C., Barlow S., Wang Z., Yan H., Jen A. K. Y., Marder S. R., Zhan X.,., 2018,, 18003
[10] Cheng P., Li G., Zhan X., Yang Y.,,2018,, 131—142
[11] Zhang G., Lin F. R., Qi F., Heumüller T., Distler A., Egelhaaf H.-J., Li N., Chow P. C. Y., Brabec C. J., Jen A. K. Y., Yip H. L.,,2022,, 14180—14274
[12] Jia B., Zhan X.,, 2020,, 1179—1181
[13] Liu Y., Zhao J., Li Z., Mu C., Ma W., Hu H., Jiang K., Lin H., Ade H., Yan H.,.,2014,, 5293
[14] Zhao J., Li Y., Yang G., Jiang K., Lin H., Ade H., Ma W., Yan H.,,2016,, 15027
[15] Vohra V., Kawashima K., Kakara T., Koganezawa T., Osaka I., Takimiya K., Murata H.,,2015,, 403—408
[16] Wan Q., Guo X., Wang Z., Li W., Guo B., Ma W., Zhang M., Li Y.,.,2016,, 6635—6640
[17] Zhang S., Ye L., Zhao W., Yang B., Wang Q., Hou J.,.,2015,, 248—256
[18] Deng D., Zhang Y., Zhang J., Wang Z., Zhu L., Fang J., Xia B., Wang Z., Lu K., Ma W., Wei Z.,.,2016,, 13740
[19] Ross R. B., Cardona C. M., Guldi D. M., Sankaranarayanan S. G., Reese M. O., Kopidakis N., Peet J., Walker B., Bazan G. C., van Keuren E., Holloway B. C., Drees M.,,2009,, 208—212
[20] Zhu J., Ke Z., Zhang Q., Wang J., Dai S., Wu Y., Xu Y., Lin Y., Ma W., You W., Zhan X.,.,2018,, 1704713
[21] Wang W., Yan C., Lau T. K., Wang J., Liu K., Fan Y., Lu X., Zhan X.,,2017,, 1701308
[22] Lin Y., He Q., Zhao F., Huo L., Mai J., Lu X., Su C. J., Li T., Wang J., Zhu J., Sun Y., Wang C., Zhan X.,.,2016,, 2973—2976
[23] Wang J., Zhan X.,,2021,, 132—143
[24] Meng L., Zhang Y., Wan X., Li C., Zhang X., Wang Y., Ke X., Xiao Z., Ding L., Xia R., Yip H. L., Cao Y., Chen Y.,,2018,, 1094—1098
[25] Zhang G., Zhang K., Yin Q., Jiang X., Wang Z., Xin J., Ma W., Yan H., Huang F., Cao Y.,.,2017,, 2387—2395
[26] Luo Z., Bin H., Liu T., Zhang Z.-G., Yang Y., Zhong C., Qiu B., Li G., Gao W., Xie D., Wu K., Sun Y., Liu F., Li Y., Yang C.,,2018,, 1706124
[27] Shi X., Zuo L., Jo S. B., Gao K., Lin F., Liu F., Jen A. K. Y.,,2017,, 8369—8376
[28] Wang Y., Price M. B., Bobba R. S., Lu H., Xue J., Wang Y., Li M., Ilina A., Hume P. A., Jia B., Li T., Zhang Y., Davis N. J. L. K., Tang Z., Ma W., Qiao Q., Hodgkiss J. M., Zhan X.,, 2022, 34, 2206717
[29] Xu X., Li Z., Zhang W., Meng X., Zou X., Rasi D. D. C., Ma W., Yartsev A., Andersson M. R., Janssen R. A. J., Wang E.,,2018,, 1700908
[30] Wang J., Wang W., Wang X., Wu Y., Zhang Q., Yan C., Ma W., You W., Zhan X.,.,2017,, 1702125
[31] Li T., Dai S., Ke Z., Yang L., Wang J., Yan C., Ma W., Zhan X.,., 2018,, 1705969
[32] Chandrabose S., Chen K., Barker A. J., Sutton J. J., Prasad S. K. K., Zhu J., Zhou J., Gordon K. C., Xie Z., Zhan X., Hodgkiss J. M.,.,2019,, 6922—6929
[33] Dai S., Li T., Wang W.,Xiao Y., Lau T., Li Z., Liu K., Lu X., Zhan X.,., 2018,30, 1706571
[34] Bi P., Wang J., Cui Y., Zhang J., Zhang T., Chen Z., Qiao J., Dai J., Zhang S., Hao X., Wei Z., Hou J.,,2023,, 2210865
[35] Yuan J., Zhang Y., Zhou L., Zhang G.,Yip H., Lau T., Lu X., Zhu C., Peng H., Johnson P., Leclerc M., Cao Y., Ulanski J., Li Y., Zou Y.,2019,, 1140—1151
[36] Ou Z., Qin J., Jin K., Zhang J., Zhang L., Yi C., Jin Z., Song Q., Sun K., Yang J., Xiao Z., Ding L.,,2022,, 3314—3320
[37] Sun R., Wu Y., Yang X., Gao Y., Chen Z., Li K., Qiao J., Wang., Guo J., Liu C., Hao X., Zhu H., Min J.,,2022,, 2110147
[38] Chen T., Li S., Li Y., Chen Z., Wu H., Lin Y., Gao Y., Wang M., Ding G., Min J., Ma Z., Zhu H., Zuo L., Chen H.,,2023, 35, 202300400
[39] Li S., Zhang R., Zhang M., Yao J., Peng Z., Chen Q., Zhang C., Chang B., Bai Y., Fu H., Ouyang Y., Zhang C., Steele J. A., Alshahrani T., Roeffaers M. B.J., Solano E., Meng L., Gao F., Li Y., Zhang Z. G.,,2023, 35, 2206563
[40] Cai Y., Xie C., Li Q., Liu C., Gao J., Jee M. H., Qiao J., Li Y., Song J., Hao X., Woo H. Y., Tang Z., Zhou Y., Zhang C., Huang H., Sun Y.,,2023, 35, 2208165
[41] Yang X., Sun R., Wang Y., Chen M., Xia X., Lu X., Lu G., Min J.,, 2023, 35, 2209350
[42] Chen H., Zhang Z., Wang P., Zhang Y., Ma K., Lin Y., Duan T., He T., Ma Z., Long G., Li C., Kan B., Yao Z., Wan X., Chen Y.,, 2023, 16, 1773—1782
[43] Liu F.,Zhou Z., Zhang C., Vergote T., Fan H., Liu F., Zhu X.,.,2016,, 15523—15526
[44] Yao H., Cui Y., Yu R., Gao B., Zhang H., Hou J., Design,.,2017,, 3045—3049
[45] Wu J., Xu Y., Yang Z., Chen Y., Sui X., Yang L., Ye P., Zhu T., Wu X., Liu X., Cao H., Peng A., Huang H.,,2019,, 1803012
[46] Chen S., Yao H., Hu B., Zhang G., Arunagiri L., Ma L., Huang J., Zhang J., Zhu Z., Bai F., Ma W., Yan H.,.,2018,, 1800529
[47] Wu Y., Bai H., Wang Z., Cheng P., Zhu S., Wang Y., Ma W., Zhan X.,.,2015,, 3215—3221
[48] Xiao B., Tang A., Cheng L., Zhang J., Wei Z., Zeng Q., Zhou E.,2017,, 1700166
[49] Holliday S., Ashraf R. S., Wadsworth A., Baran D., Yousaf S. A., Nielsen C. B., Tan C., Dimitrov S. D., Shang Z., Gasparini N., Alamoudi M., Laquai F., Brabec C. J., Salleo A., Durrant J. R., McCulloch I.,.,2016,, 11585
[50] Wadsworth A., Ashraf R. S., Abdelsamie M., Pont S., Little M., Moser M., Hamid Z., Neophytou M., Zhang W., Amassian A., Durrant J. R., Baran D., McCulloch I.,.,2017,, 1494—1500
[51] Xiao B., Tang A., Zhang J., Mahmood A., Wei Z., Zhou E.,.,2017,, 1602269
[52] Tang A., Xiao B., Chen F., Zhang J., Wei Z., Zhou E.,,2018,, 1801582
[53] Liu Y., Zhang Z., Feng S., Li M., Wu L., Hou R., Xu X., Chen X., Bo Z.,,2017,, 3356—3359
[54] Deng M., Xu X., Duan Y., Yu L., Li R., Peng Q.,, 2023, 35, 2210760
[55] Feng S., Zhang C., Liu Y., Bi Z., Zhang Z., Xu X., Ma W., Bo Z.,., 2017,, 1703527
[56] Fei Z., Eisner F. D., Jiao X., Azzouzi M., Röhr J. A., Han Y., Shahid M., Chesman A. S. R., Easton C. D., McNeill C. R., Anthopoulos T. D., Nelson J., Heeney M.,,2018,, 1705209
[57] Li H., Zhao Y., Fang J., Zhu X., Xia B., Lu K., Wang Z., Zhang J., Guo X., Wei Z.,.,2018,, 1702377
[58] Lin Y., Zhang Z.-G., Bai H., Wang J., Yao Y., Li Y., Zhu D., Zhan X.,.,2015,, 610—616
[59] Xu H., Yang Y., Zhong C., Zhan X., Chen X.,,2018,, 6393—6401
[60] Wang J., Li T., Wang X., Xiao Y., Zhong C., Wang J., Liu K., Lu X., Zhan X, Chen X.,,2019,, 26005—26016
[61] Wang J., Xu H., Xiao Y., Li T., Wang J., Liu K., Lu X., Zhan X., Chen X.,2020,, 105705
[62] Yang Y., Wang J., Xu H., Zhan X., Chen X.,,2018,, 18984—18992
[63] Yang Y., Jiang X., Zhan X., Chen X.,2019,, 257—267
[64] Wang J., Cai G., Jia B., Lu H., Lu X., Zhan X., Chen X.,,2021, 9, 6520—6528
[65] Wang Y., Qian D., Cui Y., Zhang H., Hou J., Vandewal K., Kirchartz T., Gao F.,,2018,, 1801352
[66] Blom P. W. M., Mihailetchi V. D., Koster L. J. A., Markov D. E.,,2007,, 1551—1566
[67] Riedel I., Parisi J., Dyakonov V., Lutsen L., Vanderzande D., Hummelen J. C.,,2004,, 38—44
[68] Shen Y., Hosseini A. R., Wong M. H., Malliaras G. G.,,2004,, 16—25
[69] Mai J., Xiao Y., Zhou G., Wang J., Zhu J., Zhao N., Zhan X., Lu X.,,2018,, 1802888
侧链的简单调制使近红外吸收的非富勒烯受体实现更高的短路电流密度
王家成1,蔡贵龙3,张亚静1,王嘉宇2,路新慧3,占肖卫2,陈兴国1
(1. 湖北省有机高分子光电功能材料重点实验室, 武汉大学化学与分子科学学院, 武汉 430072; 2. 北京大学材料科学与工程学院, 北京 100871; 3. 香港中文大学物理系, 香港 999077)
为了探究相同共轭骨架中不同位点引入不同侧链对相应小分子非富勒烯受体光电性能的影响, 本文通过在中心核茚并二噻吩并[3,2-b]噻吩(IDT)单元和端基(1,1-二氰基亚甲基)绕丹宁单元上同时引入正辛基柔性侧链并以噻吩稠合苯并噻二唑(BTT)单元为“-桥”而构建了一个新的A--D--A型非富勒烯受体(JC11). 与以往研究得到的两个A--D--A型非富勒烯受体(A2和JC1)相比, 在中心核和端基同时引入适宜长度的正辛基侧链不仅赋予JC11更有利的分子间堆叠, 而且使JC11比在中心核引入刚性更强和空间位阻更大的己基取代苯基侧链的A1表现出了更大的吸收红移和更好的结晶度; 同时也比在端基仅引入短链乙基侧链的JC1表现出了更好的溶解性能, 避免了其与PTB7-Th给体共混时出现过度聚集而导致相分离尺度过大. 此外, JC11在 给体/受体共混膜中展现了比A2和JC1更高的摩尔消光系数(高达1.14×105L∙mol-1∙cm-1)和更高的电子迁移率(e=1.01×10-3cm∙V-1∙s-1)以及更平衡的电子/空穴迁移率(e/h=2.72). 因此, 基于PTB7-Th∶JC11的太阳能电池器件经优化后实现了10.09%的光电转换效率(PCE)和高达24.20 mA/cm2的短路电流密度(SC), 超过PTB7-Th∶A2和PTB7-Th∶JC1优化器件8.20%和8.16%的PCE以及低于20 mA/cm2的SC值. 研究结果表明, 调节共轭主链侧链是一种简单且有效的调控非富勒烯小分子受体材料的吸收性能和结晶性能的分子设计策略, 可大幅提高异质结有机太阳能电池的SC和PCE.
A--D--A结构;非富勒烯受体;侧链工程;有机太阳能电池
O649.5
A
10.7503/cjcu20230163
网络首发日期: 2023-06-05.
联系人简介:陈兴国, 男, 博士, 教授, 主要从事光电功能材料的分子设计与合成及性能研究. E⁃mail: xgchen@whu.edu.cn
占肖卫, 男, 博士, 教授, 主要从事有机高分子光电功能材料的设计合成、器件构筑与性能研究. E-mail: xwzhan@pku.edu.cn
2023-04-01
国家自然科学基金(批准号: 51973163)资助.
Supported by the National Natural Science Foundation of China(No.51973163).
(Ed.: N, K, M)
