新型(4-HBA)SbX5·H2O 类钙钛矿单晶及其卤素结构对发光特性的调控
2023-02-17庄必浩靳子骢田德华朱遂意曾琳茜范建东娄在祝李闻哲
庄必浩,靳子骢,田德华,朱遂意,曾琳茜,范建东,4,*,娄在祝,*,李闻哲,*
1暨南大学,信息科学与技术学院电子科学与工程系,新能源技术研究院,广州 510632
2暨南大学,新型半导体与器件广东省高等学校重点实验室,广州 510632
3暨南大学,纳米光子学研究院,广州 511443
4山东大学,晶体材料国家重点实验室,济南 250100
1 Introduction
Organic-inorganic hybrid perovskite materials are developing rapidly because of their unique photoelectric conversion properties. Among them, the power conversion efficiency (PCE)of solar cells based on organic-inorganic lead halide perovskite has exceeded 25%1–3. Likewise, the antibonding of lead element special 6s2inert electron pairs coupling to halide p orbitals would lead to the excellent luminescent properties, such as high quantum yield and narrow half peak width of luminescence spectrum4–6. At present, the photoluminescence quantum yield(PLQY) of organic-inorganic hybrid lead halide perovskite has exceeded 98%7–9. Although great progress has been made in the study of organic-inorganic hybrid lead halide perovskite, a series of problems such as thermal stability and lead toxicity also deserve deeply explored10–13.
The general structural formula of organic-inorganic perovskite is ABX3(A is organic cation, B is metal cation, X is halogen anion)14. For B-site cations, the metal ions with ns2electronic configuration are commonly used to construct perovskite, such as Ge2+, Sn2+, Pb2+and Sb3+15. Compared with other metal ions, Sb5+ion has the advantages of high chemical stability and low chemical toxicity16–19. For example, an orally active composition of meglumine antimonate (MA) and βcyclodextrin (β-CD) is prepared at a molar ratio of antimony(Sb): β-CD of 7 : 1, allow large animals to take large doses of Sb5+20. Antimony-based materials have been extensively studied,such as the antimony-based superconductor CsV3Sb5, where detailed high-voltage transport measurements reveal a more complex relationship between charge-density-wave and superconductivity21. The research on the optical properties of antimony-based perovskite has made a great breakthrough recently, for example, (Ph4P)2SbCl5single crystal shows 87%PLQY broadband red-light emission at 648 nm15and(TMA)2SbCl5·DMF single crystal shows 67% broadband red light emission at 630 nm22.
For halogen coordination ions, their lone-pair electrons usually hybridize with the empty orbitals in the central ions to form hybrid orbitals, which leads to the interaction between halogen coordination ions and central ions. Among them, the interaction between various halogen coordination ions and central metal ions affects the octahedral distortion, which could furtherly tune the optical properties. For example, Ye et al.regulated the emission wavelength in the range of 580–840 nm by regulating the proportion of halogens in FAPbBrxI3-xSCs23.At present, the structural instability of organic-inorganic lead halide perovskite is due to the widespread use of A-site cations with small radius, such as methyl ammonium, ethyl ammonium,methylamine, etc. High temperature accelerates the A-site cations breaking away from the lattice and perovskite decomposition24.
In this paper, the 4-HBA (4-hydroxybenzylamine) with benzene derivative is used as A-site to construct a series of novel single crystal (4-HBA)SbX5·H2O by solvothermal method. The insertion of 4-HBA expands the metal octahedral frames so that(4-HBA)SbX5·H2O has a high stable 0D structure. The PLQY of(4-HBA)SbCl5·H2O single crystal is significantly enhanced than that of (4-HBA)SbBr5·H2O single crystal.
2 Experimental
2.1 Preparation method
(4-HBA)SbBr5·H2O: 0.075 g 4-HBA and 0.189 g SbBr3were placed in a glass vial of 5 mL. 750 μL of acetonitrile, 500 μL of hydrobromic acid (48% (w, mass fraction) in water) and 300 μL of deionized water were added into the glass vial to prepare the precursor solution. The precursor solution was placed on hot plate and heated to 140 °C for 600 min. The temperature decreased from 140 to 100 °C at the rate of 0.6 °C·h-1. And then the precursor cooled from 100 to 60 °C at the rate of 0.9 °C·h-1.During this process, the critical crystal nucleus extends in the direction of the three directions. After 5 h the precursor is reduced to room temperature, the surface impurities are washed with hydrobromic acid, and finally (4-HBA)SbBr5·H2O single crystal is obtained.
(4-HBA)SbBr3Cl2·H2O: 0.075 g of 4-HBA, 0.040 g of SbCl3and 0.096 g of SbBr3, 750 μL of acetonitrile, 100 μL of hydrobromic acid, 400 μL of hydrochloric acid (37% (w) in water) and 270 μL of deionized water were placed in a glass vial of 5 mL. The cooling process was the same as above.
(4-HBA)SbCl5·H2O: 0.075 g of 4-HBA and 0.12 g of SbCl3,750 μL acetonitrile, 500 μL of hydrochloric acid and 300 μL of deionized water were placed in a glass vial of 5 mL. The cooling process was the same as above25,26.
2.2 Characterization method
The determination of unit-cell parameters and data collections were performed on XtaLAB Synergy-i using the scan technique with Mo Kαradiation (λ = 0.71073 Å (1 Å = 0.1 nm)), for data collection at a temperature of 295(1) K. The single crystal structure was resolved and refined by SHELXT and OLEX227–29.All H atoms were placed in geometrically calculated positions and refined using a riding model with C―H = 0.97 Å (methylene)and 0.96 Å (methyl), with Uiso(H) = 1.2 Ueq (C) or 1.5 Ueq(methyl C). The visual structure of Fig. 1 can be obtained by importing the obtained single crystal data into VESTA software,and the simulated X-ray diffraction pattern can be obtained by using the powder diffraction pattern calculation function in VESTA. X-ray photoelectron spectroscopy (XPS) was measured with Thermo K-Alpha+. All XPS spectra were shifted to account for sample charging using inorganic carbon at 284.80 eV as a reference. Three kinds of single crystals were crushed and ground into powders, and then characterized by Cu Kαradiation under 40 kV and 40 mA by Bruker D8 Advantage X-ray diffractometer (XRD). The step and the time are set to 0.01° and 0.2 s respectively. The UV-Vis NIR spectra measurement of the single crystals was carried out by placing a single crystal in a double-beam spectrophotometer equipped with an integrating sphere (Japan-Shimadzu-UV-3600 plus). The PL images and spectra of the samples were recorded with the XPQY-EQE-Adv fluorescence quantum efficiency measurement system under the treatment of the integrating sphere. The PLE spectrum was obtained by the test of Edinburgh-steady-State/Transient Fluorescence Spectrometer FLS1000. The samples were excited through an oilimmersion objective lens (Olympus,UplanSApochromat, 100×, 1.4 NA) and a circular-polarized 405 nm Plus wave laser controlled by a PDL-800B driver(PicoQuant). The lifetime distribution is directly obtained from the FLIM equipment system.
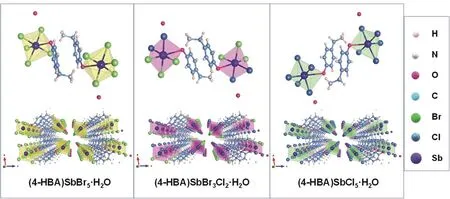
Fig. 1 Structure of the crystal lattice of (4-HBA)SbX5·H2O and arrangement along the (010) crystallographic orientation.
3 Results and discussion
3.1 Structure
As shown in Fig. 1, five halogen atoms and one oxygen atom from 4-HBA surround antimony ion and form a pseudooctahedral structure with the [SbBr5O]2-framework. The organic 4-HBA is embedded in the gap to form a (4-HBA)SbBr5·H2O single crystal with space group P-1. With the substitution of Cl-ions, the unit cell volume decreases from 821.8974 to 741.2648 Å3. The specific crystallographic parameters are shown in Table 1.
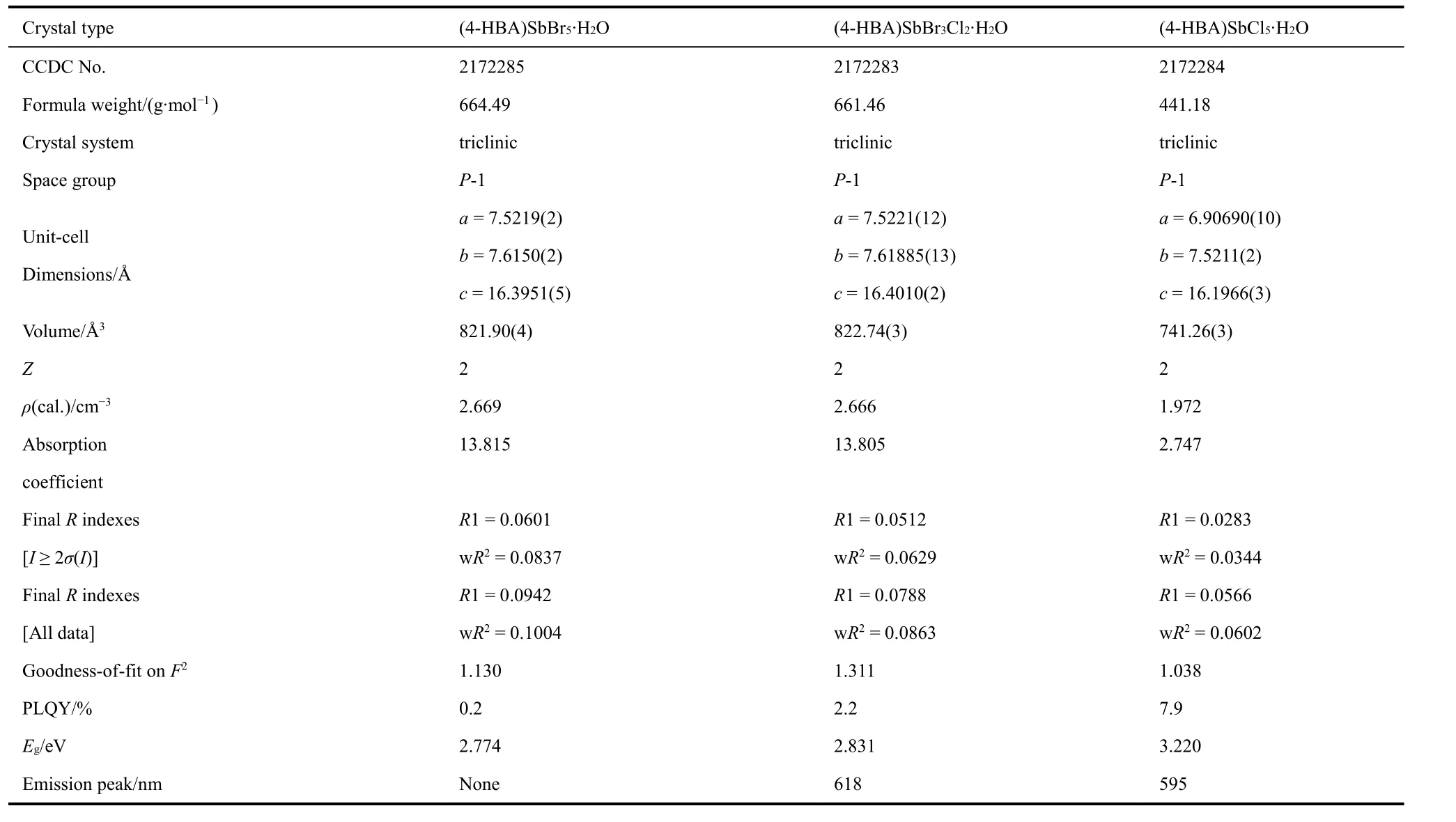
Table 1 Details of X-ray crystallographic parameters of (4-HBA)SbX5·H2O single crystals and their corresponding photophysical characteristics.
In order to further verify the structure, the powder X-ray diffraction test was carried out, and the results are shown in Fig.2. The X-ray diffraction pattern of the experimental powder is almost consistent with the X-ray diffraction pattern simulated from the single crystal data, which indicates that the crystal composition is mainly (4-HBA)SbX5·H2O single crystal. We also notice that the experimental powder X-ray diffraction pattern a) and b) have miscellaneous peaks in the range of 20°–22°, which is due to the residual part of SbBr3in the single crystal.
In order to determine the element composition and valence state of (4-HBA)SbX5·H2O single crystal, we also characterized it by X-ray photoelectron spectroscopy (XPS). As expected, the peaks of C 1s, N 1s, O 1s, Cl 2p, Sb 3p and Br 3d can be clearly detected in the full spectrum scan. For the valence state analysis of Sb element, because the peaks of Sb 3d5/2and O 1s overlap,we use the peak position of Sb 3d3/2to analyze, we can see that the binding energy of Sb 3d3/2were 540.19, 540.23, 540.28 eV,indicating that the Sb element in (4-HBA)SbX5·H2O single crystal is Sb5+30. In addition, the binding energy of Cl is much larger than that of Br, which indicates that the interaction between Cl-and Sb5+is stronger than that between Br-and Sb5+,as shown in the Fig. 3.
3.2 Optical properties
As shown in Fig. 4a–d, the absorption edge of (4-HBA)SbBr5·H2O single crystal is 450 nm. With the substitution of Cl-, the absorption edge blue-shifts toward 400 nm. For the fluorescence excitation spectra (PLE), we can see a strong peak at 350 nm and a shoulder peak at 332 nm. The excitationwavelengths of 332 and 350 nm of (4-HBA)SbX5·H2O single crystals correspond to interband absorption and exciton absorption, respectively, which come from the high energy transition of electrons in organic matter and the exciton transition of metal octahedron, respectively, which is similar to TpyInCl5perovskite24.
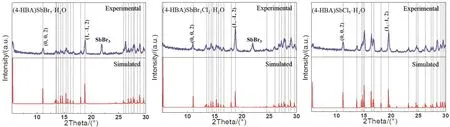
Fig. 2 Powder and single crystal XRD patterns of (4-HBA)SbBr5·H2O, (4-HBA)SbBr3Cl2·H2O, (4-HBA)SbCl5·H2O compounds..

Fig. 3 XPS spectra of (4-HBA)SbX5·H2O single crystals.
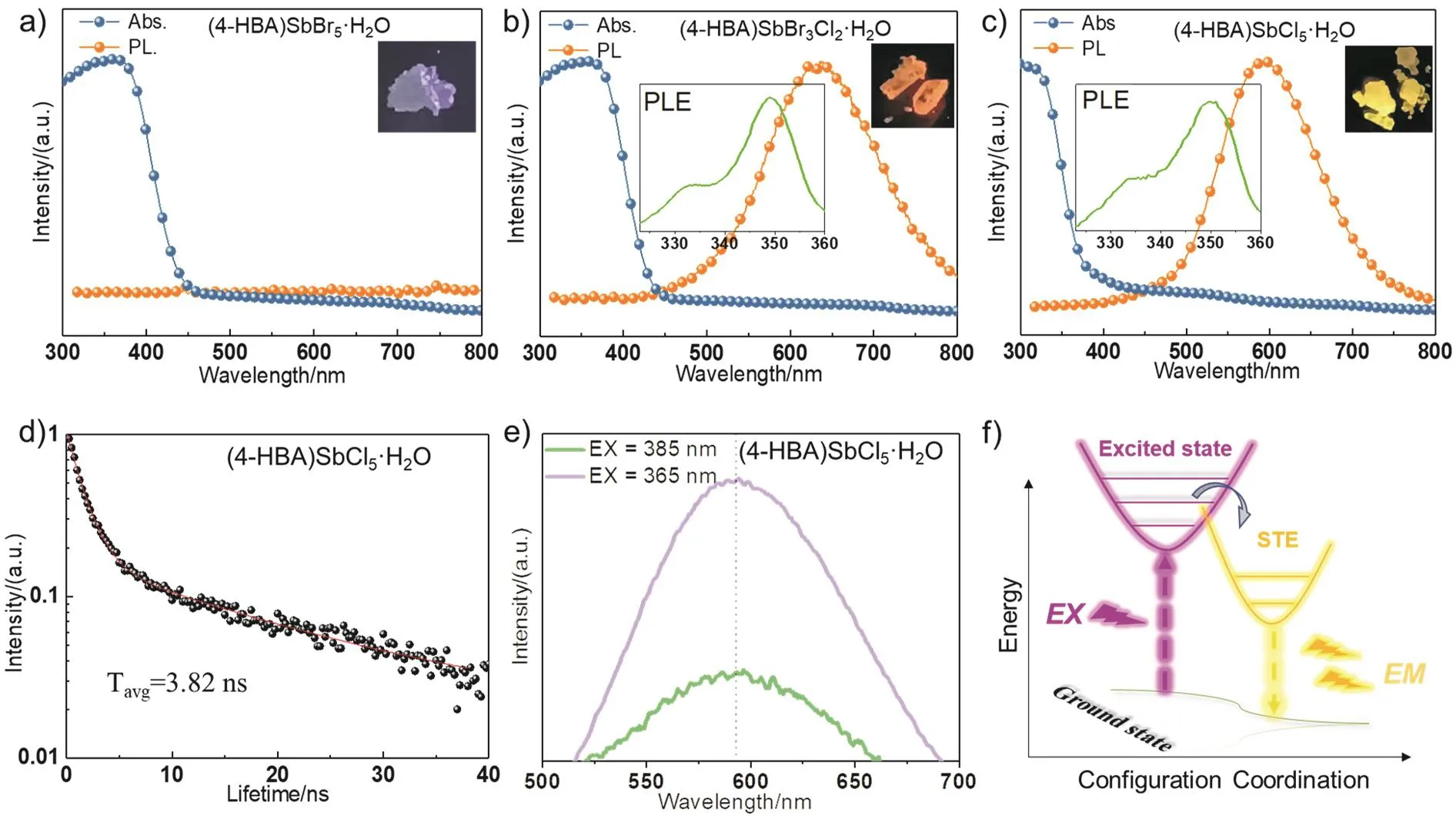
Fig. 4 UV-Vis and PL spectra of (4-HBA)SbX5·H2O single crystal, the inset shows the excitation spectra of the single crystals.
Among them, we can see that the excitation spectra of (4-HBA)SbBr3Cl2·H2O and (4-HBA)SbCl5·H2O are similar, but there is a difference in UV-Vis. This is because the exciton of(4-HBA)SbBr3Cl2·H2O relaxes from high energy level to LUMO energy level, and then recombines to HOMO energy level,emitting long wavelength fluorescence. In the case of (4-HBA)SbCl5·H2O, the exciton relaxes directly from LUMO to HOMO energy level directly. In addition, the photoluminescence spectra (PL) show that the emission peak of(4HBA)SbBr3Cl2·H2O blue-shifts from 618 to 595 nm following the Br-completely replaced by Cl-, which is due to the increase of band gap caused by chloride ion substitution. It is worth noting that the large Stokes shift between the exciton absorption and emission peaks of (4-HBA)SbBr3Cl2·H2O and (4-HBA)SbCl5·H2O (268 and 245 nm) and the broadband emission peak (FWHM of 162 and 139 nm) are typical characteristics of STE emission31. To further verify the luminescence mechanism of the single crystal, we characterized the time-resolved decay curves of (4-HBA)SbCl5·H2O, as shown in Fig. 4d. It can be seen that the average lifetime of the carriers is 3.82 ns, which is similar to the (TPA)2SbCl5single crystal which is also the STE luminescence mechanism32. In addition, we characterize the PL of (4-HBA)SbCl5·H2O single crystal at different excitation wavelengths as shown in Fig. 4e. The results show that the PL peak wavelength excited by different wavelength of 365 nm and 385 nm are at the same position, which can be attributed to the fact that after being trapped by the defect level, excitons excited by different wavelengths have the same relaxation process to the ground state. So they have the same emission spectrum, and thus STE can be further verified. The principle is shown in Fig. 4f.
We performed fluorescence lifetime imaging (FLIM) of (4-HBA)SbX5·H2O to characterize the optical properties. As shown in Fig. 5a–c, the average fluorescence lifetime of (4-HBA)SbBr5·H2O single crystal is about 12 ns. After the introducing of Cl-, the fluorescence lifetime is significantly increased to 22 ns. It should be noted that the lifetime distribution of (4-HBA)SbCl5·H2O single crystal is wider than that of both (4-HBA)SbBr5·H2O and (4-HBA)SbBr3Cl2·H2O single crystals. Besides, the improvement of PLQY likely benefit from the fact that the substitution of Br-by Cl-shrinks the size of the Sb-octahedron, which results in a limitation of the 4-HBA spatial vibration33. Furtherly, the exciton shielding is reduced,then the exciton absorption would be enhanced34. In addition,the chromaticity coordinate gamut diagram shows that the substitution of Cl-increases the color temperature from 2616 to 3106 K, and confirms the emission conversion from orange light to yellow light. Among them, the chromaticity diagram result of(4-HBA)SbBr5·H2O single crystal comes from the color of the single crystal itself, as shown in Fig. 5d–f.
3.3 Band Structure and Spin–Orbit Coupling
To explore the intrinsic relationship between the electronic structure and properties of (4-HBA)SbX5·H2O single crystals,we used density functional theory (DFT) calculation to obtain the band structure, total and orbital-resolved projected density of states. The band structure plots calculated by the generalized gradient approximation (GGA) exchange-correlation functional are displayed at the top panel of Fig. 6a–c. Note that the calculation was implemented under spin degenerate condition,and the spin–orbit coupling (SOC) effect was not involved in the calculation. We can see that when (4-HBA)SbBr5·H2O is doped by Cl-, the band gap width increases from 2.99 to 3.58 eV, which is consistent with the rules of UV-Vis absorption spectrum analysis, as shown in Table 1. The (4-HBA)SbBr5·H2O single crystal has a direct band gap, and when doped with Cl-,(4HBA)SbBr3Cl2·H2O and (4-HBA)SbCl5·H2O have an indirect band gap, which needs the assistance of phonon for the electron transition from valence band maximum (VBM) to conduction band minimum (CBM). In addition, we further exhibit the density of states in Fig. 6d–f. The results show that the VBM of(4-HBA) SbX5·H2O single crystal is mainly contributed by the P electron orbital of both halogen group element (Cl-, Br-) and O element, while the CBM of single crystal is mainly contributed by the P electron orbital of Sb element. It is worth noting that the VBM contributed by halogen element in (4-HBA)SbCl5·H2O is much larger than that of (4-HBA)SbBr5·H2O and(4HBA)SbBr3Cl2·H2O, which can be attributed to the fact that the charge combination from Sb to halogen is much more efficient than that to oxygen, which is why the PLQY of (4-HBA)SbCl5·H2O is higher than others..
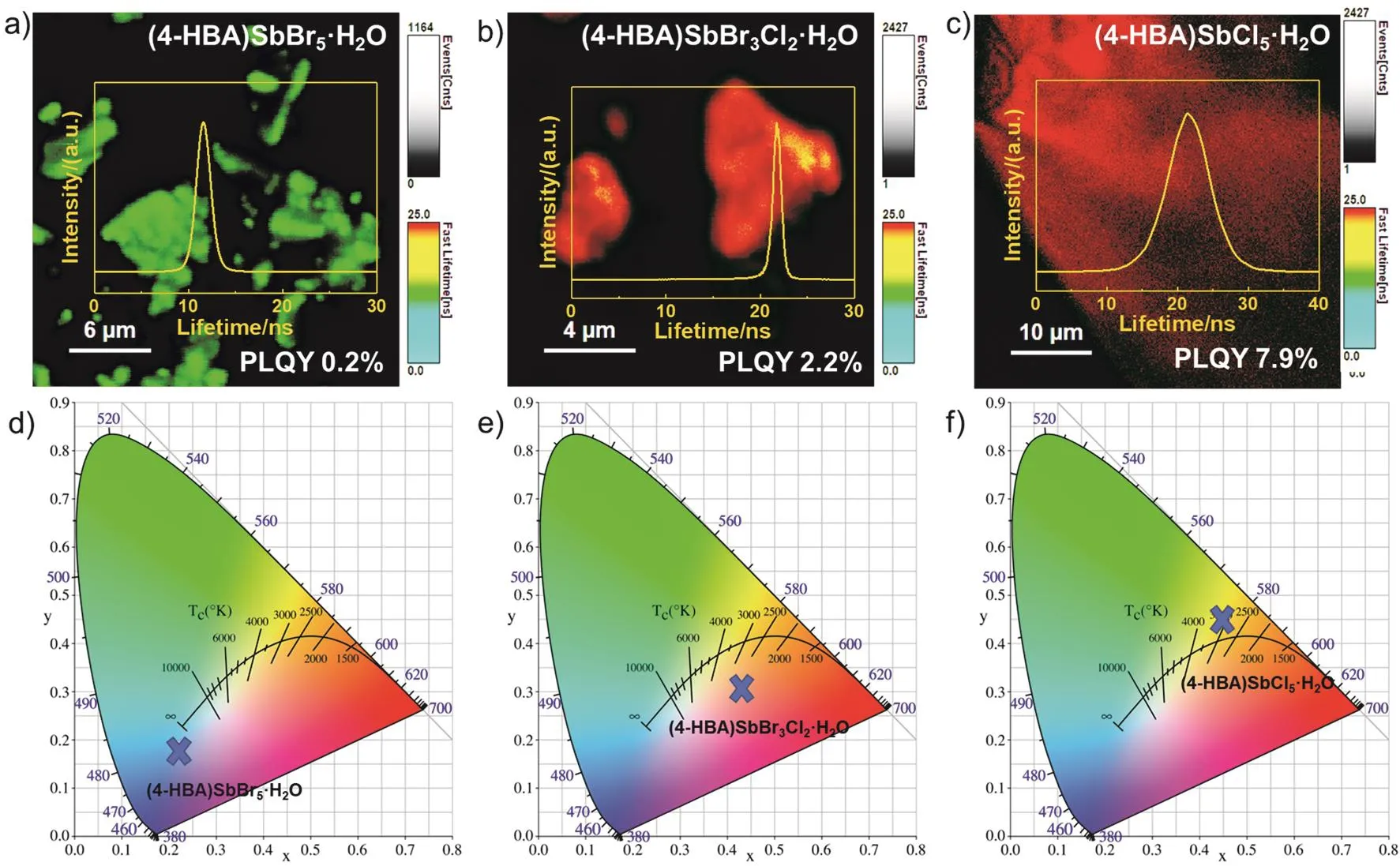
Fig 5 FLIM spectra and 1931 color space chromaticity diagram.
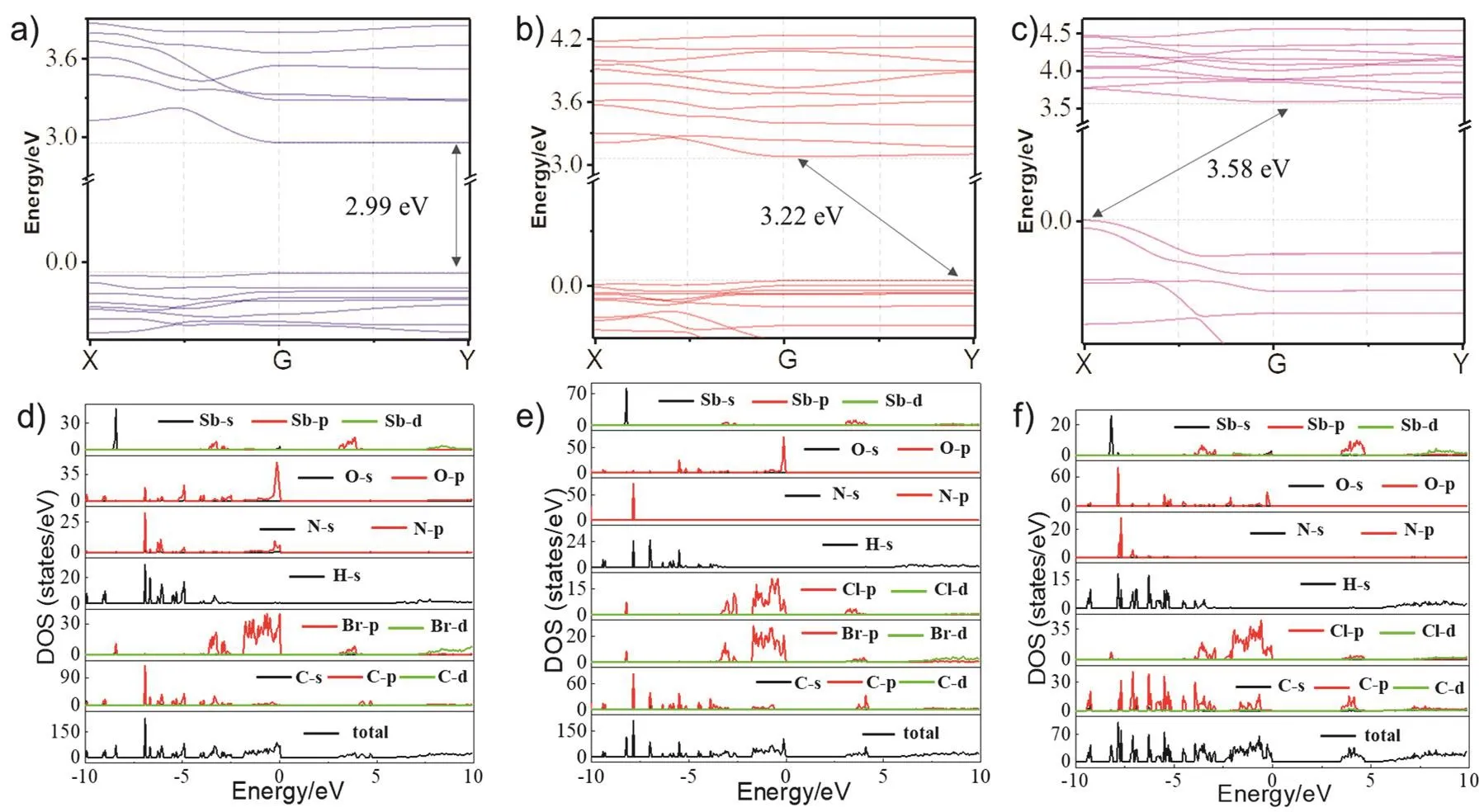
Fig. 6 Calculated band structure by the GGA-PBE exchange-correlation functional of (a) (4-HBA)SbBr5·H2O,(b) (4-HBA)SbBr3Cl2·H2O, and (c) (4-HBA)SbCl5·H2O. Total density of states (TDOS) and projected density of states (PDOS) of (d) (4-HBA)SbBr5·H2O, (e) (4-HBA)SbBr3Cl2·H2O, and (f) (4-HBA)SbCl5·H2O.
4 Conclusions
We have fabricated the emerging lead-free perovskite-like (4-HBA)SbX5·H2O single crystal. The emission of the PL spectra of (4-HBA)SbX5·H2O single crystal come from the STE of octahedron. Through the regulation of Sb-octahedral structure,the luminous color changes from orange to yellow, and the average fluorescence lifetime is extended from 12 to 22 ns. In addition, the PLQY of single crystals increase nearly 40 times from 0.2% to 7.9%. The current study provides the potential applications through octahedral structure regulation to assemble novel types of hybrid single crystals.
Acknowledgment: The authors thank the Guangdong Engineering Research Center of Thin-Film Photovoltaic Technology and Equipment and Key Laboratory of New Semiconductors and Devices of Universities in Guangdong Province.
