The amino acids differences in epitopes may promote the different allergenicity of ovomucoid derived from hen eggs and quail eggs
2023-01-23MengzhenHaoShuaiYangShiwenHanHuilianChe
Mengzhen Hao, Shuai Yang, Shiwen Han, Huilian Che*
Key Laboratory of Precision Nutrition and Food Quality, Key Laboratory of Functional Dairy, Ministry of Education,College of Food Science and Nutritional Engineering, China Agricultural University, Beijing 100083, China
Keywords:Quail egg Hen egg Ovomucoid Epitope Degranulation
A B S T R A C T Quail egg ovomucoid can inhibit activation of basophils and eosinophils, while hen egg ovomucoid has been shown to be a major allergen, named Gal d 1. At present, the differences in structure and function between two ovomucoid are unclear. We found the homology of ovomucoid in quail eggs and hen eggs reached 77%. Compared with hen egg ovomucoid, the distribution of secondary structure was different in AA52-53,AA57-58, AA66-68, AA71-72, AA131-133, AA139-140, AA157-159 and AA184-185. Among 9 epitopes of egg ovomucoid, there were different amino acids from quail egg ovomucoid in 8 epitopes. Recombination quail egg ovomucoid had trypsin inhibition activity and quail egg ovomucoid didn’t specif ically bind to serum of eggs allergic patients. Quail egg ovomucoid can significantly inhibit RBL-2H3 cells degranulation and protect cells morphology to a certain extent, indicating quail egg ovomucoid can inhibit cells activation and have potential anti-allergic effects, which is related to trypsin inhibitory activity. The difference in sensitization compare to hen egg ovomucoid may be due to amino acids differences affecting protein structure by changing antigenic epitopes.
1. Introduction
Food allergy is an immune-mediated hypersensitivity to specif ic proteins in food. In recent years, about 7%-10% of people in Western countries are suffering from food allergy [1,2]. Although prevalence of food allergy is different depended on area and diagnosis method the increasing prevalence in different are indicated food allergy has become an increasingly serious problem [3]. It is estimated that the incidence of hen egg allergy exceeds that of milk, and it is the most common immediately food allergy reactions in children in the United States and European countries [4]. Even though ovalbumin is the highest in hen egg (54%), ovomucoid (only 11%) is one of the main cause of allergy reactions [5]. Hen egg ovomucoid has thermal stability and digestive enzyme stability, resulting in some egg allergy patients also being intolerant to cooked eggs. It has been pointed out that the fragmentation of hen egg ovomucoid digested by pepsin remained antigenic, indicating that the linear epitopes on ovomucoids play a role on their antigenicity [6]. And 9 linear epitopes of hen egg ovomucoid binding to immunoglobulin E (IgE) have been identif ied with pooled sera from eight egg-allergic patients [7]. Previous studies have reported the dietary supplement consisting of proprietary blend made of quail eggs provides fast and eff icient relief of allergic rhinitis symptoms caused by the most common outdoor and indoor allergens,without adverse events [8]. Lianto et al. [9] found that quail eggs can relief the eosinophilic esophagitis diseases caused by peanut allergyin vivo, and quail eggs reduced tryptase and eosinophil cationic protein(ECP) releasing through regulating protease-activated receptors-2(PAR-2) expression. Lineweaver et al. [10] found that quail ovomucoid can act as serine protease inhibitor, which can inhibit the activation of basophils and eosinophils instead of the ovomucoid of 10 species of poultry eggs, including hen eggs [11]. However, the reason for the differences in functions of the ovomucoid in hen and quail eggs is not clear. The linear epitopes were more concentrated for hen egg ovomucoid remaining allergenic after digestion. So based on linear eptitopes for IgE in hen egg ovomucoid, comparing two sources of ovomucoid may explain the opposite functions of ovomucoid from hen and quail.
At present, for proteins that have not been reported for allergenicity, such as proteins in genetically modified crops,bioinformatics procedures are utilized to evaluate the risk of cross-reactivity with known allergen [12,13]. Bioinformatics is the comparative analysis of protein sequences intended to evaluate structural and functional relationships. Based on several publicly available database, proteins are aligned with known allergens on sequence similarity, which suggested that the two have the possibility of cross reactivity. With more and more partial sequence of protein identified by IgE in the serum from clinically allergic subjects and included in the structural database of allergenic proteins (SDAP) database, the potential epitopes on allergen and proteins are compared using bioinformatics which is more helpful than comparing with full sequence of proteins to further explore whether proteins with amino acid sequence similarity are likely to be cross-reactive with allergen [14].There is no denying that evaluating the ability of proteins binding to specific IgE from high risk clinically allergic subjects is the most intuitive way to look for cross reactivity to allergen [15].
Considering that it hasn’t been reported on allergy to ovomucoid in quail egg, the sequences of ovomucoid in quail eggs and hen eggs were compared by a variety of bioinformatics methods to align the similarity of amino acid composition and sequence. And the different amino acids between the two sequences were distributed on the 9 IgE linear epitopes located on the three-dimensional (3D)structural template of hen eggs ovomucoid. We found though the similarity of amino acids sequences between quail eggs and hen eggs ovomucoid was high, most of different amino distributed on the 9 IgE linear epitopes. And serological test performed by serum from patient allergy to hen egg evaluated there was no cross reactivity between the two proteins. Besides, in order to further study the specific components of quail eggs play an anti-allergy role, we found ovomucoid in quail eggs inhibited RBL-2H3 cells degranulation and protected morphology. All in all, our results indicated that ovomucoid in quail eggs had lower sensitization than that in hen eggs, and it has a regulatory effect on the degranulation of basophil in the stage of allergic effect.
2. Materials and methods
2.1 Production and purification of recombinant quail egg ovomucoid
The codon encoding quail egg ovomucoid (XP_015731887.1)was optimized, and was cloned into the pPIC9K vector using EcoR I and Not I restriction enzymes (Thermo Fisher Scientific, Waltham,Massachusetts, USA). Final confirmation for the gene insertion was obtained by restriction analysis of the recombinant plasmid (pPIC9Kovomucoid OVM) with EcoR I and Not I. The cloning strategy was to introduce His tag on the C-terminus. For the protein production in the prokaryotic expression system GS115 cells were kindly provided by Prof. Liang, China Agricultural University, China. A single positive colony of GS115 harboring pPIC9K-OVM construct was inoculated into 5 mL of yeast extract peptone dextrose (YPD) medium supplemented with G418 (4.0 mg/mL). Cells were cultured at 28 °C under constant shaking (250 r/min) in buffered glycerol-complex(BMGY) medium for 2-6 h. Cells were harvested by centrifugation(5 000 r/min, 5 min at 4 °C, Eppendorf centrifuge 5430 R, Hamburg,Germany), and cells were resuspended in buffered methanol-complex(BMMY) medium. Glycerinum and methanol were added to culture to 1% respectively at 28 °C for 5 days. For protein purification, culture supernatant was harvested by centrifugation (5 000 r/min, 8 min at 4 °C).The supernatant of the resulting crude extract was collected by centrifugation and fractionated by 30%-50% saturation of ammonium sulfate. The pellet was resuspended in poly (ethylene glycol)8000 (pH 8.0) and dialyzed against the same buffer. The protein solution was applied to an ion-exchange column (Q-Sepharose Fast Flow, GE Healthcare, USA), followed by gradient elution with 0.0-0.5 mol/L NaCl. Finally, the protein solution was concentrated and purified on a Ni affinity chromatography column (Ni NTA Sepharose 6FF,Solarbio, China), in accordance with the manufacturer’s instruction, at room temperature and a flow rate of 1 mL/min. Elution of the bound fraction was achieved with 20 mmol/L Tris-HCl (pH 8.0) containing 0.3 mol/L imidazole. Protein concentrations were determined by BCA protein assay kit (Cwbiotech Co., Ltd., Beijing, China).
2.2 Analysis of physicochemical properties and amino acids composition
The NCBI database was searched for complete amino acids sequence of ovomucoid in quail eggs and hen eggs. Proteins molecular weight, isoelectric point (pI), instability index and hydrophilicity were analyzed by ProtParam tool in ExPASy software. The domains of the two proteins were analyzed through Pfam database, the amino acids modification was analyzed in Uniprot database, and amino acids composition in sequence was compared using Bioedit.
2.3 Analysis of protein homology and sequence properties
The protein basic local alignment search tool (BLAST) in NCBI was used to align amino acids sequences of the two proteins,and the similarity was analyzed. The amino acids sequence of quail ovomucoid was aligned in SDAP allergen database. In the DNAStar Protean system, 4 properties of the amino acid sequence were considered as the parameters for epitope prediction, including hydrophilicity, flexibility, accessibility, and antigenicity. The hydrophilicity prediction was performed according to the methods described by Hopp et al. [16], and Kyte et al. [17]. Moreover, the properties of flexibility, surface accessibility, and antigenicity were analyzed using the methods described by Karplus et al. [18],Emini et al. [19], and Jameson et al. [20], respectively.
2.4 Advanced structural analysis of proteins and spatial positioning of amino acids
The secondary structure of the two proteins was predicted according to SOMPA method. Thus, rather accurate 3D models were built for quail egg and hen egg ovomucoid using the alignment mode in the SWISS-MODEL website of the subtilisin carlsberg: OMTKY3 Complex as a template. Furthermore, the 3D model of quail egg and hen egg ovomucoid were obtained after molecular dynamics simulation and energy minimization. The visualization software PyMol was used to view the position of the known 9 epitopes of hen egg ovomucoid included in SDAP database and the different amino acids between the two proteins in the tertiary structure.
2.5 Human sera
The collection and use of serum have been reviewed and approved by the Human Research Ethics Committee of China Agricultural University (CAU-HR2021015). Sera from 6 Chinese hen egg allergic patients with a convincing history of egg anaphylaxis were used to evaluate the ability of quail egg and hen egg ovomucoid to bind to IgE. The characteristic information of hen egg allergic patients is shown in Table S1. Blood samples were centrifuged at 3 000 ×gand 4 °C for 10 min following coagulation for 1 h at room temperature to obtain sera. The sera were stored at -80 °C before analysis. The blood collection process has been approved by the patients.
2.6 Serological test
Western blot was performed according to the method described by Guo et al. [21]. The sera of 6 egg-allergic patients were mixed in equal volumes. Proteins were subjected to 15% sodium dodecylsulphatepolyacrylamide gel electrophoresis (SDS-PAGE), and the separated proteins were transferred to polyvinylidene fluoride (PVDF)membranes (MilliporeSigma, Merck KGaA, Darmstadt, Germany).The membranes were blocked in Tris-buffered saline containing 0.1%Tween-20 (Xilong Science Co., Ltd., Shantou, Guangdong Province,China) and 5% non-fat milk powder (Inner Mongolia Yili Industrial Group Co., Ltd., Inner Mongolia Autonomous Region, China), and then incubated with sera overnight at 4 °C. Non-fat milk powder was used to block non-specific binding sites. Next, the membranes were incubated with specific peroxidase-conjugated goat anti-human IgE antibody (Sigma-Aldrich Inc., St. Louis, Missouri, USA) for 2 h at room temperature. Finally, the membranes were incubated in enhanced chemiluminescence (ECL) reagent (Pierce ECL Western blotting Substrate, Thermo Fisher Scientific Inc., Waltham,Massachusetts, USA) and then exposed and developed. The image in a chemiluminescence developer, and use ImageJ software for semi-quantitative analysis.
2.7 Evaluation of trypsin inhibitory activity
As described by Sharmila et al. [22], the method of determining the inhibitory activity of ovomucoid on trypsin is modified as follow.Grouping: standard group (without sample), quail ovomucoid group(addition of quail ovomucoid), egg ovomucoid group (addition of egg ovomucoid), inhibitor group (addition of trypsin inhibitor) and the blank groups corresponding to standard group, each sample group and inhibitor group respectively. Hen egg ovomucoid was purchased from Sigma-Aldrich (St. Louis, MO, USA).
Standard group: 0.5 mLNα-benzoyl-L-arginine 4-nitroanilide hydrochloride (BAPNA, Sigma-Aldrich, St. Louis, MO, USA),0.3 mL distilled water; each sample group: 0.5 mL BAPNA, 0.2 mL distilled water and 0.1 mL sample; inhibitor group: 0.5 mL BAPNA,0.2 mL distilled water and 0.1 mL inhibitor. Blank group: add 0.1 mL of 30% acetic acid on the basis of adding the same components to the standard group, sample group and inhibitor group, respectively.
Mix solution of each group and incubate at 37 °C for 10 min; add 0.1 mL trypsin solution (0.1 mg/mL) to each tube, mix and incubate for 10 min; add 0.1 mL acetic acid to the standard group, sample group and inhibitor group, and measure the absorbance at 410 nm.The calculation formula of the inhibition rate is as follows:

In the formula:iis inhibition rate;Aris absorbance of standard group;Abris absorbance of standard blank solution;Asis absorbance of sample or inhibitor solution;Absis absorbance of sample or inhibitor blank solution.
2.8 Cells proliferation assay
RBL-2H3 cells were derived from the National Experimental Cell Resource Platform (Beijing, China). Cells were cultured in Eagle’s minimum essential medium (MEM) containing 15% fetal bovine serum (FBS) and 1% penicillin/streptomycin/amphotericin B, and incubated at 37 °C in a humidified atmosphere with 5% CO2.
The effects of ovomucoid on cells proliferation were determined by CCK8 assay. RBL-2H3 cells at an initial density of 1 × 104cells/well were seeded into the 96-well plates. After 12 h, cells were treated with 100 µL quail or hen egg ovomucoid (100, 250, 500 and 1 000 µg/mL, respectively) for 2 h, then 10 µL CCK8 (Sigma, St.Louis, MO, USA) was added and incubated for 2 h. The absorbance of each well was measured at 450 nm with a microplate reader.Control group was added with 100 µL of MEM basic medium and 10 µL CCK8.
2.9 β-Hexosaminidase release assay
RBL-2H3 cells at an initial density of 5 × 104cells/well were seeded into the 96-well plates. After 12 h, cells were incubated with 100 µL C48/80 (0, 40, 80, 100, 120 µg/mL, respectively, dissolved in Tyrode’s buffer; Sigma, St. Louis, MO, USA) for 2 h at 37 °C.After placing on ice for 15 min, 30 µL of supernatant was collected and transferred to a 96-well plate and incubated with 50 µL ofp-nitrophenyl-N-acetyl-β-D-glucosaminide (1.3 mg/mL in 0.1 mol/L citric acid buffer, pH 4.5, Merck Pte. Ltd., Darmstadt, Germany) for 1 h at 37 °C. The reaction was terminated by adding 200 µL stop solution (0.1 mol/L Na2CO3/NaHCO3, pH 10.0). Each well absorbance was measured at 405 nm using a microplate reader. In addition, add 100 µL 1% Triton X-100 to the cells to determine the release ofβ-hexosaminidase, and set the release rate of Triton X-100 group as 100%. The formula of the release ofβ-hexosaminidase calculation is:

RBL-2H3 cells at an initial density of 1 × 106cells/well were seeded into the 24-well plates. After 12 h, cells were incubated with 500 µL quail ovomucoid or egg ovomucoid (0, 100, 250, 500,1 000 µg/mL, respectively, prepared from MEM basic medium)for 2 h at 37 °C, then washed with Tyrode’s buffer. Add 500 µL C48/80 (120 µg/mL, Tyrode’s buffer dilution) to each well and incubate for 2 h. After the reaction was terminated, the absorbance at 405 nm was measured, and the release rate ofβ-hexosaminidase in each group was compared. Trypsin inhibitor group (50 µg/mL) was used as control.
2.10 Cells morphometry
In cells degranulation experiment, after terminating the reaction,the supernatant was aspirated, the cells were fixed with 4%formaldehyde overnight, and the cells morphology in each group was observed under an electron microscope and photographed(magnification: 10 × 25).
2.11 Statistical analysis
All experiments were performed in triplicate and repeated at least 3 times. Results are presented as mean ± SD. Data between different treatment groups were analyzed by using the One-way analysis of variance (ANOVA) with a significance set toP< 0.05, a very significance set toP< 0.01.
3. Results
3.1 Physicochemical properties and function of ovomucoid
The complete amino acid sequences of ovomucoid in quail eggs(XP_015731887.1) and hen eggs (NP_001295423.1) were obtained from the NCBI database, and their physicochemical properties were shown in Table 1. Both proteins are composed of 210 amino acids and have similar molecular weights and pI. The instability index of quail egg ovomucoid is predicted to 40.35 by ExPASy, which is higher than that of hen egg ovomucoid. It indicated that ovomucoid from hen egg is a stable protein but that from quail egg is an unstable protein.

Table 1 Physicochemical properties of ovomucoid.
The types of amino acids in the two ovomucoid are 19, lacking Trp (Fig. 1). Compared with hen egg ovomucoid, the proportions of Glu, Thr and Tyr are increased in quail egg ovomucoid, while those of Ala and Asp and Leu are decreased. The differences in amino acids composition may affect the spatial structure of the protein or the binding site in the sequence.

Fig. 1 Analysis of amino acids composition on ovomucoid.
The amino acids modifications of the two proteins were compared through the Uniprot database. As shown in Table S2, both proteins have 9 disulfide bonds, which are at the same positions. Compared with quail ovomucoid, there is also a glycosylation site at AA93 in hen egg ovomucoid. The glycosylation sites of both proteins use asparagine as connection point to formN-glycosidic bonds.
Both ovomucoid proteins have 3 Kazal-type serine protease inhibitor domains, which are located on the similar positions (Table 2).Compared with hen egg ovomucoid, AA172-173 in quail egg ovomucoid is also a trypsin binding site.

Table 2 Comparison of two ovomucoid on Kazal-type serine protease inhibitor domain.
3.2 Homology analysis of ovomucoid in quail eggs and hen eggs
The two amino acid sequences were subjected to protein BLAST(Fig. 2), the similarity reaches 77% (162/210). Allergen prediction was performed in the SDAP allergen database, and only the allergen Gal d 1 (hen egg ovomucoid) and quail egg ovomucoid are homologous (E= 1.6 × 10-68,E< 0.01 represents homology) which showed that at the level of primary structure, quail egg ovomucoid is only homologous to hen egg ovomucoid in allergen database, but the structural differences between the two require further analysis.Although hen egg ovomucoid (Gal d 1) is known as a major allergen and is 77% similar to quail egg ovomucoid. Food allergic reactions are usually caused by the binding of allergen and antibody, and the allergenicity of homologous proteins can also be significantly affected by the difference in the epitopes bound to the antibodies. The 9 IgE epitopes of egg ovomucoid (Gal d 1) were obtained from SDAP database (Table 3). As shown in Table 3, in the 9 epitopes, 8 epitopes have the different amino acids in two proteins. The differences in amino acids composition of ovomucoid in quail eggs and hen eggs may change the original epitope or affect the spatial structure, thereby reducing the allergenicity.

Fig. 2 Homology analysis of ovomucoid from quail eggs and hen eggs. Query: hen egg ovomucoid; Sbjct: quail egg ovomucoid.
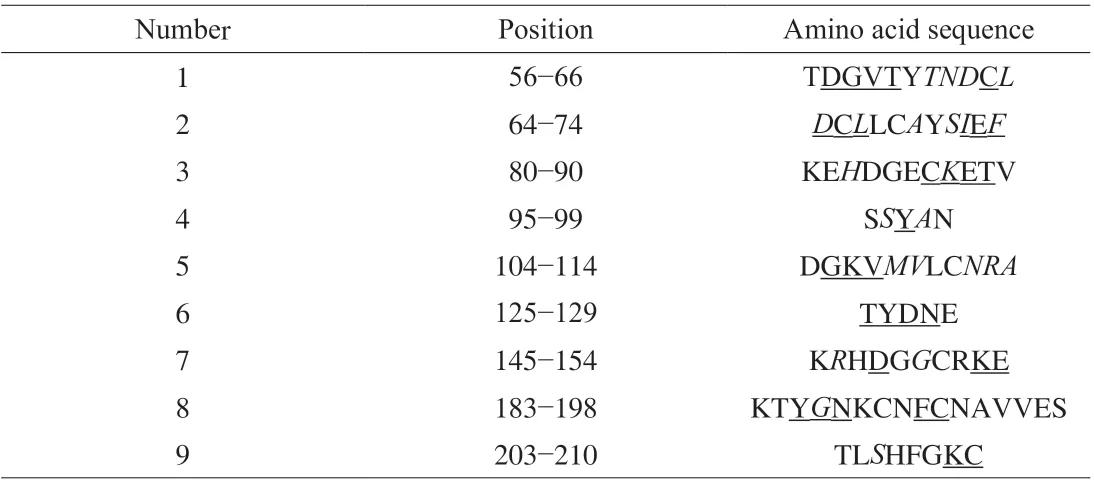
Table 3 IgE-binding fragments of ovomucoid, the allergen in eggs.
3.3 Analysis of the structure of ovomucoid
The overall hydrophobicity of the two ovomucoids is similar.The hydrophobic region is mainly distributed in signal peptide region(AA0-24), and the hydrophobicity in AA24-210 is poor (Fig. 3a, b).The hydrophilic distribution of ovomucoid in quail eggs and hen eggs is broad and similar (Fig. 3c, e). The hydrophobic region is mainly distributed in signal peptide part of the N-terminus of the sequence,which is consistent with the Kye & Doolittle analysis results. In AA45-65 and AA170-180, the plasticity of hen egg ovomucoid is higher than that of quail egg ovomucoid, and it is more prone to folding and bending. Compared with quail egg ovomucoid, hen egg ovomucoid has a higher antigen index in AA115-125 and AA130-140.

Fig. 3 Analysis of sequence properties and secondary structure of ovomucoid. Hydrophobicity analysis (a, b), scan-window size = 13; sequence analysis (c, e)and secondary structure analysis (d, f) of ovomucoid on quail (a, c, d) and hen egg (b, e, f).
Using the SOPMA method in ExPASY to predict the secondary structure of the protein, as shown in Table S3, in the two proteins,the proportions of the 4 structural forms are similar, mainly random coils, and the total proportions ofα-helix andβ-sheet are only about 30%. AA13-14, AA21-23, AA42-43, AA71-72, AA88-87 and AA145-146 in quail egg are random coil that changed fromβ-sheet.In contrast, AA25-27, AA115-118 and A157-159 in quail egg areβ-sheet changed from random coil (Fig. 3d, f). Although the ratio of each structure is similar, the structure distribution of the two proteins is different, which may be the reason for the difference in the sensitization of the two proteins.
3.4 Homologous modeling of the 3D structure of ovomucoid
2ovo.1.A and 1z7k.1.B are as similar templates for quail egg and hen egg ovomucoid respectively in SWISS-MODEL. GMQE score(0-1) reflects the accuracy of using the template to build the model.The higher the score, the higher the reliability of the model. It was found that in AA27-86, AA90-151, and AA155-210, quail egg ovomucoid and hen egg ovomucoid were constructed using 1z7k.1.B,1z7k.1.B, and 2ovo.1.A, respectively, which had the highest accuracy and basically matched the three Kazal-type serine protease inhibitor domains (AA29-86, AA94-151, AA162-210) of ovomucoid. Among them, the similarity of quail egg ovomucoid modeling in the three regions are 58.06%, 83.87%, 89.29%, and the similarity of hen egg ovomucoid modeling are 61.67%, 77.42%, 94.64%, respectively.

Fig. 3 (Continued)
There isα-helix in each area (cartoon) of ovomucoid on quail(Fig. 4) and hen eggs (Fig. 5). Use the Laplace conformation diagram to analyze the conformational rationality of ovomucoid. In Fig. 4a,in AA25-86, the amino acids coverage in allowed area reaches 95%.Only one point is located in disallowed area, two points are located in critical area, and the others are mostly located in green allowed area. In Fig. 4b, excepttwo points in AA90-151 are located in critical area, the others are all in allowable area. In Fig. 4c, only two points in AA155-210 are in critical area, and the rest are in allowable area.In Fig. 5, in (a) AA25-86, (b) AA90-151, (c) AA155-210, only two points are located in critical area, all others are located in green allowable area, in Fig. 5a, b, and c, respectively. The amino acids coverage rate reaches 97%. The results showed that the constructed 3D models of ovomucoid in quail eggs and hen eggs were reasonable and stable.
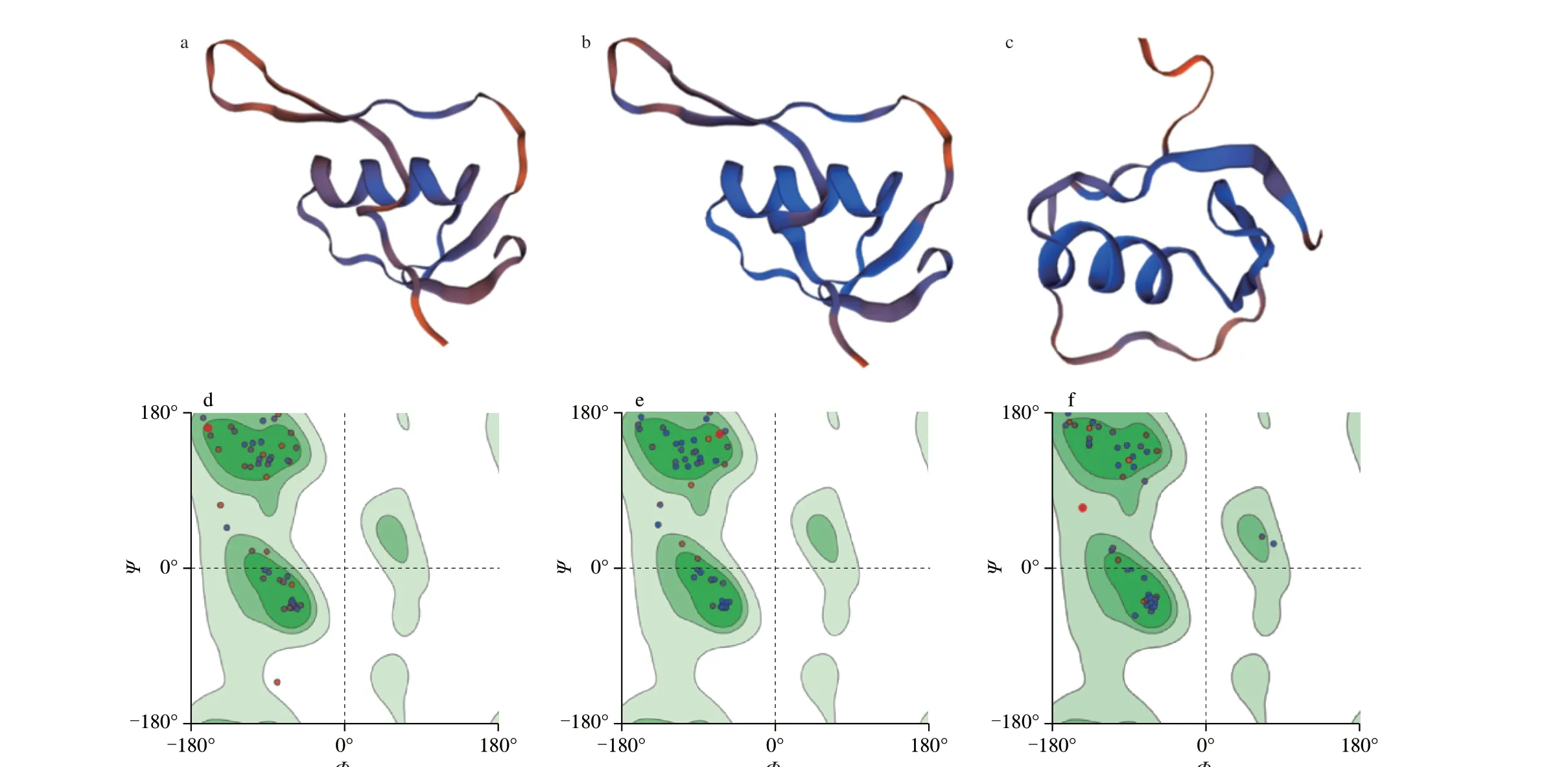
Fig. 4 3D structure and Ramachandran plot analysis of ovomucoid in quail eggs. (a-c) and (d-f) are the models and Laplace conformation diagrams of areas in AA25-86 (a, d), AA90-151 (b, e), AA155-210 (c, f), respectively.
3.5 The spatial location of different amino acids in the two ovomucoids

Fig. 5 3D structure and Ramachandran plot analysis of ovomucoid in hen eggs. (a-c) and (d-f) are the models and Laplace conformation diagrams of areas in AA25-86 (a ,d), AA90-151 (b, e), AA155-210 (c, f), respectively.
In Pymol software, locate the 9 known IgE epitopes in hen egg ovomucoid on the 3D model (Fig. 6). There were 3 epitope peptides in AA27-86 (left), which were mainly located inα-helix,β-sheet and random coil, but in the pink (α-helix) and green (random coil),there were different amino acids in the two protein. In AA90-151(middle), there were four epitopes distributed in random coils and the junction withα-helix,β-sheet andβ-turn. The different amino acids of the two proteins were located in the three epitopes ofβ-sheet,β-turn and random coil. There were two epitopes in AA155-210 (right),which were located inα-helix andβ-sheet. The different amino acids in the two were located at random coil and the junction withα-helix.Generally speaking, most of antigenic epitopes of hen egg ovomucoid were located inβ-sheet,α-helix and the junctions with random coil. However, among the 9 IgE epitopes of hen egg ovomucoid,there were 8 epitopes in the sequence containing amino acids that were different from that in sequence of quail egg ovomucoid. The differences of amino acids in epitopes and different distribution of secondary structure may affect the local 3D structure of protein and epitopes, and even affect the functions of protein.

Fig. 6 3D structure and amino acids localization of ovomucoid in eggs. The models from left to right are area in AA27-86, AA90-151 and AA155-210.Pink, blue, green and purple indicate epitopes. Similar amino acids in the two proteins are shown in yellow, and different amino acids are shown in red.
3.6 Trypsin inhibitory activity of ovomucoid
To evaluate the inhibitory effects of ovomucoid in quail eggs and hen eggs on trypsin, compared to control group, hen egg ovomucoid, quail egg ovomucoid and trypsin inhibitor can significantly inhibit the trypsin response (P< 0.05, Fig. 7), which indicates recombination ovomucoid of quail eggs can fold properly to form trypsin inhibitory domain.

Fig. 7 Inhibitory effect of quail egg and hen egg ovomucoid on trypsin enzyme activity. a-c means there are significant differences between the 3 groups (P < 0.05).
3.7 Potential allergenicity of quail egg ovomucoid
In order to evaluate the potential allergenicity of quail egg ovomucoid, the binding ability of ovomucoid to the serum pool of egg allergy patients was tested by Western blot (Fig. 8). Hen egg ovomucoid can bind to the serum of egg allergy patients. However, no binding of quail egg ovomucoid to the same serum had been observed, which indicated that quail egg ovomucoid was basically not allergenic.
3.8 Effects of ovomucoid on β-hexosaminidase released by RBL-2H3 cells
The effects of two ovomucoids on the activity of RBL-2H3 cells were determined (Fig. 9a). After processing RBL-2H3 cells,ovomucoid in quail eggs and hen eggs (100, 250, 500, 1 000 µg/mL)had no significant effects on cells viability, and can be used in subsequent experiments.
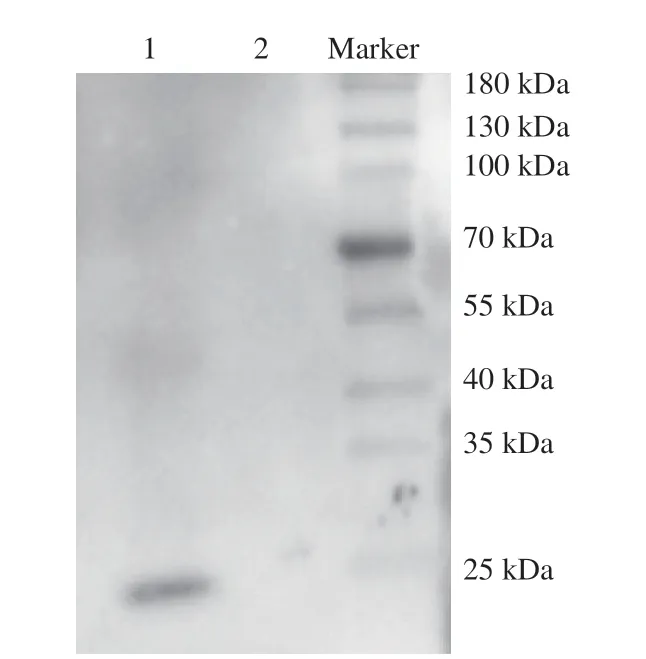
Fig. 8 Evaluation of the binding ability of ovomucoid with the serum pool of egg allergy patients. 1, egg ovomucoid; 2, quail egg ovomucoid.
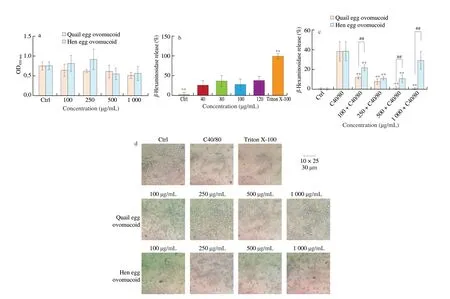
Fig. 9 Effects of ovomucoid on RBL-2H3 cells activity, degranulation and the morphology of RBL-2H3 cells. (a) Cell proliferation assay; (b) C48/80 concentration optimization, ** P < 0.01 vs. C48/80 group; (c) the effect of ovomucoid on the release rate of β-hexosaminidase,** P < 0.01 vs. C48/80 group, ##P < 0.01 vs. quail egg treatment group; (d) light microscopic view of cell morphology.
In order to determine the concentration of C48/80 that can stimulate RBL-2H3 cells to degranulate, different concentrations of C48/80 (0, 40, 80, 100, 120 µg/mL) were used to stimulate cells to degranulate (Fig. 9b). The release levels ofβ-hexosaminidase in C48/80 treatment groups at different concentrations were significantly different from that in Triton X-100 group (P< 0.01),and were significantly higher than that in control group (P< 0.01).There were no significant effects among different concentrations of C48/80 groups. At 120 µg/mL, the release rate ofβ-hexosaminidase in cells was the highest, reaching 38%. In order to determine the effects of ovomucoid in quail eggs and hen eggs on allergic reactions, 120 µg/mL C48/80 was used to stimulate RBL-2H3 cells.Quail egg ovomucoid significantly inhibited RBL-2H3 releasingβ-hexosaminidase in dose-dependent from 100 to 1 000 µg/mL(P< 0.01). When the concentration of quail egg ovomucoid was 1 000 µg/mL, the inhibitory rate ofβ-hexosaminidase was better than that in trypsin inhibitor group, indicating that different concentrations of quail egg ovomucoid can inhibit RBL-2H3 cells degranulation.Low concentration of hen egg ovomucoid (100, 250, 500 µg/mL) can significantly inhibit the release ofβ-hexosaminidase (P< 0.01), but it had no effect on the level ofβ-hexosaminidase at 1 000 µg/mL (Fig. 9c).
Changes in cell morphology with different treatment were consistent with releasing rate ofβ-hexosaminidase. After RBL-2H3 degranulation, cell morphology has changed from fusiform to circular.Quail egg ovomucoid (100, 250, 500, 1 000 µg/mL) can protect RBL-2H3 cells morphology, and as the concentration increased,the proportion of fusiform cells increased significantly, which was consistent with the results of degranulation experiment. Hen egg ovomucoid did not show a significant protective effect on cell morphology.
4. Discussion
Allergy is a global health problem that can cause rhinitis, asthma,urticaria, vasodilation, diarrhea and even life-threatening [23]. Eggs are one of the common foods that induce children’s allergies. Hen egg ovomucoid has heat stability and digestive enzyme stability, which has high tolerance to heat, high concentrations of urea and organic solvents in neutral and slightly acidic solutions [24]. Hen egg ovomucoid can resist the degradation of digestive enzymes and maintain a certain degree of sensitization, which was consistent with the results of ExPASy tool that hen egg ovomucoid was a stable protein.
The different sequence and structure of ovomucoid in hen and quail eggs also effect allergenicity. Although sequence of ovomucoid in quail eggs is 77% similar to that in hen eggs, among the 9 IgE epitopes of hen egg allergen ovomucoid, there are different amino acids in 8 epitopes. Gu et al. [6] also pointed out that the fragments of hen egg ovomucoid digested by pepsin still remained allergenic, which indicated that the binding of antibodies to ovomucoid was controlled by linear epitopes. The amino acid differences among the 8 IgE epitopes of egg ovomucoids may change the original epitope or affect the spatial structure, thereby reducing the allergenicity, and even causing function differences in two proteins. And we found there was no cross reactivity between ovomucoid from quail egg and hen egg by serological evaluation. These further indicated that quail ovomucoid is not potentially allergenic due to different amino acid sequence in 9 epitopes of hen egg ovomucoid. Synthetic peptides or site-directed mutation of amino acids can be used to study the effect of different amino acid on the two ovomucoids on their structure, allergenicity and bioactivity.Besides, we found the glycosylation sites of ovomucoid from two type eggs were different. Ovomucoid is highly glycosylated, and its glycosyl component makes it quite stable to trypsin degradation and heat treatment. In addition to the 4 glycosylation sites in ovomucoid,hen egg ovomucoid had another site at AA93. Whether glycosylation of ovomucoid affects the structure and properties of protein is unclear. Some researchers have studied the effect of carbohydrate chain of ovomucoid on its allergenicity. It has reported that the glycan on an allergen is crucial for IgE binding, but not for leading to allergy symptoms [25]. The potential effect of carbohydrates on the allergenicity of egg protein is still one of the focuses of debate. In our study, it has not been found that increased glycosylation sites improved specificity IgE binding to ovomucoid in quail eggs.
Both quail egg ovomucoid and egg ovomucoid had 3 Kazal-type serine protease inhibitor domains, which were in the similar positions,and were connected to each other by intermolecular disulfide bonds,expressing as a globulin molecule [26]. The trypsin binding site in hen egg ovomucoid was at AA89-90 of the second domains, while AA89-90 and AA172-173 of the second and third domains respectively in quail egg ovomucoid were the trypsin binding sites, respectively. It has been reported that the first and third domains of hen egg ovomucoid were relatively ineffective inhibitors of several serine proteinases [27].In this study, the quail egg and hen egg ovomucoid significantly inhibited trypsin reaction. Studies have shown that hen egg ovomucoid had strong inhibitory effects on porcine and cattle trypsin, but had no inhibitory effects on chymotrypsin [27]. Feeney et al. [11] found that ovomucoid in quail eggs had effects of resisting trypsin-like serine proteases, while ovomucoid in hen eggs had no effects on the protease activity of serine protease family. At present, more than 100 Kazal-type protease inhibitors have been discovered, but their specific structures and functions have not been fully studied. Mainly due to the cruel evolutionary pressure of protease inhibitors, their active sites are highly variable [28], resulting in the diversity of protease inhibitor functions and even some inhibitors have lost their original functions.
Serine protease has an important effect on promoting allergic reactions. In challenging of allergy, mast cells and basophil degranulate to release serine protease, such as trypsin, chymotrypsin and carboxypeptidase A [29], which can bind to PAR2 to cause mast cells and basophil further degranulation resulting in local inflammation on airway and skin [30]. So as serine protease inhibitorin vitro, whether ovomucoid from hen and quail eggs can inhibit mast cells and basophil degranulation has been further explored.
Mast cells and basophils are main effector cells in allergy so RBL-2H3 is generally regarded as a convenient and reliable model for allergic reaction research. RBL-2H3 cells can respond to many different stimulations (such as cytokines, bacterial products,neuropeptides, venom components, C48/80, etc.) and participate in various physiological and pathological processes [31]. A number of clinical studies have shown that quail eggs orally can alleviate symptoms of allergic asthma and rhinitis [8]. The United States granted patent US2015/0057232A1 in 2015, indicating the role of quail eggs in regulating immune cell functions (especially eosinophils and neutrophils) [32]. In this study, quail egg ovomucoid can inhibit the RBL-2H3 releasingβ-hexosaminidase in dose-dependent.With the increase of the concentration of quail egg ovomucoid,the morphology of RBL-2H3 cells was getting closer and closer to fusiform shape, indicating that quail egg ovomucoid has the potential of anti-allergic effects. Hen egg ovomucoid (1 000 μg/mL) had no effects on degranulation of cells, and egg ovomucoid at different concentrations did not significantly change cells morphology. Lianto et al. [9] found that quail eggs can significantly reduce the activation of PAR-2 receptors, inhibit phosphorylated nuclear factor κB (NF-κB)downstream signals, and can also inhibit the polarization of Th2, ILC2 and NKT cells and their cytokine levels to Inhibit allergic inflammatory processes. In the follow-up work, we can also analyze the effects of quail egg ovomucoid on PAR-2 receptor, Ca2+mobilization, NF-κB signaling pathway and other aspects through animal experiments, and further clarify the role of quail egg ovomucoid in anti-allergic
5. Conclusion
All in all, this study shows that the amino acid differences between quail egg and hen egg ovomucoid mainly exist in the IgE epitopes of egg ovomucoid. The difference in the secondary structure of the two may cause changes in the spatial structure.Recombinant quail egg ovomucoid obtained from system of GS115 Pichia had trypsin inhibitory activity. And no IgE cross-reactivity was found between recombinant quail egg ovomucoid and hen egg ovomucoid. Recombinant quail egg ovomucoid inhibiting the degranulation of RBL-2H3 cells protecting cells morphology, have potential therapeutic effects on allergic diseases, which enrich the nutritional and functional properties of quail eggs, and also promotes the prevention and treatment of allergic diseases. For people who are allergic to hen egg ovomucoid, quail eggs maybe a good nutritional substitute for hen eggs.
Conflicts of interest
None of the authors has a conflict of interest in relation to this work.
Acknowledgments
This study was supported by the Beijing Municipal Natural Science Foundation of China (7202100).
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://doi.org/10.1016/j.fshw.2022.09.028.
杂志排行
食品科学与人类健康(英文)的其它文章
- The role of probiotics in prevention and treatment of food allergy
- Roles of fermented plant-, dairy- and meat-based foods in the modulation of allergic responses
- The role of gut microbiota and its metabolites short-chain fatty acids in food allergy
- Association of nutrients intake during pregnancy with the risk of allergic disease in offspring: a meta-analysis of prospective cohort studies
- Purif ication and immunoglobulin E epitopes identif ication of low molecular weight glutenin: an allergen in Chinese wheat
- Determination of egg and milk allergen in food products by liquid chromatography-tandem mass spectrometry based on signature peptides and isotope-labeled internal standard