Therapeutic effects of epigallocatechin and epigallocatechin gallate on the allergic reaction of αs1-casein sensitized mice
2023-01-23QinqinZhngXiofengYuLinghnTinYnjunCongLinfengLi
Qinqin Zhng, Xiofeng Yu, Linghn Tin, Ynjun Cong,*, Linfeng Li
a Beijing Advanced Innovation Center for Food Nutrition and Human Health, College of Food and Health, Beijing Technology and Business University, Beijing 100048, China
b Department of Dermatology, Beijing Friendship Hospital, Beijing 100050, China
Keywords:αs1-casein Epigallocatechin Epigallocatechin gallate Antianaphylaxis
A B S T R A C T To investigate the anti-αs1-casein allergy mechanism of two tea-derived polyphenols, epigallocatechin(EGC) and epigallocatechin gallate (EGCG), BALB/c mice were sensitized and challenged with αs1-casein and nutritional intervention was given by EGC and EGCG during the sensitization provocation phase. The main evaluation indexes used were levels of mast cell proteases, histamine, and specifi c antibody immunoglobulin E (IgE),as well as cytokine secretion and pathological observation. The results showed that both EGC and EGCG signifi cantly reduced levels of mast cell protease, histamine, specifi c IgE antibodies, and Th2 cytokines in allergic mice. The histopathology results showed that both EGC and EGCG markedly reduced the degree of lesions in the intestine, thymus, spleen, and lung. The conclusions from this study can provide a theoretical basis for the mechanism by which tea polyphenols regulate food allergens.
1. Introduction
Milk is one of the eight internationally recognized allergic sources. A milk allergy is a common food allergy in infants and young children, and has an incidence rate of 2%-3% in infants under one year old [1]. While children over 3 years old will have a certain immune tolerance to milk, their morbidity rate is about 0.3% [2]and it is seriously harmful to the health of infants and young children. There is presently no radical clinical cure for milk allergy and strict allergen avoidance is usually adopted, however, there are drawbacks to avoidance. Due to the prevalence of food allergens in the average daily diet, it is very diff icult to completely avoid ingestion which can make it difficult to meet the normal nutritional needs of infants and adolescents [3]. Therefore, safe and effective products that can prevent or treat food allergies need to be found. Casein is the main allergen in milk, and there is higher sensitization withαs1-casein compared toαs2-,β-, andκ-casein [4], and therefore, there is signif icant research focus on its allergic mechanism.
Tea is rich in potential bioactive compounds such as f lavane-3-ol,prothiothrin, flavonol, theaflavins, theanosaccharins, 1-theanine,methylxanthine, gallic acid, and polyamines [5]. These components of tea play an important role in human health, including the prevention of cancer, coronary heart disease and obesity [6,7]. The effect of tea on health is mainly related to phenolic compounds [8].Polyphenols in tea can not only inhibit histamine released from mast cells, but can also reduce the level of specif ic IgE in allergy bodies and improve the immune function of the body [9-11]. However,which tea polyphenols have antiallergic effects remains to be further studied. Polyphenolic compounds account for about 65%-80% of tea polyphenols, and are mainly found in four forms epicatechin gallate (ECG), epigallocatechin gallate (EGCG), epicatechin (EC),and epigallocatechin (EGC) [12]. The content of EGC accounts for about 15%-20% of the total catechins, and the content of EGCG accounts for about 50% of the total catechins. Catechins have many functions, a high application value and strong biological activity [13-15].Tea polyphenols are a relatively safe food additive and will not have negative effects on human health [16-18].
In this study, based on a model of BALB/c mice sensitized byαs1-casein, mice were gavaged daily with EGC and EGCG during the challenge stage. The levels of mast cell protease (MCP-1),histamine, specific IgE antibody and cytokines were assessed,and pathological sections were observed to investigate the antiallergic mechanism of EGC and EGCG. Tea polyphenols with strongin vivoactivity ways are used to develop new anti- and hypoallergenic milk products and may also provide a theoretical basis for improving the safety of milk products.
2. Materials and methods
2.1 Effects of EGC and EGCG on αs1-casein sensitized mice
Fifty female BALB/c mice aged 4 weeks were purchased from Beijing Viton Lihua Company (license: SCXK (jing) 2006-0009).BALB/c mice were fed adaptively in the specific pathogen-free(SPF) animal room for a week at a controlled ambient temperature of (23 ± 3) °C and humidity of 40%-70%. The mice were selected into the 5 following groups according to their body weight: saline negative control (NC),αs1-casein allergic model, EGC (Sigma Aldrich(Shanghai) Trading Co., Ltd., China) treatment, EGCG (Sigma Aldrich (Shanghai) Trading Co., Ltd.) treatment, and disodium cromoglycate anti-allergy treatment. Each group had 10 mice and were weighed before gavage each week. During the first 4 weeks,every 7 daysαs1-casein (Shanghai Yuanye Biotechnology Co., Ltd.)(2 mg/each, plus cholera toxin (CT) (Sigma Aldrich (Shanghai)Trading Co., Ltd.)) were given orally with the negative control group given saline with same bulk. After the 4th week, the EGC and EGCG treatment groups were given intragastric administration once a day for 14 days until the 42ndday of high-dose stimulation. The doses administered were 50 mg EGC or EGCG (The dose selection is based on the result ofin vitroexperiments, i.e. the IgE-binding ability of the complex after EGC or EGCG andαs1-casein reaction). The drug control group was injected with 1 mg/mL disodium tryptophan (Sigma Aldrich (Shanghai) Trading Co., Ltd.), an anti-allergic drug, before a high-dose stimulation [19]. At the 6th week, the mice were given a high dose of 5-10 times dose after fasting overnight. The schematic diagram of the animal experiment is shown in Fig. 1.
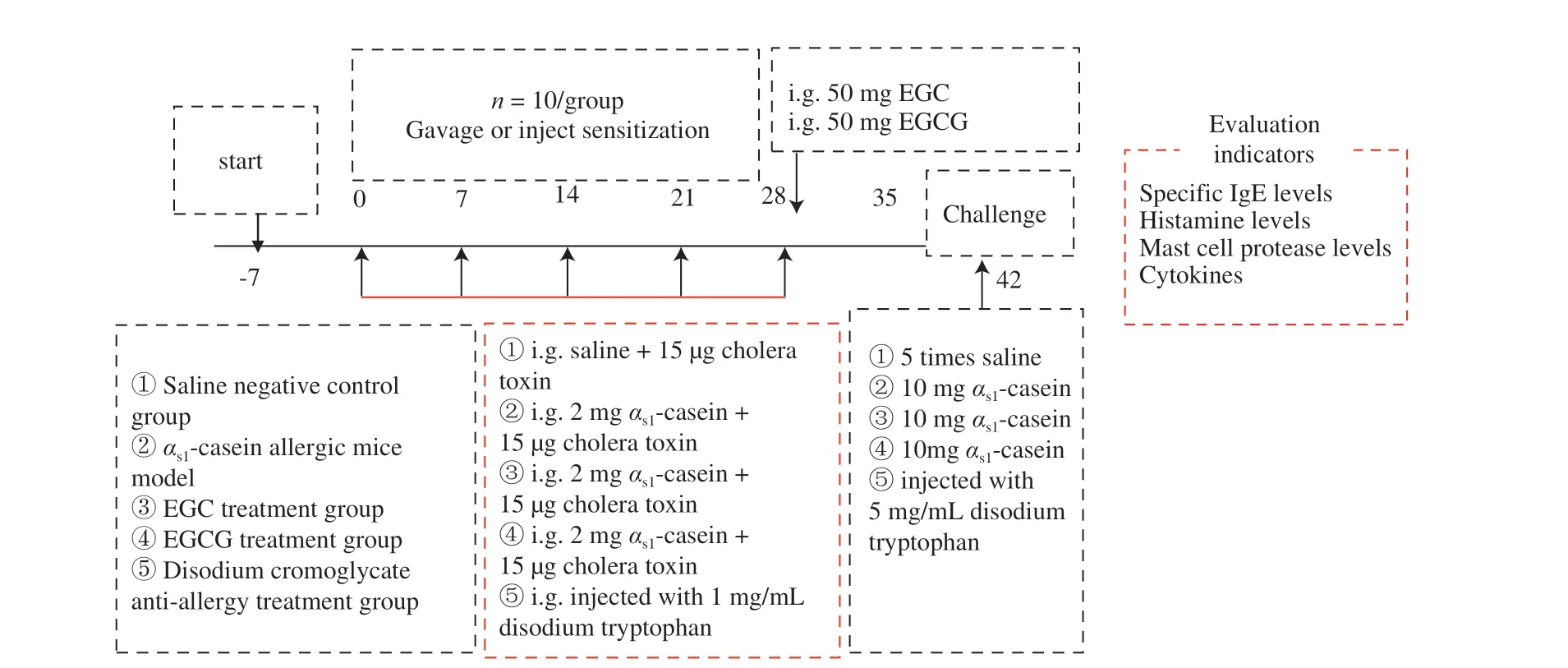
Fig. 1 EGC and EGCG interfere with the αs1-casein sensitized mice model.
The change in body temperature was measured before and after stimulation [20]. On the last day of the experiment, blood was collected from the medial canthus vein of each group, put into centrifuge tubes with or without EDTAK2 (Shanghai Yuanye Biotechnology Co., Ltd.), and gently mixed. Samples were kept overnight at 4 °C and centrifuged for 10 min at 5 000 r/min at 4 °C on the next day to separate serum or blood plasma which were then stored separately at -20 °C. The serum was used to determine levels of specific IgE antibodies, histamine release levels, and mast cell protease levels.
2.2 Determination of MCP-1 levels
An enzyme-linked immunosorbent assay (ELISA) kit was used according to manufacturers’ instructions [21].
2.3 Histamine release
A histamine kit was used according to manufacturers’instructions [21].
2.4 Determination of specific antibody IgE levels
An indirect ELISA based on a previously published method was developed and optimized to detect specific IgE levels [22].
(1) Antigen encapsulation:αs1-casein (Sigma Aldrich (Shanghai)Trading Co., Ltd.) diluted to 10 µg/mL in carbonate pH 9.6 was used as the encapsulation antigen, and 100 µL/well was added to the enzyme label plate. The antigen was incubated for 12 h at 4 °C.
(2) Washing: The encapsulation liquid was poured off and the plate was washed three times with PBST (0.02 mol/L phosphate buffer at pH 7.5 with 0.1% Tween-20) at 250 μL/well with constant temperature and agitation for 3 min. After each wash, the liquid was poured off and the plate tapped several times on absorbent paper until no obvious drops of liquid were seen in the wells.
(3) Sealing: Each well was sealed with 150 µL PBST with 1%bovine serum albumin (BSA, Sigma Aldrich (Shanghai) Trading Co., Ltd.) and incubated at 37 °C for 1 h, after which the liquid was removed and the wells washed with PBST as in step (2).
(4) Primary antibody: The serum was diluted 1:1 000 with PBST with 1% BSA (antibody diluent), 100 µL was added to each well, and the plate was incubated at 37 °C for 2 h.
(5) Washing: The plate was washed 6 times with PBST, 250 µL/well,3 min per wash. After each wash, the liquid was poured off and the plate tapped several times on absorbent paper until no obvious drops of liquid were seen in the wells.
(6) Enzyme-labeled secondary antibody: HRP-sheep anti-mouse IgE (Beijing Friendship Zhonglian Biotechnology Co., Ltd.) was diluted 1:4 000 with antibody diluent, 100 µL was added to each well,and the plate was incubated at 37 °C for 1.5 h.
(7) Washing: The same washing protocol was used as step (5).
(8) Color development: After the final wash, 100 µL of substrate solution (10 mL of 0.1 mol/L pH 6.0 phosphate buffer, 100 µL TMB(Sigma Aldrich (Shanghai) Trading Co., Ltd.) solution (60 mg TMB dissolved in 10 mL dimethyl sulfoxide), and 15 µL 30% hydrogen peroxide was added to each well, and the plate was incubated at room temperature and in the dark for 20 min, until a blue color developed.
(9) Termination reaction: 50 µL of 2 mol/L sulfuric acid was added to each well to terminate the reaction, and the color changes from blue to yellow
(10) Determination: The optical density at 490 nm was recorded within 30 min
2.5 Determination of cytokine levels
The levels of interleukin (IL)-4, IL-5, IL-10, and interferon-γ (INF-γ)in the supernatant were all determined using an ELISA kit [22].
Lymphocytes were extracted from the spleens of mice in each group. The spleens were crushed and centrifuged at 4 °C for 5 min.The supernatant was then discarded, and 1640 complete medium (90%1640 incomplete medium and 10% fetal bovine serum, and 1% triple antibody (containing penicillin, amphotericin B and streptomycin))was added to reach a final cell concentration of 2 × 105cells/mL.Into each well of a 96 well plate, 100 µL cell suspension was added,with or without 100 µL/mLαS1-casein, with anαS1-casein well used as the positive control. After 72 h of continuous culture in a CO2cell incubator (Haier Biomedical Co., Ltd.), the cell supernatant was collected and the levels of cytokines IL-4, IL-5, IL-10 and IFN-γ were determined.
2.6 Histopathological observation
After a large stimulation dose, mice were sacrificed by cervical dislocation. The jejunum, thymus, spleen, and lung were dissected and fixed in formalin solution. Paraffin-embedded sectioning was performed and routine HE staining was used to observe whether each organ was diseased [23].
2.7 Statistical analysis of data
In each group of experiments, a blank control and three parallel experiments were performed which were repeated thrice. Microsoft Excel 2010 software was used for chart making, and SPSS 17.0 was used for data analysis.
3. Results
3.1 Body temperature changes
Compared with the negative control group, the body temperature of mice in other groups decreased significantly, but the decrease in the EGC, EGCG, and drug groups was less than that of theαs1-casein model group, indicating that they might inhibit allergic reaction to some extent. There were no significant differences between the four groups (P> 0.05) (Fig. 2).

Fig. 2 Changes in body temperature of mice in each mice group.
3.2 MCP-1 levels
The levels of MCP-1 in the plasma of the EGC and EGCG groups were significantly lower than that of theαs1-casein allergic model group, which suggests that EGC and EGCG could inhibit the release of mast cell degranulation in food-allergic mice (P< 0.05) (Fig. 3).Compared with the allergy model mice, the level of MCP-1 in the plasma of mice in the EGC and EGCG groups decreased by 13.64%and 24.56%, respectively. Compared with the drug control group, the MCP-1 level was significantly higher in the EGC group (P< 0.05),and while it was also higher in the EGCG group, the difference was not significant.

Fig. 3 MCP-1 levels in plasma of each mice group.
3.3 Assessment of histamine release
Histamine levels in the plasma of EGC and EGCG group mice were significantly lower than that of the allergy model group(P< 0.05). Compared to allergy model mice, histamine content in the EGC and EGCG groups was reduced by 21.10% and 32.93%,respectively. Compared with the drug control group, histamine levels were significantly higher in the EGC group (P< 0.05) and slightly,but not significantly higher in the EGCG group (P> 0.05) (Fig. 4).
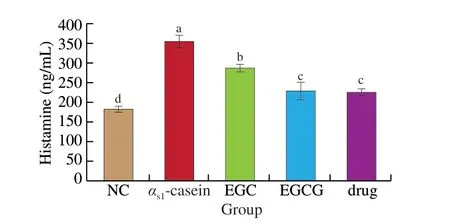
Fig. 4 The histamine content of plasma from each mice group.
3.4 Determination of specific IgE levels
The optical density value of the experimental group was 2.1 times greater than that of the negative control group, which was positive. The OD450nmof specific antibody IgE in the EGC and EGCG groups was significantly lower than that in the allergy model group.Compared to the allergy model group, the serum-specific IgE level in the EGC and EGCG groups decreased by 26.13% and 35.44%,respectively. Compared to the drug control group, the serum-specific IgE level was significantly higher in the EGC group (P< 0.05), and slightly, but not significantly higher in the EGCG group (P> 0.05),shown in Fig. 5.

Fig. 5 Specific IgE levels in sera of each mice group.
3.5 Determination of cytokine levels
Compared to the allergy model group, the IL-4 levels in the EGC and EGCG groups were significantly lower, indicating that both could inhibit the release of cytokine IL-4 inαs1-casein allergic mice. The IL-4 levels in the EGC, EGCG, and drug groups were reduced by 14.98%, 29.63%, and 29.98%, respectively. Compared to the drug control group, the IL-4 level was significantly higher in the EGC group (P< 0.05) and slightly, but not significantly higher in the EGCG group (P> 0.05). This shows that the inhibitory effect of EGCG on the release of cytokine IL-4 in allergic mice is like that of disodium cromoglycate (Fig. 6).

Fig. 6 IL-4 levels in each mice group.
Compared to the allergy model group, the IL-10 levels in the EGC and EGCG groups were significantly lower, indicating that they could inhibit the release of cytokine IL-10 inαs1-casein allergic mice.The IL-10 level in the EGC, EGCG, and drug groups decreased by 28.30%, 21.49%, and 18.31%, respectively. Compared to the drug control group, the IL-10 level was significantly lower in the EGC group (P< 0.05), and slightly, but not significantly lower in the EGCG group (P> 0.05), indicating that the inhibitory effect of EGC and EGCG on the release of cytokine IL-10 in allergic mice was slightly higher than that of disodium cromoglycate (Fig. 7).

Fig. 7 IL-10 levels in each mice group.
Compared to the allergy model group, IL-5 levels in the EGC and EGCG groups were not significantly lower. The content of IL-5 in the EGC, EGCG, and drug groups was slightly, but not significantly lower (P> 0.05). Compared to the drug group, the content of IL-5 in the EGC and EGCG groups was slightly, but not significantly higher(P> 0.05) (Fig. 8).

Fig. 8 IL-5 levels in each mice group.
Compared with the allergic model group, IFN-γ levels increased slightly, but not significantly in the EGC, EGCG, and drug groups(P> 0.05). Compared to the drug group, IFN-γ levels in the EGC and EGCG groups were not significantly different (P> 0.05) (Fig. 9).
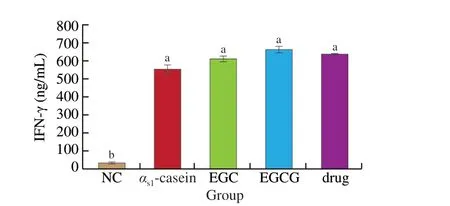
Fig. 9 IFN-γ levels in each mice group.
3.6 Histopathological observation

Fig. 10 Pathological sections of the lungs, spleen, thymus, and small intestine of mice from each treatment group.
As shown in Fig. 10, pathological observation showed that in the negative control (NC) group, all organs had normal morphology.Comparing the pathological sections of the mice from each group, it can be seen that all treatments produced local alveolar septal collapse and compensatory alveolar expansion, but the degree of alveolar lesions in the EGC, EGCG, and drug groups was lower than that seen in theαs1-casein allergic model. Lymphoid foci were seen in the marginal sinus of the spleen in EGC, drug andαs1-casein allergic model groups, but almost no lymphoid foci in spleen in EGCG group.Lymphoid foci were seen in the thymic medulla of mice in the EGC andαs1-casein allergic model groups, but almost no lymphoid foci in thymic medulla in EGCG group and drug group. Inflammatory cells had infiltrated the intestinal mucosal stroma of mice in the EGC,EGCG, drug andαs1-casein allergic model groups.
4. Discussion
Food sensitization is a cascade of related allergies. After an allergen enters the body, it is internalized by antigen-presenting cells, processed into peptides, then presented to T cells. T cells can regulate the response of several immune cells, including B cells and T helper lymphocytes (Th1, Th2). Th2 type cells secrete IL-4, IL-5,IL-10, and other cytokines, which indirectly promote the immune response. Th1 type cells mainly secrete IFN-γ which inhibits the Th2 allergic reaction. Both cell types can restrict each other and balance between the two is a basic part of immune regulation [24]. When the balance between the subsets is disrupted, Th1 cells become Th2 cells,resulting in an increase in specific IgE antibodies (which promotes anaphylaxis). The secretion of interleukins causes B cells to mature and form plasma cells that produce IgE, which binds to specific Fc+receptors present on the surface of mast cells or basophils. After a second exposure, the allergen cross-links with IgE, triggering a release of a variety of mediators and promoting the onset of allergic symptoms in the physiological environment [25].
The possible intervention point can be inferred from the allergic cascade reaction, and the results of this experiment show that EGC and EGCG have an obvious intervention effect on the sensitization process in the challenge stage. We have shown that levels of histamine, MCP-1 and specific IgE antibody in the serum of mice treated with EGC and EGCG were significantly lower than those in theαs1-casein allergic model group. In EGC or EGCG treatment groups, Th2 cytokines decreased and Th1 cytokines increased,promoting balance between Th2 and Th1 cells and reducing the occurrence of allergic reactions. Lee et al. [26] found that processed aloe gel decreased levels of Th2 cytokines and IgE in the serum of ovalbumin allergic mice, and that it had a certain inhibitory effect on the occurrence of food allergy. By comparison, we found that the decrease of serum-specific IgE antibody and Th2 cytokines in allergic mice could inhibit the allergic reaction in mice. However,the role that IFN-γ plays is still under debate. Morafo et al. [27]suggested that IFN-γ could play a protective role against the induction of allergic reactions. Studies conducted by Perrier et al. [28] showed that although high levels of IFN-γ were produced in cells isolated from spleen and mesenteric lymph nodes after allergen stimulation,IFN-γ could not inhibit strong allergic reactions in animals allergic toαs1-casein. Studies by Barbara et al. [29] also seemed to confirm that this cytokine can inhibit allergies. Whether the increase of IFN-γ content in EGC and EGCG treatment groups seen in this study is involved in the allergic intervention mechanism in the provocation phase requires further study.
In a food allergy, allergen-specific T cells migrate from the spleen to mesenteric lymph nodes, resulting in gastrointestinal inflammation. The histopathology of small intestine, lung, spleen,thymus, and other organs of the mice in each group were observed and it was found that the pathological changes in the EGC and EGCG treatment group were less pronounced than those in theαs1-casein allergic model group. Magrone et al. [30] concluded that the common features of the anti-allergic effect of polyphenols include the restoration of T regulatory/T helper 17 cell hubs and the production of the anti-inflammatory cytokine IL-10. This provides further evidence that polyphenols have certain preventive and therapeutic effects on allergic reactions.
In a previous study, the effect of tea polyphenols on the sensitization of milk allergens has been reported. Wu et al. [31]showed that the IgE-binding and IgG-binding activities ofβ-lactoglobulin were decreased upon tea catechins bindingin vitro.Pessato et al. [32] found that whey proteins-EGCG complexes obtained in both pH conditions showed a reduced IgE-binding capacity toβ-lactoglobulin and BSA, suggesting that complexation with EGCG could be a promising strategy to reduce the allergenicity of whey proteins. Furthermore, we confirmed the anti-αs1-casein allergy mechanism of two tea-derived polyphenols, EGC and EGCG by animal experiment.
In this study, the main allergenαs1-casein sensitization mice model was used to evaluate a specific polyphenol compound’s antifood allergy activity. The allergenic protein entering the body binds to the allergen-specific IgE on the surface of mast cells and basophils,causing effector cells to release allergic mediators such as histamine and mast cell protease, resulting in allergic symptoms such as ear scratching, shortness of breath, hypothermia, and convulsions, and can even lead to shock and death in mice. Animal experiments showed that the allergic reaction symptoms of mice treated with EGC and EGCG were significantly relieved, the number of erect hairs and shortness of breath decreased, and the symptoms of hypothermia were also alleviated.Thein vivoexperiments showed that while EGC and EGCG had a certain therapeutic effect on the allergic reaction ofαs1-casein sensitized mice,their preventive effect requires further verification.
5. Conclusion
EGC and EGCG, polyphenols found in tea, could significantly reduce mast cell protease, histamine, specific IgE antibody, and Th2 cytokine levels in allergic mice. Pathological results showed that they significantly reduced the degree of pathological changes in the intestine, thymus, spleen, and lung. These results can provide a theoretical basis for understanding the pathways by which tea polyphenols regulate the allergenic sensitization reactions. Future studies will aim to uncover their anti-allergic mechanism at the signal transduction and gene expression level.
Declaration of competing interest
All the authors declare no conflicts of interest.
Acknowledgments
This work was supported by the National Key Research and Development Program of China (2019YFC1605002), and the National Natural Science Foundation of China (31872886).
杂志排行
食品科学与人类健康(英文)的其它文章
- The role of probiotics in prevention and treatment of food allergy
- Roles of fermented plant-, dairy- and meat-based foods in the modulation of allergic responses
- The role of gut microbiota and its metabolites short-chain fatty acids in food allergy
- Association of nutrients intake during pregnancy with the risk of allergic disease in offspring: a meta-analysis of prospective cohort studies
- Purif ication and immunoglobulin E epitopes identif ication of low molecular weight glutenin: an allergen in Chinese wheat
- Determination of egg and milk allergen in food products by liquid chromatography-tandem mass spectrometry based on signature peptides and isotope-labeled internal standard