Porphyra haitanensis polysaccharide (PH) attenuates cell hyperplasia via remodeling the cross-talk between Hippo/YAP and mTOR pathways
2023-01-21ChongWngWngleiLinZhihuSunYimingSunYnoWngLinglinFu
Chong Wng,Wnglei Lin,Zhihu Sun,Yiming Sun,Yno Wng,Linglin Fu,*
a Food Safety Key Laboratory of Zhejiang Province,School of Food Science and Biotechnology,Zhejiang Gongshang University,Hangzhou 310018,China
b The Aff iliated High School to Hangzhou Normal University,Hangzhou 310030,China
Keywords:Porphyra haitanensis Polysaccharide Hippo mTOR Cancer
ABSTRACT Porphyra polysaccharide is a special kind of nutrient showing multiple physiological functions including regulating cell proliferation,but the detailed mechanisms are not fully revealed,impairing its further development and applications.This work was to purify and characterize the Porphyra haitanensis polysaccharide (PH),investigate its physiological function,and demonstrate the underlying mechanisms.The purified PH was f irst characterized by Fourier-transform infrared spectroscopy.Then an intestinal epithelial cell model was established,in which PH significantly suppressed cell hyperplasia.Specifically,PH activated the Hippo/YAP pathway,which subsequently activated mTOR pathway,however mTOR activated Hippo in the absence of PH.Moreover,both the inhibition of Hippo by YAP1 knock-down and the suppression of mTOR by rapamycin impaired PH function.These results indicated that PH attenuated hyperplasia activity by remodeling the cross-talk between Hippo/YAP and mTOR pathways,which revealed potential targets and approaches for treating hyperplasia-related diseases and provided novel ways to utilize P.haitanensis as well as other related functional foods.
1.Introduction
As an abundant source of proteins,polysaccharides,vitamins and minerals,the red algal genusPorphyrais an important food in many parts of the world and thus has become an important economic alga [1-4].Porphyrais widely distributed in eastern Asian countries and has been widely used in folk medicine for treating various diseases [5].Modern research also revealed the diverse physiological functions ofPorphyrasuch as anti-cancer,anti-oxidation,and immune-regulation activity [6,7].
Polysaccharide is a special kind of nutrient inPorphyrathat provides significant physiological activities by regulating critical signaling pathways.For example,P.yezoensispolysaccharide has been demonstrated to inhibited MCF-7 cell proliferation by downregulating NF-κB and upregulating mTOR signaling pathway [8].P.yezoensisandP.haitanensispolysaccharides have been shown to modulate immune homeostasis by NF-κB-dependent immunocyte differentiation [9].However,the understanding of the detailed molecular mechanisms ofPorphyrapolysaccharide activities is still limited nowadays.
The dysregulation of crucial signaling pathways can result in uncontrolled cell growth,hyperplasia,and subsequential tumorigenesis.In recent years,many studies have found that the Hippo and mTOR signaling pathways play extremely important roles in regulating cell proliferation [10,11].The Hippo pathway inhibits cell proliferation and promotes apoptosis,the impairment of Hippo pathway may lead to several types of cancer [12].The Hippo pathway comprises a core kinase cassette that consists of a pair of related serine/threonine kinases,MST1/2 and LATS1/2,together with the adaptor protein SAV1.When the pathway is activated,these kinases are activated and phosphorylate the transcriptional co-activator YAP1 and TAZ,making them remaining in the cytoplasm and undergoing rapid degradation in proteasome,so as to suppress the expression of downstream target proliferation-promoting genes includingCTGF,Ankrd1,etc.[13,14].On the other hand,by integrating signals from nutrients,energy status,and growth factors,the mTOR pathway regulates many cellular processes,including autophagy,ribosome biogenesis,and metabolism,and is a key regulator of cell growth,proliferation and development [15].mTOR is a large protein kinase that nucleates at least two distinct multi-protein complexes,mTORC1 and mTORC2.The mTORC1 pathway regulates cell growth and development through downstream effectors such as 4EBP1,p70S6K and Cyclin D1 [16].Therefore,both Hippo and mTOR are potential targets of anti-hyperplasia therapy,and the development of novel regulating methods for the two pathways and the understanding of the detailed molecular mechanisms can facilitate the controlling of diverse kinds of cancer.
Colon cancer is the most common incident cancer and the most common cause of cancer death in the United States [17],which can initiate from the hyperplasia of intestinal epithelial cells.However,the effect ofPorphyra haitanensispolysaccharide (PH) on controlling colon cancer and the underlying molecular mechanisms is rarely reported.It was hypothesized that PH may attenuate cell hyperplasia and thus suppress cancer,which is mediated by the crucial cell signaling pathways.
Overall,this study aimed to investigate the effect and underlying molecular mechanisms of PH on suppressing cell hyperplasia and treating colon cancer.The polysaccharide was separate fromP.haitanensisand applied to an established colon cancer cell model.Furthermore,the involvement and cross-talk of Hippo and mTOR signaling pathways were revealed.These results deepened the understanding of the molecular mechanisms of colon cancer,provided potential targets and methods for cancer therapy,and facilitated the development of functional foods based onPorphyrapolysaccharide.
2.Materials and methods
2.1 Materials
P.haitanensisharvested from Dongtou,Zhejiang province(China) were purchased from a local market.Rapamycin (Rapa)was bought from Fushen Biotechnology (Shanghai,China).LATS1 and Phospho-LATS1 (Thr1079) antibodies were bought from Cell Signaling Technology (MA,USA),p70S6K,Phospho-p70S6K(PS371) antibodies were from Abcam (San Francisco,CA,USA),TAZ antibody was from Santa Cruz Biotech (CA,USA),andβ-actin antibody was from Boster Biological Technology (Wuhan,China).
2.2 Purification of PH
PH was extracted and purified fromP.haitanensisaccording to a previous report [18].In brief,95% ethanol (1:5,m/V) was first used to remove the pigments and small lipophilic molecules from crudeP.haitanensis.The residue was then extracted with 20 volumes of distilled water at 95 °C for 2 h for three cycles and concentrated three times by rotary evaporation.After that,the polysaccharide was precipitated by 3 volumes of 95% ethanol and collected by centrifuging at 800 r/min,10 min.The pellet was dissolved in distilled water and was dialyzed (3 500 Damwcut-off) against ultrapure water.The crude product was further purified by DEAEcellulose 52 chromatography and Sephadex G-100 gel filtration.The lipopolysaccharide was removed using Thermo Scientific™ PierceTMHigh Capacity Endotoxin Removal Resin (Thermo Fischer Scientific,USA) according to the instructions.The final product was collected after freeze-drying,yielding the purified polysaccharide PH.
2.3 Measurement of the chemical content of PH
The contents of carbohydrate,sulfate,uronic acid,and protein were measured by the anthrone-sulfate method [19],barium chloridegelatin method [20],sulfate-carbazole method [21],and the Lowry method [22],respectively.The monosaccharide composition was determined on a gas chromatography-mass spectrometer (Trace GC Ultra DSQII,Thermo Fisher Scientific,Waltham,MA,USA)equipped with a TR-5MS capillary column (30 m × 0.25 mm,0.25 μm) [23].
2.4 Measurement of the molecular weights of PH
The molecular weight of polysaccharide was measured using high-performance gel permeation chromatography on a Waters 1000 HPLC system,with a Waters 2414 refractive index detector [24].Dextran standards with different molecular weights (5,25,150,410,and 1 100 kDa) were used to define the standard curve.
2.5 Fourier-transform infrared spectroscopy (FTIR)
The FTIR of polysaccharide samples were recorded on a Nicolet 380 infrared spectrometer (Thermo Fisher,USA) with KBr pellets in the range of wave numbers 400-4 000 cm-1.
2.6 Cell culture
The CMT93 cells were originally obtained from American Type Culture Collection (Virginia,USA).The cells were maintained in DMEM (KeyGENBioTECH,Nanjing,China) containing 10% fetal bovine serum and 1% Penicillin-Streptomycin Solution (Sangon Biotech,Shanghai,China),and cultured at 37 °C with 5% CO2.
2.7 Cell proliferation assay
To test the proliferation ability,cells were seeded in 96-well culture plates (5 × 103cell/well).After attachment,the cells were treated with different concentrations of PH (0.2,0.4,or 0.8 mg/mL) or PBS as a negative control for 24,48,or 72 h.And then the MTT assay was performed to evaluate cell proliferation as previously described [25].
2.8 Western blot
Cells were planted in 6-well culture plates and treated with PH(0.4 mg/mL),Rapa (100 nmol/L),or PBS as indicated for 48 h.Then the cells were harvested,the total protein was isolated,and the Western blot analysis was conducted as previously reported [26].40 μg of total protein extract was loaded for each sample.
2.9 Knock-down of YAP1 gene in CMT93
The sequence of the short hairpin RNA (shRNA) targetingYAP1mRNA was obtained from MISSION®shRNA database (Sigma-Aldrich,USA) and cloned into the lentiviral knock-down vector pLKO.1-Puro (Addgene,USA).To prepare lentiviral particles,the vector was co-transfected with packaging plasmids (pLP1,pLP2,and pLP/VSVG) into HEK293T cells.Viral supernatants were harvested 48 h after transfection and applied to CMT93 with 10 μg/mL polybrene (Sigma-Aldrich,USA).24 h later,the cells were incubated with 8 μg/mL puromycin to eliminate uninfected cells.After one week of selection,the knock-down efficiency was verified by qPCR,and the cells were ready for subsequential experiments.The primers for shRNA cloning are: forward-5’-CCGGGAAGCGCTGAGTTCC GAAATCCTCGAGGATTTCGGAACTCAGCGCTTCTTTTTG-3’,reverse-5’-AATTCAAAAAGAAGCGCTGAGTTCCGAAATCCTC GAGGATTTCGGAACTCAGCGCTTC-3’.
2.10 Gene expression quantification by real-time quantitative PCR (qPCR)
Total RNA of cell samples was extracted using the E.Z.N.A.®Total RNA Kit II (Omega Bio-Tek,USA).The reverse transcription of RNA to cDNA was performed using the HiScript®II qRT Super kit (Vazyme Biotech,USA).The real-time quantitative PCR analysis was performed using the SYBR Green I kit (Vazyme Biotech,USA)with the LightCycler®480 II system (Roche,USA).The expression ofGADPHserved as the internal standard for calibration.The qPCR primers are listed in Table 1.
2.11 Statistical analysis
All the statistical results were analyzed with three replicates according to a completely randomized design.All data were performed by analysis of variance (ANOVA) or Student’st-test.Data were analyzed statistically by repeated measures using GraphPad Prism software,andP<0.05 was considered statistically significant.
3.Results
3.1 Purification and characterization of PH
To investigate the detailed physiological activity of PH,PH sample was extracted and purified according to a previous report [18],which includes ethanol precipitation,DEAE-cellulose 52 chromatography,and Sephadex G-100 gel filtration.To characterize the produced PH,conventional characterization approaches were used to determine the chemical content,high-performance gel permeation chromatography was used to measure the peak molecular weight,and gas chromatography-mass spectrometer was used to quantify the monosaccharide composition (Table 2).

Table 1 Primers used for qPCR analysis.

Table 2 Chemical characterization of PH.
Furthermore,the structure of PH was measured by FTIR spectra.As shown in Fig.1,PH showed special absorption bands in the 400-4 000 cm-1region.Specifically,the broad and strong peak at 3 425 cm-1was assigned to the stretching vibration of OH groups,and the weaker peak approximately appeared at 2 926 cm-1indicated C-H stretching and bending vibrations,which was consistent with the characteristic peaks of common polysaccharides [23,27].Additionally,while the absorbance peak at 1 654 cm-1was caused by water absorption,the band at approximately 1 075 cm-1was assigned to C-C and C-O vibrations,and the 1 255 cm-1peak indicated the presence of sulfate [28,29].

Fig.1 FTIR spectra of PH.
3.2 PH inhibited intestinal epithelial cell hyperplasia
To evaluate the anti-hyperplasia activity of PH,we established anin vitrocarcinoma model based on the mouse colon cancer cell line CMT93.Cultured CMT93 cells were stimulated by PH with a concentration up to 0.8 mg/mL for 24 to 72 h,thereafter the proliferation of CMT93 was evaluated by MTT assay (Fig.2).CMT93 kept growing throughout the progress,and PH significantly suppressed cell proliferation after 24 h.Notably,the inhibition effect was even more significant with the prolongation of treatment time.Moreover,PH also showed a dose-dependent manner in suppressing CMT93 proliferation,particularly,0.8 mg/mL PH showed approximately twice the inhibitory effect of 0.2 mg/mL.

Fig.2 PH inhibited CMT93 proliferation.CMT93 cells were treated with different concentration of PH (0,0.2,0.4 and 0.8 mg/mL) for 24,48,and 72 h.The proliferation of the cells was measured by MTT assay.The results were represented as mean ± SD from five replicates.The statistical difference was calculated by ANOVA.**P <0.01.
3.3 PH activated Hippo signaling pathway in intestinal epithelial cell
It has been demonstrated that Hippo signaling pathway is involved in colon cancer progression [10,30].To investigate whether Hippo pathway participates in the anti-hyperplasia activity of PH,the expressions of Hippo pathway-related genes under PH treatment were measured by qPCR.PH dose-dependently inhibited the expression ofYAP1,a core transcriptional co-activator and regulator of Hippo pathway (Fig.3A).Moreover,PH also dose-dependently inhibited the expression ofCTGFandAnkrd1,the Hippo pathway inhibited downstream target genes that participate in cancer cell proliferation and invasion [31](Figs.3B-C).When Hippo pathway is activated,the crucial kinase LATS1 keeps highly phosphorylated and the transcriptional co-activators TAZ is rapidly degraded,resulting in the inhibition of target genes [32].Western blot results showed that after PH treatment,the phosphorylation of LATS1 was increased and the protein level of TAZ was decreased (Fig.4,lane 1-2).

Fig.3 PH activated Hippo signaling pathway in CMT93.CMT93 cells were treated with different concentrations of PH (0,0.2,0.4,or 0.8 mg/mL) for 24 h,then the mRNA expression of YAP1 (A),CTGF (B),and Ankrd1 (C) were measured by qPCR.The expression of the housekeeping gene GADPH served as the internal standard for calibration.The results were represented as mean ± SD from three replicates.The statistical differences were calculated by Student’s t-test.*P <0.05,**P <0.01,***P <0.001.

Fig.4 Western blot analysis of PH activity.(A) CMT93 cells were treated with PH (0.4 mg/mL) and/or Rapa (100 nmol/L) as indicated for 48 h.An equal volume of PBS and DMSO were used as the negative control of PH and Rapa,respectively.Total cell protein was analyzed by Western blot with indicated antibodies.(B) The grayscale of protein bands was quantified by Photoshop,and the relative protein levels (grayscales compared with β-actin) were represented as mean ± SD from three replicates.
3.4 Hippo pathway mediates the anti-hyperplasia activity of PH
To further validate the direct role of Hippo pathway in PH activity,we established aYAP1knock-down CMT93 cell line (YAP1 KD) by shRNA,in which Hippo pathway was consistently activated.qPCR results verified that the transcription ofYAP1in the KD cell line decreased to less than 50% (Fig.5A),and the expressions ofCTGFandAnkrd1were also suppressed (Figs.5B-C),confirming the consistent activation of Hippo pathway.Based on this,these cells were treated by PH,and the activation of Hippo pathway in both wild type (WT) and YAP1 KD cells were assessed by related gene expression.As shown in Fig.5,while PH suppressedYAP1,CTGF,andAnkrd1expressions in WT CMT93,the decrements were not significant in YAP1 KD.Similarly,the increment of LATS1 phosphorylation and the decrement of TAZ protein by PH were also inhibited in YAP1 KD (Fig.4,lane 5-6).On the other hand,the effect of PH on cell proliferation was also assessed in the YAP1 KD cell line.Interestingly,compared with WT CMT93(Fig.2),YAP1 KD CMT93 did not show significant changes in the proliferation with PH stimulation (Fig.6),indicating that YAP1 was required in the process.

Fig.5 PH activity on Hippo signaling pathway in YAP1 KD CMT93.YAP1 KD CMT93 cells were treated with 0.8 mg/mL PH for 24 h,then the mRNA expression of YAP1 (A),CTGF (B),and Ankrd1 (C) were measured by qPCR.An equal volume of PBS was used as the negative control,and the expression of housekeeping gene GADPH served as the internal standard for calibration.The results were represented as mean ± SD from three replicates.The statistical differences were calculated by Student’s t-test.*P <0.05,**P <0.01,***P <0.001,NS: not significant.
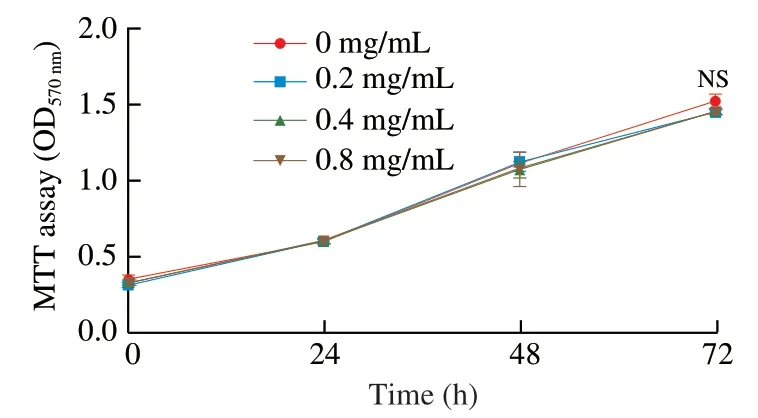
Fig.6 Hippo pathway mediates the anti-hyperplasia activity of PH.YAP1 KD CMT93 cells were treated with 0.8 mg/mL PH for 24,48,or 72 h.The proliferation of the cells was measured by MTT assay.The results were represented as mean ± SD from five replicates.The statistical difference was calculated by ANOVA.NS: not significant.
3.5 mTOR involves in the Hippo-mediated anti-hyperplasia activity of PH
mTOR is one of the most important signaling pathways regulating diverse cancer processes including colon cancer [33].We hypothesized that mTOR may also involve in the PH activity,and investigated mTOR activity after PH stimulation.Both the expression of mTOR target geneCyclin D1(Fig.7A) and the phosphorylation of the pathway crucial kinase p70S6K (Fig.4) were increased after PH treatment,and these effects can be blocked by the mTOR inhibitor Rapa,demonstrating that PH can activate mTOR.

Fig.7 mTOR involves PH activity.CMT93 cells were treated with PH (0.4 mg/mL) and/or Rapa (100 nmol/L) as indicated for 24 h.An equal volume of PBS and DMSO were used as the negative control of PH and Rapa,respectively.The mRNA expression of Cyclin D1 (A),CTGF (B),and Ankrd1 (C) were measured by qPCR.The expression of the housekeeping gene GADPH served as the internal standard for calibration.The results were represented as mean ± SD from three replicates.The statistical differences were calculated by Student’s t-test.**P <0.01,***P <0.001,NS: not significant.
Because PH regulated both Hippo and mTOR activation,we further investigated the cross-talk between the two pathways under PH treatment.As shown in Figs.7B-C,the application of Rapa significantly promoted the expression ofCTGFandAnkrd1,indicating the activation of Hippo pathway by mTOR.However,Rapa did not block the effect of PH on suppressionCTGFandAnkrd1.Consistently,Rapa directly increased TAZ protein level and inhibited LATS1 phosphorylation,but did not block the effect of PH on these proteins (Fig.4,lane 3-4).On the other hand,knock-down ofYAP1did not significantly change mTOR activity (indicated by the phosphorylation of p70S6K) in the steady-state but blocked the effect of PH on activating mTOR (Fig.4,lane 5-6).These results implied that the cross-talk between Hippo and mTOR was converted with the treatment of PH.
To further validate the role of mTOR in the anti-hyperplasia activity of PH,we blocked mTOR activity by Rapa and assessed CMT93 cell proliferation with PH treatment.As shown in Fig.8,although PH significantly suppressed CMT93 proliferation,the application of Rapa blocked this effect,indicating that mTOR was indispensable for the anti-hyperplasia activity of PHin vitro.

Fig.8 mTOR pathway mediates the anti-hyperplasia activity of PH.CMT93 cells were treated with 0.8 mg/mL PH and/or 100 nmol/L Rapa as indicated for 24,48,or 72 h.An equal volume of PBS and DMSO were used as the negative control of PH and Rapa,respectively.The proliferation of the cells was measured by MTT assay.The results were represented as mean ± SD from five replicates.The statistical differences were calculated by Student’s t-test.**P <0.01,NS: not significant.
4.Discussion
Porphyrapolysaccharides are special compounds that have various physiological functions including anti-cancer activity,but the detailed molecular mechanisms are unclear.In this study,we evaluated the activity of PH in suppressing intestinal epithelial cell hyperplasia and investigated the underlying mechanisms.We found that Hippo and mTOR,two crucial signaling pathways for tumorigenesis and progression,were associated with PH activity.
We first found that PH significantly suppressed CMT93 proliferation,suggesting that PH had strong anti-hyperplasia activity in thein vitrocell model.However,in some other previous studies,it has been shown that PH can promote the proliferation of several types of mice lymphocytes and thus maintain immune homeostasis [9,34].Therefore,PH exhibits contrary activities on different cell types.This could be due to the discrepant pathways that dominate these different cell types.In immune cells,NF-κB is a dominant pathway regulating cell proliferation,whereas in colon cancer cells,Hippo and mTOR could be more important.Consistently,previous studies have shown that NF-κB mediated the immune-regulatory activity of PH,whereas in the present study Hippo and mTOR were required for PH function.Furthermore,a robust immune system can suppress tumors,and cancer will lead to the dysregulation of immune system [35],so the overall effects of PH were consistent in these different studies.
We first applied qPCR and Western blot to assess the effect of PH on Hippo pathway.When Hippo pathway is activated,upstream kinases phosphorylate LATS1/2,which further phosphorylate YAP1 and TAZ,making them remaining in the cytoplasm and undergoing rapid degradation in proteasome [32].After PH stimulation,the decrease of TAZ protein and the phosphorylation of LATS1 were observed,indicating the Hippo pathway was activated by PH.Moreover,the expression of downstream target genesCTGFandAnkrd1were also decreased.Notably,these genes are responsible for cell proliferation,and their overexpression may result in cancer [14].So the promotion of Hippo could be associated with the antihyperplasia activity of PH.
In addition to downstream target genes,the expression of the critical regulatorYAP1was also inhibited by PH.Based on this,we hypothesized thatYAP1mediated the effect of PH on Hippo.To validate the hypothesis,we established a YAP1 KD CMT93 cell line.In this cell line,the effects of PH on Hippo pathway were all impaired.In addition,the anti-tumor activity of PH was also blocked.Therefore,these loss-of-function results all demonstrated that YAP1 was required for PH function,and Hippo/YAP pathway mediated the anti-hyperplasia activity of PH.Based on this,we suggested that Hippo/YAP could be a promising target for colon cancer therapy by PH as well as other functional components in food.
To further investigate the involvement of other pathways in PH activation,we evaluated the activation of mTOR pathway after PH treatment.mTOR is a key promoter of cell growth,development,and function,and the regulations of mTOR are widely used in cancer therapy.For example,Rapa can specifically inhibit mTOR activation and thus has been tested in treating several types of cancer [36-38].In this study,we found that Rapa significantly inhibited Hippo pathway,demonstrating that mTOR activated Hippo pathway,which has also been revealed by several other studies [39,40].However,although the background Hippo activation was decreased,Rapa did not impair the relative activation of Hippo by PH,indicating that mTOR did not participate in PH-induced Hippo activation.On the contrary,the application of PH activated mTOR pathway,however the knock-down ofYAP1did not directly regulate mTOR,but blocked the activation of mTOR by PH.Moreover,the suppression of mTOR by Rapa blocked the anti-proliferation activity of PH on CMT93,indicating that mTOR is indispensable for the anti-hyperplasia function of PH.Consistantly,the abuse of Rapa has been shown to increase the risk of specific types of cancer [37].Overall,these results demonstrated that mTOR and Hippo can activate each other,while mTOR was upstream in the steady-state,Hippo was upstream with PH treatment.Nevertheless,how the normally growth-promoting pathway mTOR facilitates the anti-growth activity of PH and how the relationship between Hippo and mTOR converted under PH treatment requires far more investigations.
5.Conclusion
In summary,this study revealed the detailed molecular mechanism of the anti-hyperplasia activity of PH (Fig.9).On the one hand,PH decreased the expression ofYAP1and thus activated Hippo pathway.Thereafter mTOR was activated due to the cross-talk between Hippo and mTOR.Both the activation of Hippo and mTOR resulted in the suppression of intestinal epithelial cell hyperplasia,and thus mediated the anti-cancer activity of PH.On the other hand,although mTOR can activate Hippo,this process did not participate in PH activity.This study elucidated the biological activity of PH on intestinal epithelial cells and demonstrated the underlying mechanisms,provided theoretical basis for the regulation of Hippo/mTOR pathway and epithelial cell proliferation and shed light on further relevant investigations.This study also revealed Hippo/mTOR pathways as the potential targets for colon cancer treatment,and provided novel ways to utilizeP.haitanensisas well as other related functional foods,which provided significant practical value in the development of functional foods and novel medicines.
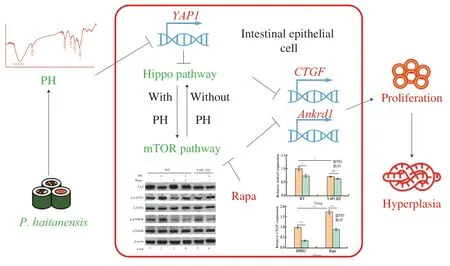
Fig.9 The molecular mechanism of the anti-hyperplasia activity of PH.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgments
This work was supported by the National Key R&D Program of China (2017YFD0400203);the National Natural Science Foundation of China (31801452);the Zhejiang Provincial Science and Technology Foundation of China (2018C02025 and 2019C02069);the Zhejiang Provincial Natural Science Foundation of China (LGN21C200013);the China Postdoctoral Science Foundation (2019M662108);and the Scientific Research Fund of Zhejiang Provincial Education Department (Y201942404).
杂志排行
食品科学与人类健康(英文)的其它文章
- Colloidal nanoparticles prepared from zein and casein:interactions,characterizations and emerging food applications
- Biological factors controlling starch digestibility in human digestive system
- Preparation methods,biological activities,and potential applications of marine algae oligosaccharides: a review
- Development of hyaluronic acid-based edible film for alleviating dry mouth
- Mushroom β-glucan and polyphenol formulations as natural immunity boosters and balancers: nature of the application
- Preparation of multicore millimeter-sized spherical alginate capsules to specifically and sustainedly release fish oil
