Comparison of vegetable oils on the uptake of lutein and zeaxanthin by ARPE-19 cells
2023-02-11JeonghunBaekChunWaiMaiWeiMengLimLaiChunWong
Jeonghun Baek, Chun Wai Mai, Wei Meng Lim, Lai Chun Wong
1School of Medicine, International Medical University, Kuala Lumpur 57000, Malaysia
2Department of Pharmaceutical Chemistry, School of Pharmacy, International Medical University, Kuala Lumpur 57000, Malaysia
3Department of Pharmaceutical Technology, School of Pharmacy, International Medical University, Kuala Lumpur 57000, Malaysia
Abstract
● KEYWORDS: lutein; zeaxanthin; adult retinal pigment epithelial-19 cells; vegetable oil
INTRODUCTION
The macular xanthophylls lutein (LUT), zeaxanthin(ZEA), and meso‐ZEA abundant in the human macula lutea and eye lens, aids in maintaining eye health and preventing ophthalmic illnesses[1‐2]. LUT and ZEA can only be obtained through diet and occur abundantly in green leafy vegetables, orange and yellow fruits[3]. In contrast, meso‐ZEA is a rare dietary component that only occurs in the human eye as a result of a metabolic conversion from dietary LUT.Although about 20‐30 carotenoids have been discovered in human blood and tissue[3], only LUT and ZEA are specifically taken up by the retinal pigment epithelial (RPE) cells,preferentially deposited in the human macula, and transferred to photoreceptors[4]. Collectively LUT, ZEA and mexo‐ZEA forms the predominant carotenoids in the macular pigment(MP). High levels of MP density have been positively correlated with improved visual function[5], and lower rates of retinal degeneration[6]and retinitis pigmentosa[7]. Age‐related macular degeneration (AMD), which results from retinal degeneration,causes 8.7% of blindness globally[8]. It is the most prevalent cause of permanent vision loss, particularly in the elderly.Oxidative stress‐related damage to the RPE is thought to be a precursor in the development of AMD[9]. Because the RPE is constantly subjected to excessive levels of oxygen tension and blue light, it is especially vulnerable to oxidative stress. In fact, cholesterol oxidation to form oxysterols is thought to be induced by oxidative stress in the RPE by enzymatic and free radical reactions. It has been shown that certain oxysterols such as 7‐ketocholesterol (7‐KC), mainly formed by cholesterol auto‐oxidation and localized in the RPE, may play a functional role in cataracts and AMD[10].
The two types of AMD are the “dry” (non‐exudative) and “wet”(exudative) forms[11]. The early stage of the dry form is defined by alterations in the RPE, pigmentation, lysosomal lipofuscin accumulation, and formation of extracellular yellowish drunsen deposits. In the advanced stage of dry AMD, the RPE cells in the macular break down due to drunsen maculopathy.Drusen maculopathy is caused by accumulation of lipofuscin,reactive oxygen species, complement alternative‐related inflammation, and reduced extracellular matrix remodeling and phagocytosis. Because RPE cells provide metabolic support to the photoreceptor cells, these photoreceptor cells lose their function and vision from this area of the retina is gradually diminished. This condition is called geography atrophy. Wet AMD on the other hand is a neovascular disease phenotype. Choroidal neovascularization happens whereby aberrant vessels from the underlying choriocapillaris invades the subretinal space. Rapid and irreversible vision loss happens when these leaky newly formed blood vessels cause accumulation of fluid under and within the retina. Dry AMD can change to wet AMD, and it is also uncertain why one person develops dry AMD, whereas the other develops its wet counterpart. There is no cure for AMD. Intravitreal injections are the only effective treatment for wet AMD, but these procedures carry a high risk of infection and endophthalmitis.On the other hand, no treatments are available for dry AMD.In clinical trials, LUT and ZEA supplements delayed the development of AMD to the advanced stage[12‐13]. Notably,the Age‐Related Eye Disease Study 2 is a clinical trial study involving 4203 participants range in age from 50 to 85y with bilateral intermediate AMD or advanced AMD in one eye. A statistically significant decrease in development to advanced AMD (P<0.05) was observed in the LUT+ZEA supplement study cohort, in comparison to the cohort that did not receive the supplement. With the supplement, the development of advanced AMD and geography atrophy decreased by 10% and 11%, respectively, compared with no supplement[14]. Therefore,LUT and ZEA were considered a better alternative to replace β‐carotene in the original Age‐Related Eye Disease Study supplement as β‐carotene increases the risk of lung cancer in past smokers[15]. Studies have shown that LUT and ZEA protect the macula by acting as mechanism of defense against oxidative stress[16].
The bioavailability of these carotenoids is however affected by multiple factors. During digestion, the carotenoids are liberated from the food source and incorporated into mixed micelles before absorption by intestinal epithelial cells.The micellarization of LUT and ZEA and their uptake by enterocytes are dependent on various components such as the food matrix, processing factors, diet’s fat content, interaction and competition with other dietary compounds, nutritional status, host’s gut health and genotype[17]. Therefore, patients may not achieve the recommended dietary allowance for LUT and ZEA of 6 mg/d for decreased disease risk[18]. Hence,ways to increase uptake of these carotenoids by the RPE for delivery to photoreceptors could be valuable for AMD therapy.A potential method is by utilizing oils. Lipids can improve the bioavailability of carotenoids by increasing carotenoid solubility in the mixed micelles[19]. Hence, it is beneficial to incorporate oils into meals or supplements of LUT and ZEA to increase the absorption of these important nutrients. In addition to improved bioaccessibility, LUT and ZEA supplements in oil formulation increases the stability of the carotenoids compared to tablet or capsule form[20].Besides improving bioavailability, the other beneficial effects of oils to eye health are increasingly being recognized. Among the potential applications are topical fatty acids for dry eye treatment[21]and lipophilic vehicles in eye drops. Moreover,when retinal adult retinal pigment epithelial (ARPE)‐19 cells are incubated with vegetable oilsin vitro, the vegetable oil fatty acids were found to incorporate into the cells, enhancing membrane fluidity. Therefore preventing or repairing a possible dysfunction of the RPE[22]. Owing to the potentially desirable biological properties of oils for the eye, it would be useful to identify oils which could also provide the best uptake of LUT and ZEA in cells. The optimum oil may be applied in the future in ocular treatments such as eye drops, intravitreally or suprachoroidally injections to exert favourable biological properties as well as to deliver LUT and ZEA to the RPE.Hence the objective of this work is to compare the uptake of LUT and ZEA by cultured ARPE‐19 cells in different oils.We hypothesize that the uptake of these carotenoids by the cells will be dependent on the type of oil. The vegetable oils tested in this study are coconut, corn, peanut, olive, sunflower,soybean, castor and linseed oils.
MATERIALS AND METHODS
Ethical Approval This research was approved by the Joint Committee on Research and Ethics of International Medical University [No.BMSI‐2019 (13)].
Materials and ReagentsHigh performance liquid chromatography (HPLC) grade methanol, 2‐propanol,dichloromethane and methyl tert‐butyl ether were purchased from Merck (New Jersey, USA), while dimethyl sulfoxide(DMSO) was from Chemolab supplies (Selangor, Malaysia).All other materials were purchase from Sigma Aldrich (St.Louis, MO, USA).
Cell CultureThe human RPE cell line, ARPE‐19 (ATCC®CRL‐2502TM) was obtained from the American Type Culture Collection (Rockville, MD, USA), and cultured according to the previously established protocol[23]. Briefly the ARPE19 were cultured in Dulbecco’s Modified Eagle Medium‐F‐12 supplemented with 10% foetal bovine serum and 1% penicillin–streptomycin in a humidified 5% CO2incubator maintained at 37℃. Cells less than passage 30 were used in the experiments.
Preparation of LUT, ZEA and oil stock solutions for cell treatmentStock solution of LUT (Supelco PHR1699), ZEA(USP 1733122) and each of the oil was prepared separately in DMSO. The concentration of stock LUT and ZEA was each 100 mmol/L, and the concentration of the stock oil was 100 μL/mL.The vegetable oils used in this study were coconut oil (Sigma C1758), corn oil (Sigma C8267), peanut oil (Sigma P2144),olive oil (Sigma O1514), sunflower oil (Sigma 88921),soybean oil (Sigma S7381), castor oil (Sigma 259853) and linseed oil (Sigma 430021).
Treatment of Cells with LUT, ZEA and OilsTo evaluate the role of the oils in LUT and ZEA uptake, ARPE‐19 cells were first seeded at 3×105/mL on a 6‐well flat bottom plate of Dulbecco’s Modified Eagle Medium complete media for 24h before treatment. The cells were then treated for 48h with LUT, ZEA, and respective oil at the effective concentration of 247 μmol/L, 49 μmol/L and 1% (v/v) respectively. The maximum DMSO concentration in each well was 1% (v/v).Control wells contained 1) Culture medium + LUT (247 μmol/L)+ ZEA (49 μmol/L) + 1% (v/v) DMSO, 2) Culture medium+ 1% (v/v) DMSO. The experiments were performed at least three independent times.
Extraction of LUT and ZEATo quantify the level of LUT and ZEA in the cells after the incubation, the cells were harvested according to the reported procedure[24]with modification. The cells were scrapped off from the well plates and transferred with the culture medium to a centrifuge tube.The cells were spun at 1.5 krpm for 5min. The supernatant was removed, and the cell pellets were rinsed twice with ice‐cold phosphate‐buffered saline. After centrifuge, supernatant was removed and 2‐propanol: dichromethane (2:1 v/v) was added to the cells for 30min to extract the LUT and ZEA. The extracts were dried under nitrogen gas and the dried extracts stored in the dark at ‐20℃ until HPLC analysis. Just before HPLC analysis, the dried extracts were resuspended in 1200 μL mobile phase (methanol:methyl tert‐butyl ether 9:1 v/v) and were filtered with 0.22 μm nylon filter. Sample handling,homogenization, and extraction were conducted under low lighting whenever possible to minimize isomerization.
Quantification of LUT and ZEALUT and ZEA was quantified by HPLC using reported method from the literature[24]. Chromatography was performed on an Agilent 1260 Infinity HPLC system (Agilent Technologies, US),interfaced with a quaternary pump, autosampler, and diode array detector (DAD). Agilent OpenLAB CDS ChemStation software (Rev.C.01.04) was used for data analysis. Separation was carried out with a YMC C30carotenoid column (250 mm×4.6 mm internal diameter, 3 μm particle size) at room temperature.The mobile phase consisted of methanol:methyl tert‐butyl ether (10:90) delivered at flow rate of 0.9 mL/min under isocratic condition. The injection volume was 20 μL and the run time for each injection was 20min. Each of the sample was injected three times and the mean peak area of the three injections for each of the sample was calculated. LUT and ZEA was monitored at a wavelength of 450 nm and quantified using the external standard curve constructed for the carotenoids. The LUT/ZEA mixed stock standard solution was prepared in mobile phase with concentration 100 μg/mL and diluted accordingly with mobile phase to the standard curve concentration (0.1 μg/mL to 40 μg/mL).
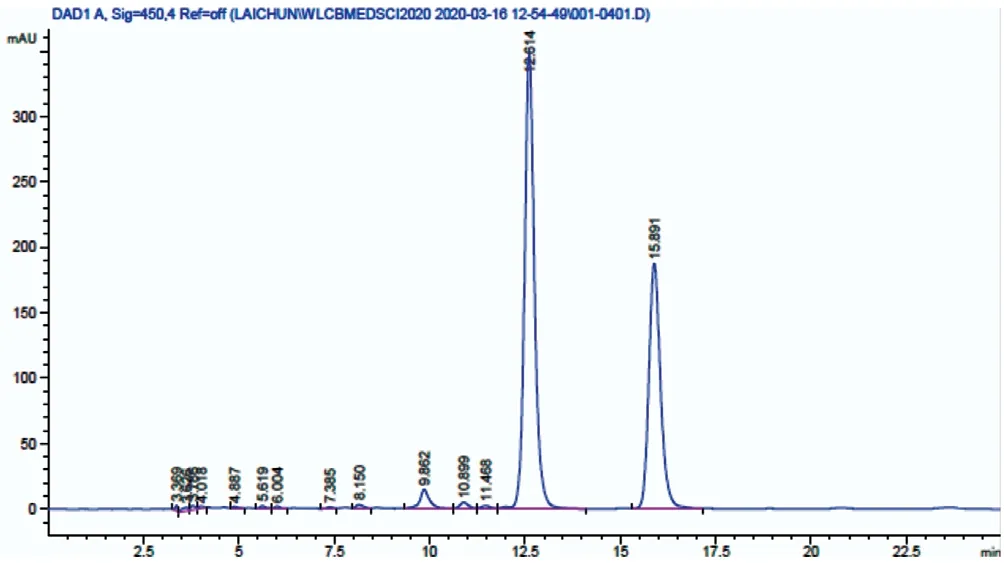
Figure 1 The HPLC chromatogram of LUT and ZEA standard at concentration 20 μg/mL The retention time for LUT and ZEA is 12.6min and 15.9min, respectively. HPLC: High performance liquid chromatography; LUT: Lutein; ZEA: Zeaxanthin.
Observation of Morphological ChangesTo evaluate the extent of morphological changes, ARPE‐19 cells were treated with LUT and ZEA in the oils as per procedure above.After incubation, the cells were examined using a Nikon Eclipse Ti‐U inverted microscope (Tokyo, Japan) at 10×magnification.
Statistical AnalysisData are presented as mean±standard deviation of the mean. The difference of means between different treatment oils were performed by Student’st‐test using Microsoft Excel Statistical Analysis tool from three independent experiments. AnyPvalue below 0.05 (P<0.05)was considered statistically significant.
RESULTS
Quantification of LUT and ZEA using HPLCOrdinary least‐square linear regression was used to plot the calibration curve for LUT and ZEA in the concentration 0.1‐40 μg/mL. A linear relationship was established between the peak area and concentration over this range, as indicated by the correlation coefficient (r2) value of 0.9987 and above. The lowest concentration on the calibration curve was taken as the limit of detection value. The limit of quantification, calculated from the standard deviation of the response and the slope of the calibration curve was 1.16 and 3.45 μg/mL for LUT and ZEA,respectively. The chromatogram of LUT and ZEA standard at 20 μg/mL is shown in Figure 1.
Uptake of LUT and ZEA by ARPE-19 Cells in OilsThe uptake of LUT and ZEA by ARPE‐19 cells were measured by HPLC. Among the oils tested, the highest uptake of LUT and ZEA was observed in coconut oil and the lowest uptake in linseed oil (Table 1).
Observation of Morphological ChangesThe oils and DMSO at the concentration used in the cell culture experiments did not cause changes in cell morphology, as observed under microscope (Figure 2). No difference in cell morphology was seen in cells incubated with 1% DMSO only, LUT+ZEA+1%DMSO, LUT+ZEA+1% oil+1% DMSO, and 1% oil+1%DMSO only. The observation suggests that the concentration of oils used in this study was not cytotoxic.
Figure 2 Microscope image of ARPE-19 cells treated with LUT and ZEA in the oils A: No significant morphological change was seen between cells treated with and without LUT and ZEA; B: No significant morphological change was also seen between cells treated with respective oil with and without LUT and ZEA. LUT: Lutein; ZEA: Zeaxanthin.
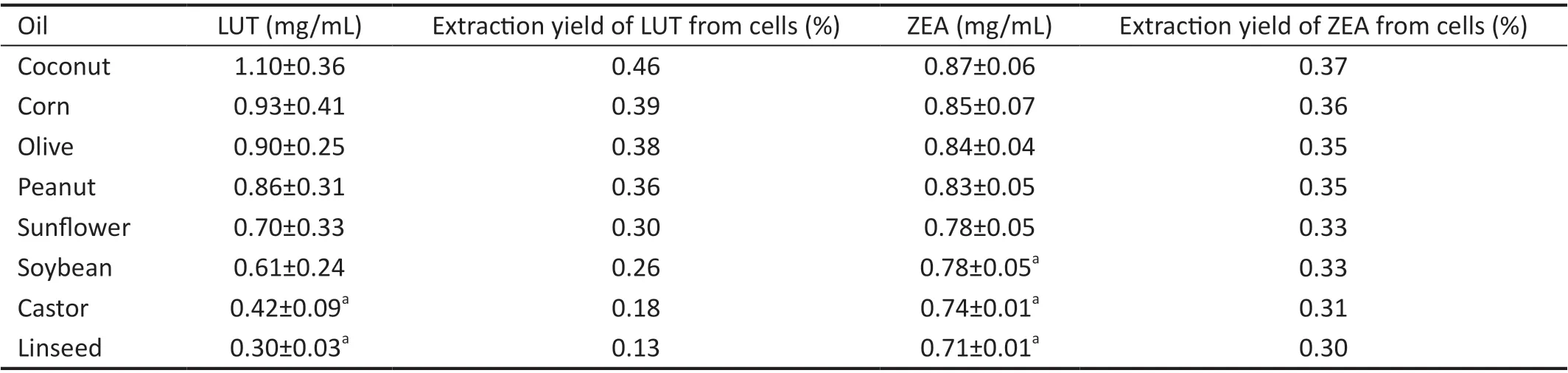
Table 1 Concentration of LUT and ZEA uptake by ARPE-19 cells
DISCUSSION
The RPE, sandwiched between the neural retina and the choroid, occupies a functionally crucial location in the human eye. It is present as an epithelial monolayer of pigmented,hexagonally packed cuboidal cells. It performs a wide variety of functions to maintain normal vision including altering and recycling retinoids in the visual cycle, phagocytosis and recycling of photoreceptor disc materials, and preserving retinal integrity and photoreceptor survival[25]. The RPE also plays an important role to transport nutrients from the choroidal capillaries to the photoreceptors. LUT and ZEA, two of such important nutrients are xanthophyll carotenoids which are specifically taken up by the RPE, preferentially accumulated in the human macula, and transferred to photoreceptors[4]. The beneficial properties of LUT and ZEA in maintaining healthy vision have been attributed to their ability to preserve the macula against oxidative stress through antioxidant effects,filtering out blue light, scavenging reactive oxygen species[1,26],and modulatingAluRNA level[23].
可以看出,该值是一无穷大的值,实际应用中,无论采取数字电路还是模拟电路,都不可能实现无限大的增益,而且过大的增益会导致系统可能出现震荡导致安全隐患[8];另一方面,理想的比例谐振控制器只在基波频率处才有很大的增益,对于基波频率附近的输入信号增益几乎为零,然而现实中,电网频率难免有所波动,这就导致了理想比例谐振控制器难以现实[9]。
Lipids play important roles in maintaining eye health. In the body, a minimum quantity of lipid is needed for optimal bioavailability of LUT and ZEA[27‐28].In vitroas well andin vivostudies have shown that the fatty acid composition of edible oils modulates the oral bioavailability of LUT and ZEA in the body by facilitating the intestinal absorption of these carotenoids. Some oils have also been shown to protect against 7‐KC induced cytotoxicity associated with age‐related diseases including AMD and cataracts[29‐30]. In addition, retinal cells are very rich in lipids which are essential in the retina structure and function. The membrane phospholipids of the retina uniquely have the most concentration of long‐chain polyunsaturated fatty acids (PUFAs); around 33% of the total phospholipids in humans[31]. The highest amount of omega‐3 and omega‐6 long chain PUFAs in the retina are docosahexaenoic acid and arachidonic acid, respectively. Retinal phospholipids also contain monounsaturated fatty acids (19% of the total phospholipids in humans) and saturated fatty acids (SFAs;42% of the total phospholipids in humans). Animal studies and epidemiological studies suggests that diet influences the composition of retina fatty acid[32].
Owing to the beneficial properties of oils in eye health, this work was conducted to compare the uptake of LUT and ZEA by RFE cells in various oils. Treatment of ARPE‐19 and human conjunctival epithelial cells with undiluted vegetable oils have been reported previously to evaluate the oils’ effect on the biochemical and biophysical property of the cell membrane, oxidative stress, apoptosis, and P2X7 cell death receptor activation[22,33]. However, as far as we are aware this is the first reported study comparing different type of oils on the uptake of LUT and ZEA by ARPE‐19 cells. In this study,ARPE‐19 cells were treated for 48h with LUT, ZEA, and respective oil at the effective concentration of 247, 49 μmol/L and 1% (v/v) respectively.
Membrane fluidity is the viscosity of the lipid bilayer component of a cell membrane, and it is dependent on the types of lipids that compose it. Among the multiple factors that impact the membrane fluidity are membrane structure,curvature, microviscosity and its phase, lipid structure,packing, and composition[34]. The benefit of increased membrane fluidity not only leads to a more flexible membrane but may also lead to improvement in drug cell delivery. Thus,vegetable oil vectors may enhance the delivery of drugs into cells by altering membrane fluidity, which depends on the fatty acid makeup of the membrane[33]. PUFAs and SFAs in the membrane lipids are known to influence membrane fluidity by changing the microviscosity of the membrane. Higher PUFA/SFA ratios in membrane lipids reduces the microviscosity of membranes thus increasing the fluidity. The membrane fluidity of ARPE‐19 cells increased when the cells were incubatedin vitrowith argan oil, camelina oil, and the camelina‐argan‐corn oil mixture (2:1:1 by volume), compared to control (refined neutral olive oil)[22]. It was observed that the fatty acids in the oils incorporated into the retinal cells and increased the fluidity of the plasma membrane. There was a significant positive correlation between membrane fluidity and the membrane lipid PUFA/SFA ratio.
Among the oils used in this study, the PUFA/SFA ratio from the highest value to the lowest was linseed, sunflower, soybean,corn, peanut, olive and coconut[35]. On the contrary, the highest uptake of LUT and ZEA was observed in coconut oil followed by corn oil, olive oil, peanut oil, sunflower oil, soybean oil,castor oil and lastly linseed oil. This suggest that the uptake of LUT and ZEA does not correlate with increased membrane fluidity. Interestingly, coconut oil which has the lowest PUFA/SFA value was found in this experiment to have the highest LUT and ZEA uptake. Our results suggest that besides PUFA/SFA values, other components of the oils may play a more important role in enhancing LUT and ZEA uptake.
Hydrophilic compounds that are poorly absorbed are primarily absorbed through passive paracellular permeation which is controlled by tight junctions. Certain fatty acids have been found to alter tight junctions. Capric acid, lauric acid and oleic acid increases the opening of tight junctions to improve paracellular permeability[36]. Among the mechanism of action has been attributed through phospholipase C‐dependent inositol triphosphate/diacylglycerol pathways[37]. Therefore,these fatty acids in coconut oil may have contributed to the higher absorption of LUT and ZEA in RPE cells via similar mechanism, in particularly as LUT and ZEA contains the polar carboxyl functional group. Lauric acid is the primary fatty acid of coconut oil with 49% composition, with the remaining component consisting of caprylic acid (8%), capric acid (7%),myristic acid (8%), palmitic acid (8%), stearic acid (2%), oleic acid (6%) and linoleic acid (2%)[38]. On the other hand, capric acid and lauric acid is not present in linseed oil, while oleic acid makes up 22.1% of the fatty acids in linseed oil[33].
Several fatty acids including lauric acid (C12H24O2) are known to be safe and effective chemical penetration enhancers(CPEs). CPEs are molecules that can interact with the constituent of skin’s outermost and rate limiting layer stratum corneum and improve its permeability. Thus, CPEs are used in transdermal drug delivery to enable the delivery of molecules into the membrane that would otherwise have poor membrane penetration. It is possible that high lauric acid content in coconut oil may have increased the permeability of the ARPE‐19 cells to LUT and ZEA through similar mechanism.Permeation enhancers may partition effectively into the lipids of the cells, and interact with the lipid layer contents to create reversible structural changes to increase permeability[39]. The effectiveness of fatty acids as CPEs in the stratum corneum is influenced by factors such as the nature of solvent and type of chain length. As a CPE, saturated alkyls of C10‐C12 with polar head group is a potent enhancer[40].
In conclusion, the result of this study proposes another beneficial application of oils in the treatment and prevention of AMD besides their positive roles to increase bioavailability of LUT and ZEA, and their cytoprotective activity against oxysterols. In this study, the uptake of LUT and ZEA by ARPE‐19 cells were found to be dependent on the type of oils,whereby the highest uptake for both carotenoids were observed with coconut oil. Therefore, among the oils tested, coconut oil is the most promising to be used in future applications for LUT and ZEA delivery to the RPE. Opportunities for future work includes mechanistic studies. This study’s limitation is that it was performed usingin vitrotest which may not accurately reflectin vivoresponses. Hence, future work may include validation of the findings in this experiment within vivoexperiment.
ACKNOWLEDGEMENTS
Conflicts of Interest: Baek J,None;Mai CW,None;Lim WM,None;Wong LC,None.
猜你喜欢
杂志排行
International Journal of Ophthalmology的其它文章
- Visual perception alterations in COVID-19: a preliminary study
- COVID-19 pandemic impact on ocular trauma in a tertiary hospital
- Apolipoprotein A1 suppresses the hypoxia-induced angiogenesis of human retinal endothelial cells by targeting PlGF
- Identifying a novel frameshift pathogenic variant in a Chinese family with neurofibromatosis type 1 and review of literature
- Recurrence risk factors of intravitreal ranibizumab monotherapy in retinopathy of prematurity: a retrospective study at one center
- Changes of optic nerve head microcirculation in high myopia
