New MgO–H2O compounds at extreme conditions
2023-09-05LanciGuo郭兰慈andJurongZhang张车荣
Lanci Guo(郭兰慈) and Jurong Zhang(张车荣)
School of Physics and Electronics,Shandong Normal University,Jinan 250014,China
Keywords: high pressure,structure prediction,crystal structure
1.Introduction
Water is abundant in the Earth,Uranus and Neptune and is a vital resource for all life,however,it is not clear how water is inside these planets.Water is mostly stored in the mantle on the Earth,[1,2]however,on Uranus and Neptune,water has separate ice sheets.[3]The study of the source and evolution of water in the body deepens the understanding of the evolution and structure of stars.
It has recently been reported that in the early Earth,when the pressure and mass were high,water may exist in the form of Mg–O–Si–H compounds.[4]As the Earth evolved,the pressure and mass decreased, and the compounds dissolved, then the water was released and poured into the mantle and crust.The sources of water in the interior of giant planet are the same as the Earth, but at higher pressure.This motivate us to explore the stability of aqueous substances at higher pressures.The inner ice mantle layer of Uranus and Neptune stores a large amount of water, and their body cores are stony cores containing a large amount of SiO2, MgO, MgSiO3and other minerals.[5,6]However,the interaction between the mantle and its rocky core is rarely studied for its high pressure.Currently, it is known that SiO2can react with H2O to form Si–O–H ternary compounds.[7]The recent report found that the MgO dissolves in H2O at about 40 GPa,which is in the H2Orich mantle layer of Uranus.[8]The reaction of magnesiumcontaining minerals and water in Uranus and Neptune at the present-day H2O-rock boundary and under high pressure has not been reported.
The lower mantle makes up 50% of the Earth’s volume and contains(Mg,Fe,Al)(Si,Al)O3perovskite[or postperovskite(ppv)and an(Mg,Fe)O ferropericlase].[9–11]MgO is one important compound in planetary mantles, and it reacts with other substances at high pressure is essential for understanding the evolution of the Earth and planetary.The core of Uranus and Neptune is a rock core, which composes a mixture of silicates and iron.[12]MgO compounds have a very large stability interval, MgO is believed to be the NaCltype,[13]and then transforms into CsCl-type(B2)at 480 GPa until 3 TPa.[14,15]H2O is thePbcmphase from 300 GPa–600 GPa,[16]H3O isCmcaphase above 450 GPa[17]and H2O2isPbcaphase at 450 GPa–600 GPa.The brucite of Mg(OH)2is a trigonal structure withP-3maphase,at 30 GPa and 800 K transforms toP-3 phase,and then toP41212 phase at 17 GPa.[18]Mg(OH)2decomposes to MgO and H2O above about 30 GPa.
In this work,we researched the MgO–H2O system at high pressure.The (MgO)2H2O, Mg(OH)2, and MgO(H2O)2stoichiometries were completed at high pressure.These results provided theoretical support for understanding Earth’s chemical reactions of the water inside the planets.
2.Method
The CALYPSO prediction method and code[19,20]are used in this work, which has been effectively employed in searching crystal structures at high pressure.[21–26]The totalenergy and electronic structure are calculated by the density functional theory(DFT)and VASP code.[27]All-electron projector-augmented wave (PAW) method and 800 eV cutoff energy are used in the Mg–O–H system.3s22p62s2, 2s22p6,and 1s are treated as valence electrons for Mg, O, and H elements, respectively.The Perdew–Burke–Ernzerhof (PBE)exchange–correlation functional and the generalized gradient approximation are used.[28,29]Phonon calculations are carried out as implemented in the PHONOPY code.[30]
To determine the phase boundaries between the solid phases, we have performed calculations to determine the Gibbs free energyG(T,P)=[U(V)+PV+Fphonon(T,V)],whereU,P,V, andFphononare energy, pressure, volume and phonon free energy, respectively.The vibrational energy and entropy are obtained from lattice dynamic calculations using harmonic approximation as implemented in the PHONOPY code.Fphonon(T,V) =kBTg(ω,V)ln[2sinh()]dω,whereg(ω,V)is the phonon density of states at frequencyωand volumeV.The minimal value ofGis found at the equilibrium volume for a givenTandP.
3.Results
For a better understanding new Mg–O–H system,we have predicted the 1 f.u.–4 f.u.structures of (MgO)2H2O, MgO–H2O, and MgO(H2O)2at 300 GPa, 400 GPa, and 600 GPa.MgO–H2O and MgO(H2O)2of the Mg–O–H system are found at 100 GPa–600 GPa.The ternary phase diagrams of Mg–H–O at 400 GPa and 600 GPa are shown in Figs.1(a) and 1(b).We predict three different ratios MgO(H2O)2, (MgO)2H2O,and Mg(OH)2.The structure of MgO(H2O)3compound is from the previous report.[31]There are only MgO(H2O)3,MgO(H2O)2, and (MgO)2H2O stable with the black balls in the ternary phase diagram and Mg(OH)2is unstable with the red ball.The convex hull of the three different compounds relative to H2O and MgO is shown in Fig.1(c) at 300 GPa,400 GPa,and 600 GPa.MgO(H2O)3is a stable compound at 300 GPa.MgO(H2O)3,MgO(H2O)2,and(MgO)2H2O are in solid line with the convex-hull meaning that they do not decompose at high pressure with respect to possible binary and elemental elements and they are thermodynamically stable at 600 GPa.It should be noted that the Mg(OH)2compound is stable relative to MgO and H2O, however, it breaks down according to the others stable compounds at 400 GPa and 600 GPa.This contradicts the previously reported knowledge that Mg(OH)2compounds decompose into MgO and H2O at 33 GPa.
The stable ranges of the two stable phases are shown in Fig.1(d),Pnmaphase of(MgO)2H2O is stable above 365 GPa andC2221of MgO(H2O)2is stable above 598 GPa.We calculate the phonon dispersions of (MgO)2H2O and MgO(H2O)2at 400 GPa and 600 GPa as shown in Fig.S1.There are no imaginary frequencies in the whole Brillouin zone of(MgO)2H2O and MgO(H2O)2and clarify the dynamically stable properties.
The crystal structures of (MgO)2H2O and MgO(H2O)2are shown in Figs.2(a) and 2(b).The structural, stability,bonding, and electronic properties are analyzed at 400 GPa.The (MgO)2H2O phase crystallizes an orthogonal structure(space groupPnma,4 f.u./cell)and exhibits an MgO8H8frame with one Mg atom linked to eight O atoms and O atoms linked to 1–2 H atoms (OH or OH2frames) forming Mg–O bonds and O–H bonds.There are two types of hydrogen atoms,two types of magnesium atoms, and one type of oxygen atom in this structure.The MgO(H2O)2builds an orthogonal structure(space groupC2221, 4 f.u.per cell) containing MgO9units with one O atom linked to two H atoms(OH2frame)or one O atom linked to four H atoms(OH4frame).There is one type of hydrogen atom in this structure,one type of magnesium atom,and two types of oxygen atoms.The Mg–O bonds length of MgO(H2O)2and(MgO)2H2O are all 1.74 ˚A–1.88 ˚A.The covalent O–H bonds length of(MgO)2H2O and MgO(H2O)2are 1.04 ˚A–1.15 ˚A and 0.98 ˚A–1.19 ˚A, respectively, compared with∼1.01 ˚A forP41212 of Mg(OH)2.[18]For MgO(H2O)2phase the coordination number of Mg is nine,which is rarely seen in minerals,the only previous report was in the compoundβ-Mg2SiO5H2.The coordination of Mg in MgO(H2O)2is the same as well as proved in theβ-Mg2SiO5H2[4]compound.The density of (MgO)2H2O and MgO(H2O)2is 6.47 g/cm3and 5.64 g/cm3, respectively.Furthermore, (MgO)2H2O and(MgO)2H2O contain 18.36 wt.% and 23.68 wt.% of water,which is larger than Mg2SiO5H2of 11.4 wt.%.

Fig.2.The crystal structure of(a)the Pnma phase of(MgO)2H2O compound; (b)the C2221 of MgO(H2O)2 compound.Detailed structural information is provided in Table S1 of supporting materials.
4.Properties
The projected density of states(PDOS)and band are calculated at 400 GPa as shown in Fig.3.The PDOS and band of predicted structures reveal the properties of the insulator feature of MgO(H2O)2and (MgO)2H2O compounds.The bandgaps of MgO(H2O)2and (MgO)2H2O are 8.21 eV and 7.93 eV at 400 GPa,respectively.In order to analyze the bonding nature of the stable compounds, the electron localization function(ELF)calculation also be analyzed as shown in Fig.4.In the new compounds,there are OH,OH2and OH4three different OH frames.The O–H bond of OH2frames is a conventional covalent bond for charge localization between the nearest H and O atoms.The O–H bond in OH and OH4frames is a weak covalent bond with a longer bonding length(1.128 ˚A–1.187 ˚A)than that in the OH2frames as shown in Fig.4.However, there is less localized changes among the Mg–O bonds,suggesting an ionicity between Mg and O atoms.There is a charge transfer of Mg atom 1.32eand H 0.62eto O at 400 GPa,indicating the ionic property between Mg and O atoms.

Fig.3.The calculated PDOS and band of(MgO)2H2O and MgO(H2O)2 compounds at high pressures.

Fig.4.Calculated ELF at 400 GPa for(a)the Pnma-(MgO)2H2O phase on(2 1 0)plane and(b)C2221-MgO(H2O)2 phase on(−5 −1 0.1)plane.
TheH–P(high pressure and high temperature) phase is shown in Figs.5(a)and 5(b)with respect to 2MgO+H2O of(MgO)2H2O and MgO+2H2O of MgO(H2O)2.MgO(H2O)2is stable from∼345 GPa–600 GPa and∼0 K–6000 K and(MgO)2H2O is stable∼314 GPa–600 GPa and∼0 K–6000 K,which under conditions of Uranus, Neptune, the proto-Earth and super-Earth.Furthermore, we find that (MgO)2H2O and MgO(H2O)2compounds exhibit the superionic,solid and liquid states of matter as shown in Fig.5.The superionic state is that H diffuses freely,O and Mg atoms are on the equilibrium sites.A solid state is when all atoms are on their equilibrium sites.Liquid state is that all atoms diffuse freely.The mean squared displacements(MSD)of hydrogen,oxygen,and magnesium atoms for the different states of(MgO)2H2O phase are shown in Fig.S2.The MSD of the diffuse freely element is a line that slopes over time,while the vibrate on the equilibrium position element is a line that balances over time.The phase boundaries of different states of(MgO)2H2O and MgO(H2O)2are identified by MSD(Fig.5).The isentropes of Neptune and Uranus proposed[32]are also shown in Fig.5.The temperature of solid states turns to superionic about 1000 K, and transforms from the superionic state to a liquid state in the temperature range∼5000 K–6000 K with pressure from 300 GPa to 600 GPa of MgO(H2O)2.Mg and O elements of(MgO)2H2O deviate from their equilibrium positions over a nearly identical range of temperatures and pressures.The Mg–O framework is important for the stability of (MgO)2H2O.The internal temperature and pressure curves of Uranus and Neptune are also shown in Fig.5 and partially coincide with the liquid and superionic states of(MgO)2H2O and MgO(H2O)2,respectively.TheP–Tconditions spanning of reference model for ice layer of Uranus and Neptune are∼20 GPa–600 GPa and∼2000 K–7000 K.Through the above analysis,the superionic regions of(MgO)2H2O and liquid region MgO(H2O)2may be in the deep mantle or mentle-core boundary of Uranus and Neptune’s interior, so they may be components of these giants.This also indirectly proves that the core-mantle boundary of Uranus and Neptune is blurry because water can react with MgO in the Earth’s core,as previously reported.
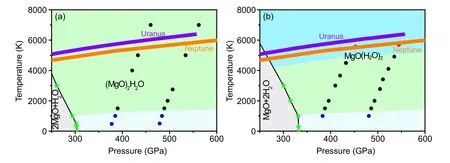
Fig.5.The pressure–temperature stability field of(MgO)2H2O and MgO(H2O)2 relative to MgO and H2O.The solid black line is the decomposition curve.Solid,superionic states and liquid phases are marked by blue,black,and red circles.The boundary made up of black lines and fluorescent stars are the decomposition line of the compound with respect to MgO and H2O.Purple line and origin line are Uranus and Neptune,respectively.
The diffusion coefficient of H of(MgO)2H2O superionic state isDH=2.8×10−8m2/s at 446 GPa, 6000 K.We calculate the electrical conductivity by Nernst–Einstein equationσ=q2HNHDH/VkBT, whereqH,V,NHare the charge of the hydrogen ion, the volume of the cell, the number of H atoms in the cell,respectively.The electrical conductivity of superionic phases(MgO)2H2O phase at a density of 6.47 g/cm3and at 446 GPa and 6000 K is around 6.84 S/cm,which is similar to Si–O–H compound in the same temperature and pressure but the order of magnitude smaller than that of number superionic water.[33]Therefore the (MgO)2H2O compound affects magnetic field and could also be similar to the Si–O–H compound in the sameP–Trange but should be weaker than that from the superionic ice in the same condition.Previous research has shown that the nondipolar and the nonaxisymmetric magnetic fields of Uranus and Neptune have been suggested to come from metallic mixtures,[34]metallic fluid hydrogen,[35]superionic ice,[6]fluid trihydrogen oxide[17]and recently silica-water compound[7]proposed.The(MgO)2H2O superionic states may be one possibility for explaining the anomalous magnetic fields of Uranus and Neptune as well as silica-water.
Before the core-mantle separation, Mg slinks into the Earth and pressures above 300 GPa, (MgO)2H2O and MgO(H2O)2likely at those pressure as well the Mg2SiO5H2.It may play the same role as Mg2SiO5H2in storing water in the early Earth.[4]The water may store in (MgO)2H2O before the core formation.As the core grows, (MgO)2H2O is no longer stable at lower pressure and has to dissociate(MgO)2H2O→2MgO+H2O.
Previous research reports there are many super-Earths with mass 1–10 times larger than our Earth outside the solar system.[36]For these super-Earths,the core-boundary pressure is also larger than the Earth, such as a super-Earth with 3 Earth masses is predicted to have a core-boundary pressure of 500 GPa.[37]For these exoplanets, (MgO)2H2O withP–Tstable range should exist as a water reservoir in the early Earth.
5.Conclusion
We have identified(MgO)2H2O and MgO(H2O)are thermodynamically stable above 300 GPa and over wide ranges of temperatures and are thus viable inside early Earth during its initial stages of differentiation.This discovery offers insights into the interconnecting substance between the mantle and its rocky core of Uranus and Neptune.The(MgO)2H2O may be a water reservoir for the proto-Earth or the super-Earths.The present results also have broad implications for the interpretation and elucidation of physical and chemical processes of the proto-Earth,Uranus,Neptune and super-Earth.
When we submitted our manuscript, we are aware of another similar work which is published inNature Communications.[31]
Acknowledgments
Project supported by the National Natural Science Foundation of China (Grant Nos.12204280 and 12147135), the Natural Science Foundation of Shandong Province of China(Grant No.ZR202103010004), and the Postdoctoral Science Foundation of China(Grant No.2021M691980).
杂志排行
Chinese Physics B的其它文章
- Interaction solutions and localized waves to the(2+1)-dimensional Hirota–Satsuma–Ito equation with variable coefficient
- Soliton propagation for a coupled Schr¨odinger equation describing Rossby waves
- Angle robust transmitted plasmonic colors with different surroundings utilizing localized surface plasmon resonance
- Rapid stabilization of stochastic quantum systems in a unified framework
- An improved ISR-WV rumor propagation model based on multichannels with time delay and pulse vaccination
- Quantum homomorphic broadcast multi-signature based on homomorphic aggregation
