Construction of Oriented Structure in Inner Surface of Small-Diameter Artificial Blood Vessels: A Review
2023-05-18YIGuanghui伊光辉CHENGXinyu成馨雨GENGMengxiang耿梦想MENGKaiZHANGKeqin张克勤ZHAOHuijing赵荟菁
YI Guanghui(伊光辉), CHENG Xinyu(成馨雨), GENG Mengxiang(耿梦想), MENG Kai (孟 凯), 2*, ZHANG Keqin(张克勤), 2, ZHAO Huijing(赵荟菁), 2*
1 College of Textile and Clothing Engineering, Soochow University, Suzhou 215123, China
2 National Engineering Laboratory for Modern Silk, Soochow University, Suzhou 215123, China
Abstract:There is an urgent need for small-diameter artificial blood vessels in clinic. Physical, chemical and biological factors should be integrated to avoid thrombosis and intimal hyperplasia after implantation and to promote successful fabrication of small-diameter artificial blood vessels. From a physical perspective, the internal oriented structures of natural blood vessels plays an important role in guiding the directional growth of cells, improving the blood flow environment, and promoting the regeneration of vascular tissue. In this review, the effects of the oriented structures on cells, including endothelial cells(ECs), smooth muscle cells(SMCs) and stem cells, as well as the effect of the oriented structures on hemodynamics and vascular tissue remodeling and regeneration are introduced. Various forms of oriented structures (fibers, grooves, channels, etc.) and their construction methods are also reviewed. Conclusions and future perspectives are given. It is expected to give some references to relevant researches.
Key words:small-diameter artificial blood vessel; internal oriented structure; direct cell behavior; vascular tissue remodeling and regeneration
Introduction
Cardiovascular disease is the leading cause of death worldwide[1-2]. According to the report of World Health Organization (WHO), approximately 17 million people worldwide die of cardiovascular disease every year[3-4]. Vasculopathy is one of the important causes of cardiovascular disease. Blood vessel by-pass grafting is an important method for the treatment of cardiovascular disease. Vascular grafts are generally divided into three types: autologous blood vessels, allogeneic blood vessels and artificial blood vessels. Among them, autologous blood vessels are the most suitable, but the number is limited, and they need to be taken from the body of patients, which will cause body damage to the patient. Allogeneic blood vessels are prone to immune rejection. At present, artificial blood vessels with an inner diameter larger than 6 mm made of synthetic polymers such as expanded polytetrafluoroethylene(ePTFE) and polyester have been successfully used in clinic[5]. Due to a slower flow velocity and a more complex hemodynamic environment, small-diameter artificial blood vessels with an inner diameter less than 6 mm are prone to forming thrombosis and intimal hyperplasia in the late stage of transplantation[6-8], and thus there has been no clinical products yet. To solve the problem of clinical applications of small-diameter artificial blood vessels, bionic construction from the aspects of structures and functions is a feasible and promising idea. Natural blood vessels consist of endothelial cells (ECs) aligned axially along the inner lumen which is surrounded by concentric layers of circumferentially aligned vascular smooth muscle cells (VSMCs) and elastic tissue, and the outer layer is mainly composed of randomly distributed fibroblasts[9]. With the in-depth study of the impact of lumen surface topography on cell behaviors[10], more and more results show that the oriented structure can effectively regulate adhesion, morphology, differentiation and proliferation behaviors of cells. The inner wall of the lumen contacts with blood directly, and its oriented structure has a direct impact on hemodynamics. In the past few decades, researchers have fabricated artificial blood vessels with various oriented structures in the inner lumen, such as oriented fibers and grooves by various methods. Results show that, compared with small-diameter artificial blood vessels with non-oriented structures, vessels with oriented structures can enhance cell adhesion and proliferation of ECs, facilitate the oriented attachment of ECs on the fibers and help VSMCs have highly oriented morphology and contractile phenotype which can ensure their activities due to the comparable geometric feature size of the fiber diameter and the directional mechanical properties[7]. What’s more, the oriented structures could increase the blood flow velocity in artificial blood vessels to avoid formation of thrombus and obvious turbulence and reduce inflammation and the adhesion of monocytes. Turbulence may cause atherosclerosis and the transport and deposition of prethrombotic substances.
At the same time, it is also proved that the development of artificial blood vessels with oriented structures mimicking natural blood vessels is one of the important strategies to solve the current problems of thrombosis and intimal hyperplasia of small-diameter artificial blood vessels[6]. In this review, oriented structures, including oriented fibers, grooves, channels,etc., in the inner lumen of small-diameter artificial blood vessels, and their construction methods are reviewed firstly. Then, the effects of oriented structures on vascular cells, including ECs, smooth muscle cells (SMCs) and stem cells, as well as on hemodynamics and vascular tissue remodeling and regeneration are introduced. Finally, the future research directions of small-diameter artificial blood vessels with oriented structures are prospected.
1 Construction of Oriented Structures
It has been proved that oriented structures at micro-level and nano-level can be used to regulate cell behaviors. The size of oriented structures at nano-level is similar to that of cell receptors. Oriented structures can therefore influence cytoskeletal organization, differentiation, gene expression and the shape of cells[11]. Techniques used to fabricate oriented structures at micro-level or nano-level to mimic the structures of natural blood vessels include electrospinning, electrostatic direct-writing, photolithography, electron beam lithography(EBL), 3D printing,etc.[11-15], enabling artificial blood vessels to possess oriented fibers, oriented grooves, oriented channels, or other oriented structures. These techniques play an important role in keeping the long-term patency of small-diameter vascular grafts.
1.1 Fabricating techniques of oriented fibers
1.1.1Electrospinning
Electrospinning is widely used due to its abundant material sources, and the size of electrospun fibers is similar to that of extracellular matrix. The preparation of random fibers is not novel, while oriented fibers can be obtained through improving the collector[16]. Lietal.[17]fabricated uniaxially oriented nanofibers made of polymers, ceramics and composites based on electrospinning. The collector shown in Fig.1 (permission from American Chemical Society, copyright 2003) was composed of two grounded conductive collecting boards with a gap ranging in width from hundreds of micrometers to several centimeters. The same potential of two conductive collecting boards led to the same force on the electrospinning jet, and the charged jet was deposited directionally in the gap between two boards.The rotating collection method was also used in the end of the 20th century to collect highly oriented electrospun fibers by crystallizing extended molecules in the jet through the high-speed rotating drum collector[18]. Mathewetal.[19]found that there was an optimal speed to obtain fibers with the highest degree of orientation, which was 1 200 r/min. In addition to meeting the conditions of high-speed collection, matching the linear velocity on rotating drum surface with the jet deposition velocity was also very important to prepare highly oriented fibers. Adhesion and proliferation of ECs can be improved by the highly oriented structure based on electrospinning. Shalumonetal.[20]used a metal stick with a diameter of 4.8 mm as a collector and prepared highly oriented fibers through a rotating collection method at the speed of about 14 000 r/min.
1.1.2Electrostaticdirect-writing
Electrostatic direct-writing has been developed into a highly controllable scaffold production technique and widely used in tissue engineering with an in-depth understanding of electrohydrodynamics and a traditional additive manufacturing principle[21]. The principle is to keep the spinning jet at the initial steady state by decreasing the collecting distance ranging from several centimeters to even several millimeters and the spinning voltage (about 1-4 kV). The spinning jet can be precisely controlled, and the fibers can be deposited orientally. The geometry of the scaffold can be regulated accurately by electrostatic direct-writing, and natural tissue can be constructed biomimetically through micro-structure or nano-structure fabrication, surface modification and biological functionalization[21]. The oriented fibers in geometry of scaffolds can provide directional guidance for the growth of cells and promote cell migration. Wangetal.[22]prepared 3D tubular structures with axially oriented microfibers by electrostatic direct-writing as shown in Fig.2(a) (permission from Wiley, copyright 2020). They cultured PC-12 cells and hippocampal neurons of mice on the tube for 14 d. Results showed that oriented fibers provided directional guidance for the growth of entire neurons, and PC-12 cells and mice hippocampal neurons wrapped evenly around single fibers to form the 3D tubular structure. Bakircietal.[23]prepared a circumferentially oriented 3D structure by depositing filaments layer by layer through electrostatic direct-writing. The related picture is shown in Fig.2(b) (permission from Wiley, copyright 2020). It was found that the structure with oriented fiber walls could promote cell migration.
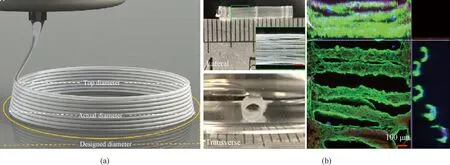
Fig.2 Electrostatic direct-writing: (a) diagram of electrostatic direct-writing; (b) morphology of 3D tubular scaffold with axially oriented structure
1.2 Techniques of fabricating oriented grooves
1.2.1Photoetching
Photoetching includes photolithography and EBL. Photolithography uses light to generate an oriented structure on surfaces, in which a photosensitive polymer, the photoresist, is exposed to ultraviolet light (UV) through a template. Cross-linking, polymerization or degradation of the photoresist is induced by exposure, resulting the formation of oriented structures of the synthetic polymers as shown in Fig.3(a) (permission from Elsevier, copyright 2014)[24]. The sizes of the oriented structures were determined by the diffraction limit of light, usually only on the micro-scale. A focused beams of electrons is used to perform a raster scan (a parallel line pattern) on the substrate in EBL. Structures with submicro-sizes can be created since the diffraction limit of electrons is much smaller than that of light used in photolithography where the entire substrate is exposed simultaneously. Artificial blood vessels with the desired groove morphology can be fabricated by using silicon wafer molds as the substrates of polymers, such as polyurethane and polycaprolactone. Seunarineetal.[25]fabricated multi-layer artificial blood vessels with oriented grooves by combining photolithography and EBL as shown in Fig.3(b) (permission from Elsevier, copyright 2008). Sizes and positions of the oriented grooves on each layer can be adjusted to promote adhesion and growth of different cells on the corresponding layers,e.g., the internal layer for ECs, the middle layer for SMCs and the outer layer for fibroblasts.
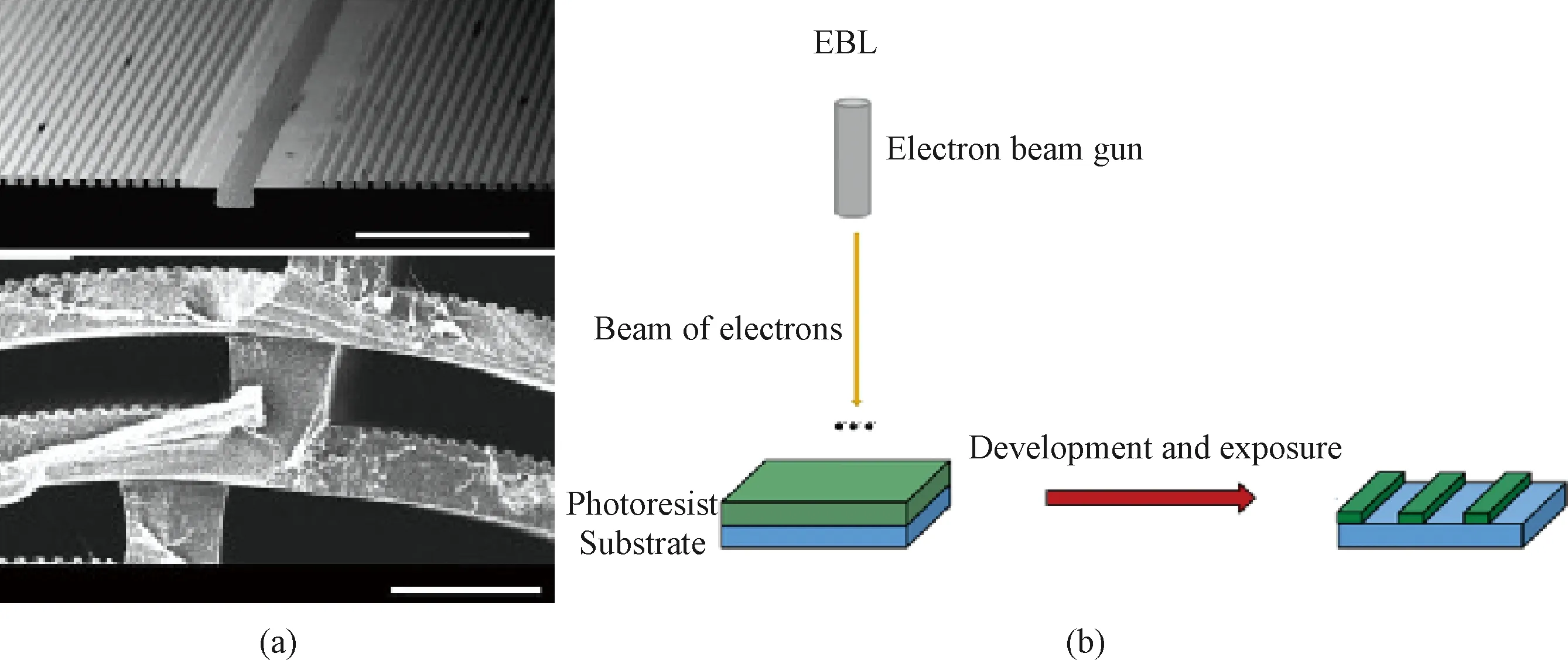
Fig.3 Photoetching: (a) morphology of artificial blood vassels with multi-layers of oriented grooves made by photolithography; (b) diagram of combination of photolithography and EBL
1.2.2Micro-imprinting
Basically, a mold with a grooved structure is used in micro-imprinting. Polymer particles are put on a substrate and heated together until the particles are melted and spread, and then the melted polymer is transferred onto a mold with a grooved structure and is pressed to form membranes with grooved structures. Huetal.[26]fabricated artificial blood vessels composed of polyether ether ketone (PPDO) membranes with grooved structures as shown in Fig.4 (permission from MDPI, copyright 2021) which could be used to regulate cell behavior and promote endothelialization.
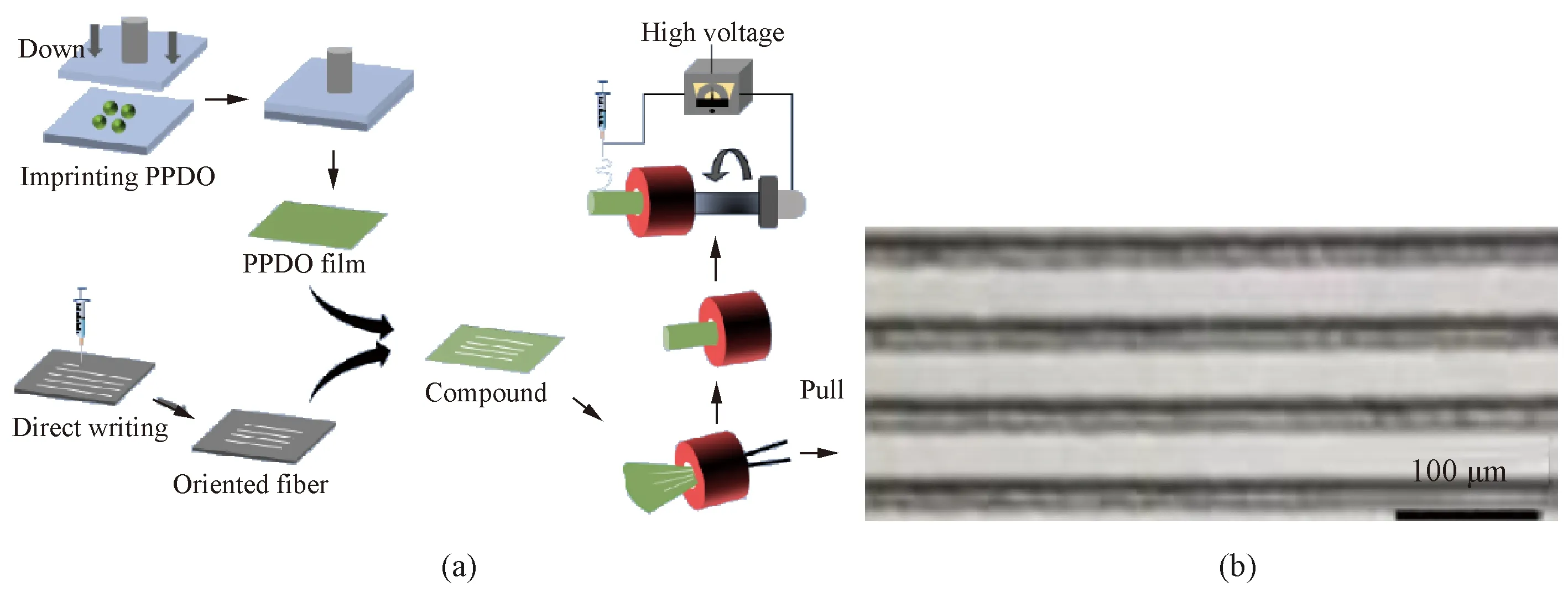
Fig.4 Micro-imprinting: (a) diagram of micro-imprinting; (b) morphology of small-diameter artificial blood vessels with controllable oriented grooves
1.3 Other techniques of fabricating oriented structures
1.3.1Methodsbasedonsolventcasting
Oriented structures at micro-level can also be constructed by methods based on solvent casting. Wuetal.[27]fabricated oriented micro-channel structures through the combination of fiber templates and solvent casting. They prepared a circumferential maltitol-fiber template by solvent casting as shown in Fig.5 (permission from Elsevier, copyright 2020), and then the fiber template was immersed in a poly(lactide-co-caprolactone) (PLCL)/CHCl3solution. After the polymer solution filled the gap between the fiber templates, the compound composed of fiber templates and the polymer was taken out, and the solvent was removed. The circumferential micro-channels in artificial blood vessels were fabricated after fiber templates were removed through immersing the compound in water.
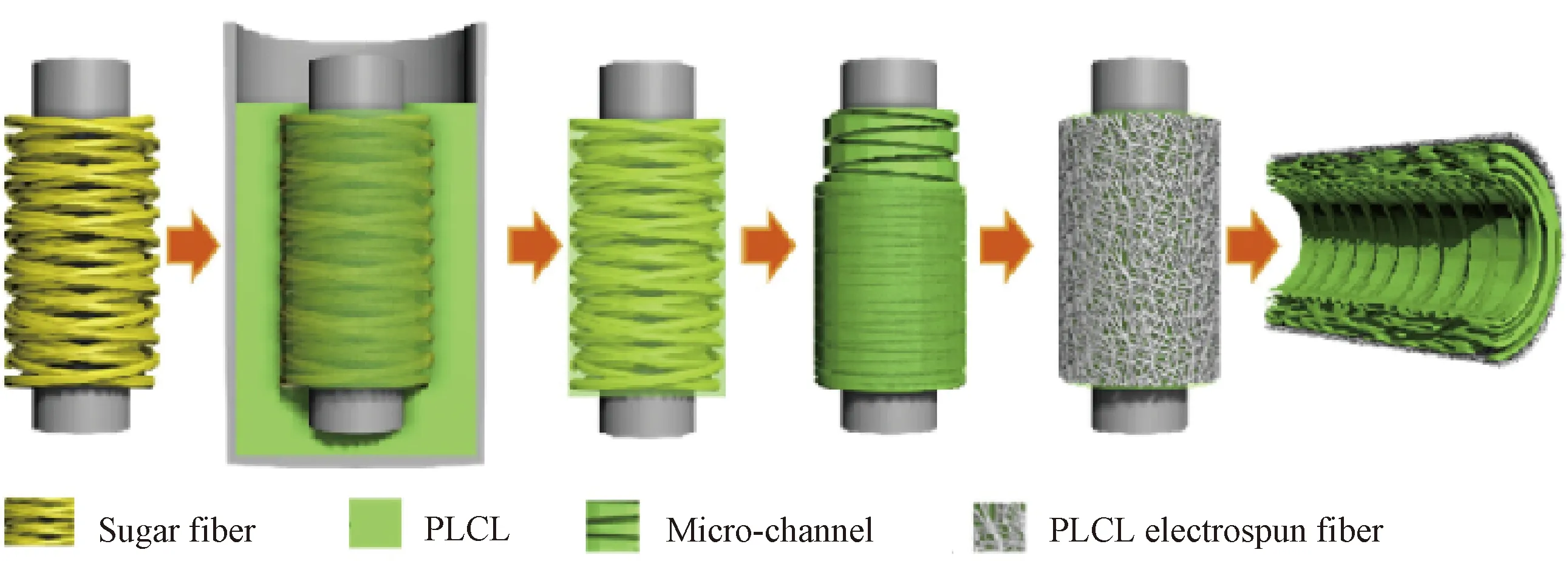
Fig.5 Morphology of circumferentially oriented structure constructed by solvent casting
1.3.2Methodsbasedon3Dprinting
The tubular scaffold with rhomboid pores and parallel structures can be prepared by 3D printing which can control the depositing position of the fibers. Van Kampenetal.[28]fabricated an automatic machinery manufacturing system based on four-axis extrusion which was similar to fused deposition modeling (FDM). Poly(ε-caprolactone) (PCL) was extruded through a nozzle with a diameter of 260 μm onto a rotating mandrel with a diameter of 2 mm. The fibers with different orientation angles and directions were prepared by controlling the depositing position of the fibers as shown in Fig.6 (permission from Elsevier, copyright 2021). Leeetal.[29]fabricated bi-layer artificial blood vessels through the combination of rapid prototyping 3D printing and electrospinning. A highly oriented electrospun nanofibrous scaffold was firstly fabricated, and then strands of molten PCL deposited between layers as the drum rotated, producing the patterned 0°/45° porous structure. Results showed that the modified samples had higher strengths and better hydrophilicities without affecting their structural integrity. Paxtonetal.[30]fabricated an artificial blood vessel with oriented structures, and the pore size and the orientation angle of the fibers could be regulated during the preparation process.

Fig.6 3D printing: (a) diagram of four-axis extrusion; (b) morphology of tubular scaffold with rhomboid pores and parallel structures
1.4 Technique comparison
Advantages and disadvantages of techniques mentioned above are summarized in Table 1. In electrospinning, jet and collection speeds should be higher, and the surface linear velocity of the collecting mandrel should match the jet speed[16, 20]. The raw material, the proportion of polymer solutions, the linear velocity of the collecting mandrel, the diameter of the injection needle, the electrospinning voltage, the working distance and the feed speed are also very important to electrospinning. In the electrostatic direct-writing, nozzle sizes, polymer concentrations, flow rates, and electric field voltages which affect the fiber diameter and the choice of subsequent curing methods are all important factors. If the direction of the device is variable and fibers need to be stacked layer by layer, the direct-writing path, the actual fiber position and heights need to be considered.
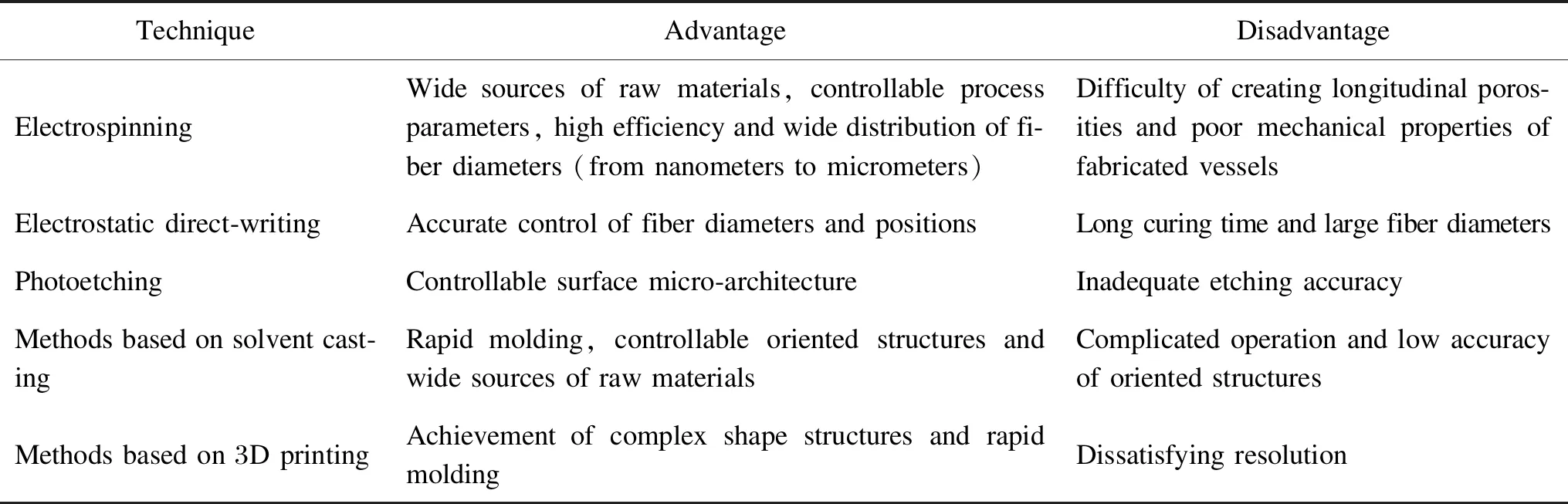
Table 1 Comparisons among techniques of fabricating oriented structures
There are gaps between techniques and practical applications. In electrospinning, the chaotic deposition of fibers at a high speed makes artificial blood vessels not necessarily produce enough pore sizes, the mechanical strength of fibers is poor, and it is difficult to remove the polymer solvent used in the preparation process. In electrostatic direct-writing, the solvent evaporation curing time is long, and the layer-by-layer stacking is challenging[21]. Although the coagulation bath can stack a large number of fibers, the function of the cells on artificial blood vessels may be affected for the fiber diameters which are above 100 μm. Classical Photoetching such as photolithography and EBL is limited by a number of optical parameters, for example diffraction limits and the depth of focus. Consequently it is not suitable for directly preparing patterned 3D scaffolds[26]. The porosity of the vessels fabricated by solvent casting is achieved by varying the amount and the size of salt particles during evaporation, which is difficult to attain precise reproducible features in micrometers and nanometers. When it comes to 3D printing, current additive manufacturing systems do not commonly implement the use of a rotational axis, which limits the application for the fabrication of tubular scaffolds[28].
2 Effect of Oriented Structures on Cells
Oriented structures can effectively regulate adhesion, morphology, differentiation and proliferation behaviors of cells including ECs, VSMCs and stem cells.
2.1 Effect on ECs
2.1.1Celladhesion
The oriented nano-structure can improve the adhesion of cells. Zorlutunaetal.[31]studied the effect of the oriented nano-structure on the adhesion of human ECs. Cells were seeded on films with longitudinally oriented grooves and cultivated at the flow shear stress for 24 h. The cell retention rates on the films with oriented nano-grooves was 16% higher than those without patterns as shown in Fig.7(a) (permission from Elsevier, copyright 2009). Gongetal.[32]designed an artificial blood vessel with a combination of concentric circular micro-grooves and radial linear micro-grooves on the inner surface as shown in Fig.7(b) (permission from Wiley, copyright 2016). The oriented structure showed good adhesion to cells. After the vessel was implanted into the rat carotid artery, ECs and SMCs in the blood were captured by radial linear micro-grooves and concentric circular micro-grooves, respectively. Then a homogeneous vascular-like tissue with excellent antithrombotic activity was formed and a new vessel with expected function was obtained.
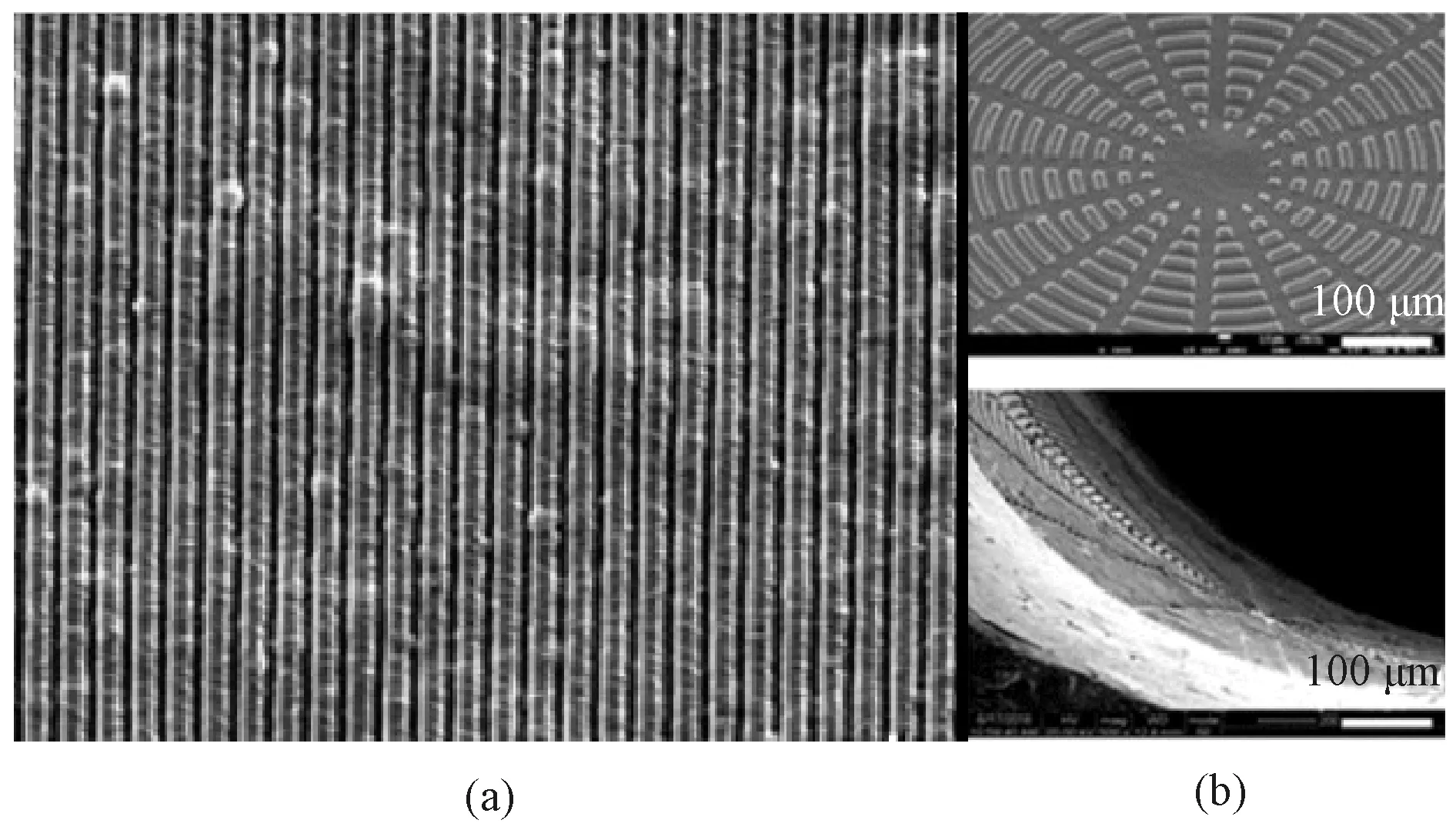
Fig.7 Oriented micro-grooves or nano-grooves: (a) thin films with oriented nano-grooves; (b) artificial blood vessels with micro-groove combination on inner surface
2.1.2Cellproliferation
The proliferation of different kinds of cells can be promoted by the oriented structure. The longitudinally oriented structure in the lumen provided necessary mechanical support for the graft and promoted growth, diffusion and migration of ECs. The oriented nanofibers can significantly enhance the adhesion and proliferation of human umbilical vein endothelial cells (HUVECs)[33-34]. Lietal.[35]proved this theory by evaluating the behaviors of HUVECs on substrates with oriented and random microfibers and nanofibers, respectively. Similarly, Huangetal.[36]fabricated a multi-layer artificial blood vessel structure composed of axially oriented fibers and random fibers. After being cultured on the inner surface of the artificial blood vessel for 3 d, HUVECs grew well, and completely covered the lumen, which showed good proliferation, diffusion of the cells and interaction.
Although the cell proliferation is promoted by the combined action of geometry, length scales, substrate materials and cell types, some studies have shown that the oriented morphology has no obvious effect on cell proliferation. Zorlutunaetal.[31]found that there was no significant difference in the number of cells between the oriented structure sample and the control group after being cultured for a period of time.
At present, there is no obvious evidence to predict the effect of the oriented morphology on cell proliferation[37-39], and there is a lack of accepted hypothesis about the mechanism of cell proliferation affected by the interaction between cells and oriented nano-structures.
2.1.3Cellalignment
The oriented morphology especially affects the geometry of cells. It can provide contact guidance, resulting in the uniform distribution and alignment of ECs, and most of the cells are elongated in a direction parallel to fibers and micro-grooves[40-43]to form tissue. Huangetal.[44]proved it by preparing substrates composed of oriented nanofibers with different sizes and comparing the cell arrangement on those substrates as shown in Fig.8 (permission from Elsevier, copyright 2013). It was found by fluorescence staining of the cytoskeleton that ECs on substrates with different fiber sizes were aligned along the oriented nanofibers, and the uniformity of the arrangement was significantly enhanced with the decrease of the fiber diameter. Wertheimeretal.[45]fabricated small-diameter artificial blood vessels composed of circumferentially and longitudinally oriented fibers as shown in Fig.9(permission from Elsevier, copyright 2021). After being cultured on oriented fibers for 6 d, the diffusion and the alignment of cells along the oriented fibers could be observed obviously. Similarly, Uttayaratetal.[46]reached the same conclusion by constructing microfibers and micro-grooves on the lumen surface of small-diameter polyurethane (PU) artificial blood vessels and seeding bovine aortic endothelial cells (BAECs) on them as shown in Fig.10(permission from Elsevier, copyright 2010).

Fig.8 Substrates consisting of oriented nanofibers with different sizes: (a) 100 nm; (b) 30 nm
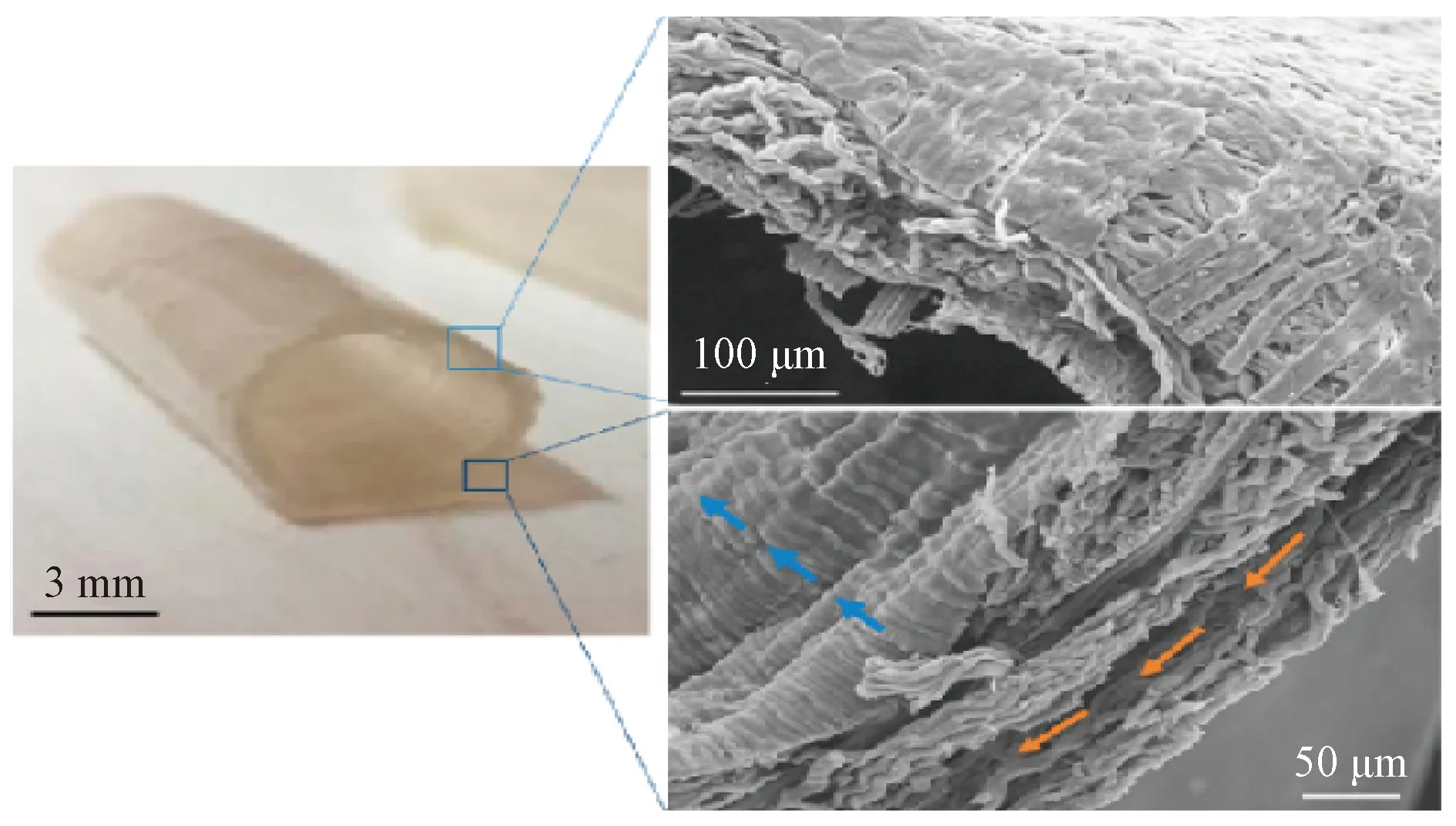
Fig.9 Anisotropic orientation section
Fig.10 Artificial blood vessels with oriented microfibers and micro-grooves on surface: (a) morphology of micro-grooves on lumen; (b) morphology of microfiber mesh on exterior
2.2 Effect on VSMCs
VSMCs can maintain a circumferentially oriented structure in natural blood vessels, playing a key role in regulating vascular tension and maintaining vascular function[47]. VSMCs exist in two distinct phenotypes: contractile and synthetic. The contractile phenotype of VSMCs is in a spindle shape and has abundant expression of alpha-smooth muscle actin (αSMA), while the synthetic phenotype is in a rhombus shape and has decreased expression ofαSMA. During the culture process of VSMCs, there are problems that the contractile phenotype gradually turns to the synthetic phenotype over time[48]. Cells grown on the axially oriented fiber structure have highly oriented morphology and relatively high proportion of the contractile phenotype which can ensure the activity of VSMCs.
Tsaietal.[49]fabricated bi-layer oriented small-diameter artificial blood vessels, in which the inner layer was composed of axially oriented fibers and the outer layer was composed of circumferentially oriented fibers as shown in Fig.11 (permission from Elsevier, copyright 2019). VSMCs were cultured on axially oriented fibers.
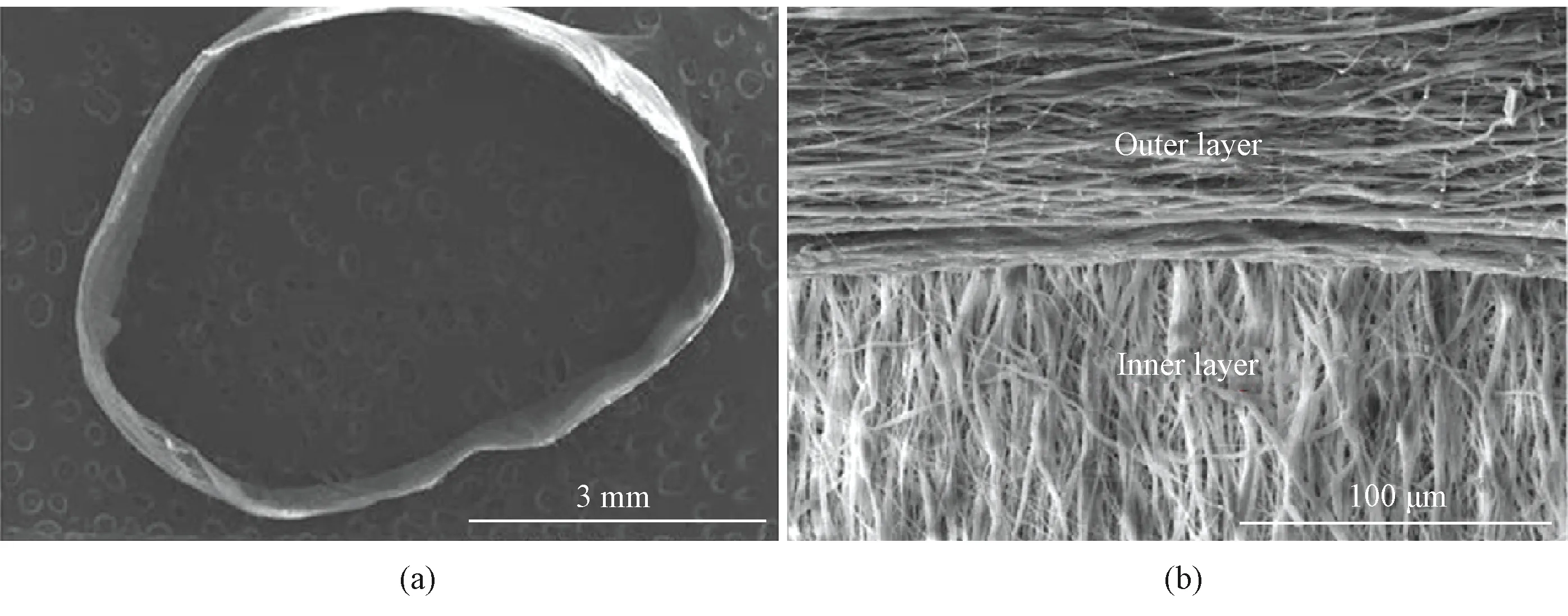
Fig.11 Artificial blood vessel with bi-layer oriented structure
Wuetal.[50]fabricated artificial blood vessels with multi-layer biomimetic structures, in which the inner layer was composed of PLCL/collagen axially oriented fibers, the middle layer was composed of poly (lactic-co-glycolide) (PLGA)/silk yarns precisely wound and aligned in a circumferential direction on the inner layer, and the outer layer was composed of PLCL/collagen random fibers to fix the whole vascular structure as shown in Fig.12 (permission from Elsevier, copyright 2018). VSMCs were seeded on random fibers and directionally oriented fibers, respectively. Results showed that after being cultured for 3 d, VSMCs on the random fibers were in disorder, while the orientation degree of VSMCs on the oriented fibers was higher and the interaction between cells was stronger.
Liuetal.[39]fabricated the three-layer biomimetic small-diameter artificial blood vessel which had an outer layer consisting of circumferentially oriented nanofibers with a thickness of (15.47±1.31) μm, a middle layer composed of random elastic nanofiber with a thickness of (127.87±2.38) μm and an inner layer composed of random nanofibers with a thickness of (19.96±1.18) μm. By seeding VSMCs on the outer layer of the artificial blood vessel, proliferation, activity and growth direction of cells were evaluated. Results showed that VSMCs grown on the oriented nanofibers were easier to be elongated and showed greater activity than VSMCs grown on the random nanofibers.
Wuetal.[27]fabricated artificial blood vessels with circumferential micro-channels which promoted migration, orientation, elongation and contractile phenotype expression of VSMCsinvitroas shown in Fig.13 (permission from Elsevier, copyright 2020). After being implanted into the rat aortic defect model, the micro-channel in the blood vessels improved infiltration and alignment of VSMCs and the directional deposition of the extracellular matrix (ECM), provided protection and support for the growth of ECs, and accelerated endothelialization.
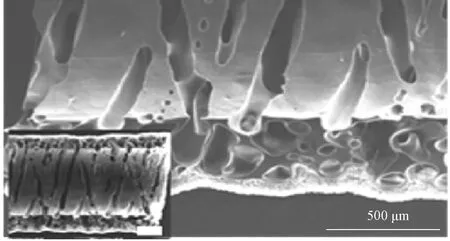
Fig.13 Circumferential micro-channel structure in artificial blood vessels
2.3 Effect on stem cells
Stem cells have the ability to self-renew and differentiate into functional cells[51-52], which can be used to supplement insufficient cells and participate in tissue repair and regeneration throughout the life cycle of the organism. The function of stem cells is controlled by their extracellular micro-environment, which can provide sites for locating stem cells and integrate complex molecular signals from internal and external cues[53-55]. It has been proved that the oriented structure can promote cell differentiation and alignment of stem cells by enhancing the expression of vascular endothelial cell (VEC)-related markers and shear response markers, and providing contact guidance.
2.3.1Celldifferentiation
The differentiation of stem cells into vascular cells can be promoted by the oriented structures of artificial blood vessels which mimic the extracellular micro-environment in natural blood vessels[56-58].
Wangetal.[59]designed a substrate composed of M13 bacteriophage biofibers with axially oriented nano-structures, and used it to simulate the natural extracellular microenvironment to induce pluripotent stem cells differentiation. The bacteriophage matrix with oriented nano-ridges or nano-grooves played an important role in inducing the elongation and the alignment of these cells along the oriented direction. It was found that the induced pluripotent stem cells (iPSCs) on the matrix first differentiated into mesenchymal stem cells (MSCs), and MSCs could be lengthened and further differentiated into osteoblasts in the absence of osteogenic supplements. Xingetal.[60]fabricated artificial blood vessels with oriented structures to mimic the anisotropy of natural blood vessels and guide human bone marrow mesenchymal stem cells (hMSCs) aligning circumferentially. The circumferential stress generated by the bioreactor further promoted the formation of the anisotropic structure of artificial blood vessels, and significantly enhanced the expression ofαSMA and cadherin in hMSCs without any biochemical stimulation. Similarly, Penningsetal.[61]fabricated biomimetic grafts with heterotypic design which held promises for functional neo-vessel regeneration by guiding the layered cellular and tissue organization into a native-like structure as shown in Fig.14 (permission from Institute of Physics, copyright 2019). A perfusable two-compartment bioreactor was designed, in which inner and outer surfaces of artificial blood vessels were placed in cell-specific media, and thus endothelial colony forming cells and multipotent MSCs could be co-cultured simultaneously on both sides of the artificial blood vessels. The lumen layer in the bioreactor induced and maintained target cell phenotypes (e.g.ECs and VSMCs). The expression of VEC-related markers (CD31) and shear response markers (COX-2, eNOS and KLF2) significantly increased. On the outer layer of artificial blood vessels, MSCs were different from VSMCs due to the circumferential multi-layer structure and the transforming growth factorβ(TGF-β), and the related expression factorsαSMA, Calponin, collagen IV and tropoelastin increased.
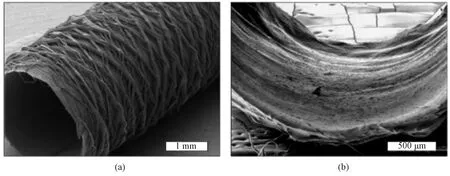
Fig.14 Morphologies: (a) scaffold without cells; (b) scaffold ladened with cells
2.3.2Cellalignment
Oriented structures especially affect the geometry of cells, help the stem cells maintain their phenotype when being cultured on the vessels, and provide contact guidance, resulting in uniform distribution and alignment of stem cells. Nakayamaetal.[9]fabricated an oriented bi-layer small-diameter artificial blood vessel with an oriented structure which derived from iPSCs, and found that iPSC-derived smooth muscle cells (iPSC-SMCs) aligned circumferentially along the oriented nanofibrils, but were morphologically disorganized on random collagen scaffolds. The average angle of alignments for iPSC-SMCs on oriented nanofibrillar collagen scaffolds was 4°±2°, while the average angle of 39°±5° for iPSC-SMCs on random collagen scaffolds. The iPSC-SMCs were shown to express theαSMA, demonstrating the ability of the cells to maintain their phenotype when being cultured on the vessels. These data demonstrate the ability of small-diameter artificial blood vessels with oriented structures to modulate cellular elongation and alignment for both primary and iPSC-derived vascular lineages.
3 Effect of Oriented Structures on Hemodynamics
The lumen structure of artificial blood vessels plays a key role in regulating the behavior of blood cells and the formation of thrombus[62]. It has been mentioned previously that the surface oriented morphology can regulate the blood compatibility of artificial blood vessels, but its internal mechanism is still unclear[63-65]. At present, the commonly accepted view is that the adsorption and the accumulation of blood-related proteins can be regulated by surface physical and chemical properties, and the behavior of blood cells can be affected by the type and the quantity of adhesion proteins. The current studies suggest another possible mechanism, that is, oriented morphology regulates blood cell behaviors based on hemodynamic effects[66-68].
3.1 Effect on blood flow velocity
It is found that the velocity gradient decreases when the fluid passes through the oriented surface, and the velocity decreases sharply when the fluid passes through the disordered structure. The blood flow will be disturbed when it flows through the surface of the disordered structure, while the oriented structure can guide the blood flow and make it flow more smoothly. Less platelet adhesion and less platelet activation are observed at the surface of oriented structures. Besides directly regulating platelet adhesion by reducing the contact area between blood and the surface of artificial blood vessels, it also indicates that appropriate oriented morphology can also indirectly inhibit platelet adhesion and platelet activation through modulating the blood flow velocity in artificial blood vessels. Wangetal.[68]proved it by constructing two kinds of surface simulation models based on oriented and disordered structures by ANSYS finite element analysis.
3.2 Effect on blood flow status
Hemorheological studies have shown that non-uniform flow caused by turbulence can lead to changes in the structure and the function of ECs, atherosclerosis and the transport and the deposition of prethrombotic substances such as low density lipoprotein (LDL), fibrinogen and von Willebrand factor (vWF) to the vascular wall[67]. Severe turbulence occurs when the blood flows through the fiber intersection in the surface of random structures. But there is no obvious turbulence in the oriented structure which parallels to the blood flow direction, and this can avoid the above problems. Wangetal.[69]drew the same conclusion by establishing the numerical model of hemodynamics according to the axially oriented structure of the lumen surface.
3.3 Effect on inflammation
Laminar shear stress in healthy natural blood vessels can regulate endothelial functions and morphology. It can make ECs which are exposed to the straight segment of the artery[70-71]align longitudinally along the flow direction and reduce the adhesion of monocytes. Further, the inflammation can be reduced. It is found that even without the shear stress, EC alignment induced by oriented nanofibers in artificial blood vessels has the same function. Huangetal.[44]cultured ECs on both oriented and random nanofiber membranes, and the ECs were exposed under inflammatory cytokines. After a period of time, it was found that the number of monocytes attached to the oriented nanofibers was 50% lower than that of the random nanofibers. Nakayamaetal.[9]fabricated a double-layer oriented artificial blood vessels, of which the inner layer was made up of longitudinally oriented nanofibers and the outer layer was made up of circumferentially oriented nanofibers. The adhesion of monocytes decreased by (0.6±0.2) times than that on artificial blood vessels with random nanofibers.
4 Effect of Oriented Structures on Vascular Remodeling
Ideal artificial blood vessels need to mimic structures and composition of natural blood vessels[70-77], as well as to have the ability of inhibiting protein deposition, blood clotting and immune rejection. To construct a bionic blood vessel with the ability of remodeling, endothelialization of the vessel is critical. Endothelium, composed of a single layer of ECs, is an intima directly exposed to blood, and plays a significant role in maintaining vascular patency through releasing regulatory factors such as nitric oxide(NO), heparin and plasminogen. Damage of endothelium will result in a series of pathological reactions, such as thrombosis, inflammation and SMC proliferation[78-80]. By driving ECs to adhere orientationally, the oriented structure promotes rapid endothelialization, inhibits the formation of thrombosis and provides a favorable environment for growth and proliferation of SMCs, as well as the expression of the contractile phenotype, ensuring the complete remodeling of artificial blood vessels. Wangetal.[68]implanted artificial blood vessels with oriented structures in the intima into rats, and a complete layer of cells was formed on the inner surface, indicating that the oriented structure could guide host cells and be rapidly endotheliallized. Similarly, Wuetal.[27]prepared artificial blood vessels with circumferential micro-channels and transplanted them into rats. It was found that the oriented micro-channels could enhance the expression of smooth muscle contractile protein and promote the formation of pebble-like cells parallelized with the direction of blood flow, which indicated the positive role of oriented structures in promoting the endothelialization of artificial blood vessels[81-83].
From the large animalinvivostudies, it can also be found that the oriented structure of the small-diameter artificial blood vessels plays a critical role in manipulating luminal formation and patency in large animals. Wangetal.[69]fabricated a specifically biomimetic intima with an oriented nanotopographical structure, and covalently immobilized anticoagulant molecules. The mixture of heparinized silk fibroin (SF-Hep) and PCL was used to produce an oriented inner layer structure, and pure PCL was used to fabricate vertically porous outer layers by a two-step cross-electrospinning. Then the artificial blood vessels were implanted onto the left carotid artery of the rabbits. Results showed that the patency of the artificial blood vessel with an oriented structure was excellent, and it was capable of remodeling endogenous endothelial cells. Kongetal.[84]fabricated a small-diameter (2 mm) artificial blood vessel with oriented structures from biodegradable chitosan and replaced a section of femoral artery in dogs. Results showed that the artificial blood vessel kept unobstructed, and there were no symptoms like intimal hyperplasia, blocking, aneurysm formation and so forth. What’s more, it could improve the growth and the attachment of VECs and provide an ideal environment for the tissue regeneration. Scherneretal.[85]fabricated a small-diameter artificial blood vessel with an oriented supramolecular fiber network structure consisting of tubular hydrogels from bio-designed cellulose. Carotid arteries of 10 sheep were replaced by the vessels. Analyses revealed that the vessel had a patency rate of 50%, and a vascular wall-like structure along the vessel was formed, which consisted of a homogeneous endothelialization of the inner vessel surface.
It was also reported that the oriented structure combined with other factors had synergistic effects, which could better promote vessel remodeling. Wangetal.[86]fabricated a biomimetic small-diameter artificial blood vessel with an oriented nano-structure combined with dopamine-mediated copper ion (DA-CuII). The DA-CuII system could significantly modulate the cell fates of vascular cells containing platelets, ECs and SMCs via catalytically producing NOinsitu, and the intimal oriented nanotopography could effectively promote the oriented growth and elongation of ECs to achieve luminal remodeling and maintain an instructive long-term patency[87].
5 Conclusions and Future Perspectives
Large-diameter artificial blood vessels have been used in clinic successfully. However, the successful application cannot be copied to that of small-diameter artificial blood vessels. The problems of intimal hyperplasia, thrombosis,etc. influence the long-term patency of small-diameter artificial blood vessels, resulting in the failure of transplantation. Fabricating small-diameter artificial blood vessels from the perspective of structural and functional bionics is still the main research approach to achieve their long-term patency. Constructing oriented structures that mimic the internal surface of natural blood vessels is beneficial to the oriented growth and the rapid proliferation of ECs, expression of SMCs’ contraction phenotypes and differentiation of stem cells. Meanwhile, the internal oriented structure can maintain the steady state of blood flow in the artificial blood vessels, guide the ECs to align longitudinally and reduce the possibility of monocytes adhesion to regulate inflammation. In summary, the oriented structure provides beneficial conditions for vascular remodeling.
Researchers have constructed oriented structures on the vascular internal surfaces through various techniques, such as electrospinning, electrostatic direct-writing, photoetching and 3D printing. The positive effects of oriented structures on rapid endothelialization, expression of SMCs’ contractile phenotypes and vascular remodeling are proved by many studies. However, with the in-depth understanding of the interactions between the inner surface of artificial blood vessels and cells, it is far from enough to only construct the inner surface with the oriented structure from the perspective of physical bionics. On the one hand, natural blood vessels have complicated physical structures. On the other hand, the blood vessel cells are influenced by various kinds of chemical factors. Growth factors, anticoagulants,etc.are all proved to play key roles in promoting vascular endothelialization and anticoagulantion. Physiological gas transmitter NO is also proved to have remarkable effects on modulating functions and regeneration of blood vessel tissue. Artificial blood vessels with NO release function play a significant role in promoting the formation of EC and SMC layers and in inhibiting calcification.
In recent years, studies on the combination of gene therapy and artificial blood vessels are also emerging, and it is regarded as a method with great potential. Therefore, combining physical oriented morphology with chemical and biological factors such as gene delivery, immunoregulation and differentiation induced by stem cells in surrounding tissue to construct small-diameter artificial blood vessels is expected to achieve long-term patency in a real sense.
杂志排行
Journal of Donghua University(English Edition)的其它文章
- Simultaneous Morphologies and Luminescence Control of NaYF4∶Yb/Er Nanophosphors by Surfactants for Cancer Cell Imaging
- Porous Graphene-Based Electrodes for Fiber-Shaped Supercapacitors with Good Electrical Conductivity
- Fabrication of High-Efficiency Polyvinyl Alcohol Nanofiber Membranes for Air Filtration Based on Principle of Stable Electrospinning
- Auto-Generation Method of Child Basic Block Structure
- Small Amplicons Mutation Library for Vaccine Screening by Error-Prone Polymerase Chain Reaction
- Proportion Integration Differentiation (PID) Control Strategy of Belt Sander Based on Fuzzy Algorithm
