Effectiveness of sacubitril-varsartan versus angiotensin converting enzyme inhibitors in patients hospitalized for acute heart failure: a retrospective cohort study of the RICA registry
2022-12-20LlanosSolerRangelManuelndezBailJosrezSilvestreJosMarFernndezRodrguezBeatrizCuestaGarcAdriArgellesCurtolvaroGonzlezFrancoAliciaCondeMartelSaraCarrascosaGarcMartanchezMartelesJosManuelCerqueiroGonzlez0NoelLorenzoVil
Llanos Soler-Rangel, Manuel Méndez-Bailón, José Pérez-Silvestre, José María Fernández-Rodríguez, Beatriz Cuesta García, Adrián Argüelles-Curto, Álvaro González-Franco,Alicia Conde-Martel, Sara Carrascosa-García, Marta Sánchez-Marteles, José Manuel Cerqueiro-González0, Noel Lorenzo-Villalba, Manuel Montero-Pérez-Barquero,
1. Llanos Soler-Rangel, Servicio de Medicina Interna, Hospital Universitario Infanta Sofía, FIIB HUIS HHEN, Madrid,Spain; 2. Servicio de Medicina Interna, Hospital Clínico San Carlos, Instituto de Investigación San Carlos (IdISSC), Spain;3. Servicio de Medicina Interna, Consorcio Hospital General Universitario de Valencia, Spain; 4. Servicio de Medicina Interna, Hospital Carmen y Severo Ochoa, Cangas del Narcea, Asturias, Spain; 5. Servicio de Medicina Interna, Hospital Carmen y Severo Ochoa, Cangas del Narcea, Asturias, Spain; 6. Servicio de Medicina Interna, Hospital Carmen y Severo Ochoa, Cangas del Narcea, Asturias, Spain; 7. Servicio de Medicina Interna, Hospital Universitario Central de Asturias,Oviedo, Spain; 8. Servicio de Medicina Interna, Hospital Universitario de Gran Canaria Dr Negrín, Spain; 9. Servicio de Medicina Interna, Hospital Clínico Universitario Lozano Blesa de Zaragoza, Zaragoza, Spain; 10. Hospital Universitario Lucus Augusti de Lugo, Lugo, Spain; 11. Service de Médecine Interne, Diabète et Maladies Métaboliques, Hôpitaux Universitaires de Strasbourg, France; 12. Servicio de Medicina Interna, IMIBIC/Hospital Universitario Reina Sofía, Córdoba, Spain
ABSTRACT BACKGROUND Sacubitril-valsartan has been shown to reduce hospitalizations and mortality in patients with heart failure(HF) and reduced ejection fraction. The PIONEER-HF trial demonstrated that initiation of the drug during acute HF hospitalization reduced NT-proBNP levels and a post-hoc analysis of the trial found a reduction in HF hospitalizations and deaths. Real-life studies in the elderly population are scarce. The aim of our study was to assess the effectiveness of sacubitril-valsartan versus angiotensin converting enzyme inhibitors (ACEI) in elderly patients who initiate this treatment during hospitalization for acute HF. METHODS We conducted a retrospective cohort study using the Spanish acute heart failure registry (RICA) comparing rehospitalizations and deaths at 3 months and 1 year among patients aged 70 years or older who had initiated treatment with sacubitrilvalsartan during hospitalization for acute HF versus those treated with ACEI. RESULTS One hundred and ninety-nine patients hospitalized between October 2016 and November 2020 were included, with a median age of 82 years and high rate of comorbidity. Of these, 107 were treated with sacubitril-valsartan and 92 with ACEI. The adjusted OR for readmission for HF at 3 months was 0.906 (95% CI: 0.241-3.404) and for the combined variable readmission for HF or death at 3 months was 0.696 (95% CI: 0.224-2.167). The adjusted OR for HF readmission at one year was 0.696 (95% CI:0.224 -2.167). and for the combined variable HF readmission or death at one year 0.724 (95% CI: 0.325-1.612). CONCLUSION Treatment with sacubitril-valsartan initiated early in hospitalization for HF in elderly patients with high comorbidity was associated with a trend towards a reduction in readmissions and death due to HF compared to treatment with ACEI, which did not reach statistical significance either at 3 months or 1 year of follow-up.
Heart failure (HF) is a very prevalent disease, affecting more than 10% of people over 70 years of age and is the main cause of admission to internal medicine wards.[1]Of these,20% to 50% have reduced left ventricular ejection fraction (LVEF).[2]After admission for acute heart failure (AHF) in this group of patients in Spain, the rate of readmissions per year for new episodes of HF is 30%-50% and the rate of mortality due to HF is 10%-20%.[3-5]
Sacubitril-valsartan is the first drug available with a compound mechanism of angiotensin receptor and neprilysin inhibition (ARNI) and has demonstrated superiority to enalapril in a composite endpoint of cardiovascular mortality and HF hospitalization.[6]According to HF treatment guidelines,[7-9]angiotensin-converting enzyme inhibitors (ACEI) or angiotensin receptor blockers 2 (ARBs) should be replaced by sacubitril-valsartan in all patients who remain symptomatic in stable conditions despite the maximum tolerated doses of these drugs.
The beneficial effect of sacubitril-valsartan is observed from the first weeks of treatment and the medication is safe to start in hospitalization once the patient is stabilized, also in those without previous treatment with ACEI or ARBs, according to the results of the PIONEER-HF clinical trial.[10]For this reason, it is currently recommended that patients admitted to the hospital for acute HF with reduced LVEF previously treated with ACEI or ARBs should be switched to sacubitril-valsartan once stable and before hospital discharge, and that those hospitalized with a new diagnosis of HF with reduced LVEF should be treated with ARNI as first-line therapy.[7,11]
However, there is concern about whether the benefit and safety profile demonstrated in these trials will be similar in patients admitted to Internal Medicine wards, as they are older, with greater comorbidity, higher prevalence of renal failure and hypotension, and consume more drugs than patients included in PARADIGM or PIONEER-HF.[12]
Therefore, we conducted a retrospective observational study with the hypothesis that initiation of treatment with sacubitril-valsartan during hospitalization for acute HF reduces rehospitalizations and mortality compared to treatment with ACEI in elderly patients admitted to internal medicine. The primary endpoint of our study was the reduction in HF hospitalizations and death at 3-month follow-up. As a secondary objective, we analyzed the reduction in HF hospitalizations and mortality at 1 year in a subgroup of patients who completed this follow-up period.
METHODS
Patients
We conducted a retrospective cohort study using the Acute Heart Failure Registry (RICA), which is a Spanish multicenter registry that includes patients discharged from hospitalization for acute HF and their follow up in a protocolized manner, including an in-person visit at 3 months and 1 year. The data are completed anonymously, through a web page (https://www.registrorica.org), and is coordinated by the working group on heart failure and atrial fibrillation of the Spanish Society of Internal Medicine(SEMI). The study protocol was approved by the Clinical Research Ethics Committee of the Hospital Universitario Reina Sofía de Córdoba and informed consent was obtained from all patients prior to inclusion. The investigators of this study adhered to the recommendations of the Declaration of Helsinki,and its subsequent modifications, for the protection of the participants.
The current study included all patients registered in the RICA as of October 1, 2016, who met the following inclusion criteria: age equal to or older than 70 years, diagnosis of HF with LVEF equal to or less than 40%, included in the RICA from an admission for acute HF, and who had started treatment with sacubitril-valsartan or ACEI upon hospitalization. Patients on treatment with sacubitril-valsartan prior to hospital admission were excluded, but not those receiving ACEI prior to hospital admission. Recruitment was completed on November 22, 2020 and the study was closed on February 22, 2021, three months after the inclusion of the last patient.
Variables
The primary outcome variables were defined as readmission for HF and the combined variable of readmission for HF and mortality at 3 months. Secondary outcome variables were HF readmission and the combined variable of HF readmission and mortality at 1 year. In addition, the following descriptive variables were collected: age, sex, medical history (hypertension, dyslipidemia, diabetes mellitus, atrial fibrillation, chronic kidney disease), HF etiology, New York Heart Association (NYHA) functional class, LVEF, blood pressure, laboratory parameters (hemoglobin, creatinine, glomerular filtration rate, potassium and NT-proBNP) and concomitant treatment for HF at discharge [diuretics, betablockers, mineralocorticoid receptor antagonists(MRAs), sodium-glucose cotransporter type 2 inhibitors SGLT2]. Potential confounding variables were considered to be baseline glomerular filtration rate and blood pressure and descriptive variables associated with sacubitril-valsartan and mortality.
Statistical Analysis
Quantitative variables are expressed as mean and standard deviation or median and interquartile range and were compared by Student'sttest or Mann-WhitneyUtest according to whether the sample distribution was normal or not. Qualitative variables are expressed as frequency and percentage.The rates of readmission for HF and death were compared between the two groups of patients using the Chi-square test. The strength of association was expressed as odds ratio (OR) with 95% confidence interval (95% CI). For this purpose, a bivariate analysis of the outcome variables between patients receiving treatment with sacubitril-valsartan or ACE inhibitors was performed, followed by a multivariate analysis including the confounding variables indicated. Possible multicollinearity effects between continuous variables that could be related were ruled out. The internal validity of this model was calibrated using the Hosmer-Lemeshow test. Statistical significance was applied with aPless than 0.05 and the SPSS 26.0 program was used.
The sample size calculation, accepting an alpha risk of 0.05 and a beta risk of 0.2 in a bilateral contrast, and estimating a loss-to-follow-up rate of 10%, indicated analyzing 122 patients treated with sacubitrilvalsartan and 240 treated with ACE inhibitors.
RESULTS
Patients
Figure 1 shows the flow diagram for patient inclusion. A total of 199 patients were included in the study, 107 in the sacubitril-valsartan arm and 92 in the ACEI arm. There were 6 and 10 missing patients, respectively, who were not included in the analysis at 3 months.
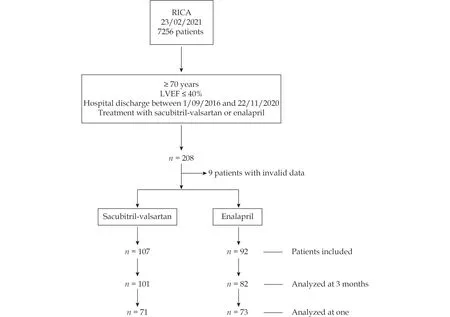
Figure 1 Flow chart. Patients included and analyzed in the study. LVEF: left ventricular ejection fraction.
In the analysis at 1 year, 71 patients could be included in the sacubitril-valsartan arm and 73 in the ACEI arm who had completed 1 year of follow-up at the time of study closure. The median follow-up was 355 days (interquartile range 150 - 393).
The baseline characteristics of the patients are shown in Table 1. We observed that in both groups the median age was 82 years and HF was associated with high comorbidity, although in the group treated with sacubitril-valsartan there was a higher prevalence of dyslipidemia, diabetes mellitus, chronic renal failure, COPD, and ischemic etiology of heart failure, as well as a higher score on the Charlson comorbidity index, and this group more frequently received treatment at discharge with betablockers, MRA, and SGLT2.
Readmissions and Mortality
Table 2 shows the rate of readmissions due to HF and the combined event of readmission due to HF or death, both at 90 days (primary endpoint) and at 1 year (secondary endpoint). Table 3 shows the ORs between sacubitril-valsartan and ACE inhibitors for the four outcome variables, as well as the results of the multivariate regression analysis, in which confounding variables were included. Only diabetes mellitus, ischemic etiology of HF, treatment at discharge with beta-blockers and treatment at discharge with SGLT2 substantially modified the ORs and are included in the final models.

Table 1 Baseline patient characteristics.

Table 2 Readmissions for heart failure and mortality at three months and at one year.

Table 3 Heart failure readmissions and mortality at three months and one year: multivariate regression analysis.
The adjusted OR for readmission due to HF at 3 months was 0.906 (95% CI: 0.241-3.404) and for the combined variable readmission due to HF or death at 3 months was 0.696 (95% CI: 0.224-2.167). The adjusted OR for readmission due to HF at one year was 0.718 (95% CI: 0.256-2.011) and for the combined variable readmission due to HF or death at one year was 0,724 (95% CI: 0.325-1.612).
DISCUSSION
The results of our study show that the introduction of sacubitril-valsartan during hospitalization for acute HF in an elderly population with high comorbidity is associated with a discrete reduction in clinical events, which did not reach statistical significance at either 3-month or 1-year follow-up.
An important limitation is that we did not reach the sample size calculated in the study design. Although it was initially planned to include twice as many patients with ACEI as with sacubitril-valsartan, we observed that the distribution between the two drugs during the study period was close to 1: 1, probably due to the progressive introduction of sacubitril-valsartan in routine clinical practice, following the recommendations of the guidelines. In addition, the outbreak of the COVID-19 pandemic made face-to-face visits difficult and even made it difficult in some cases to determine the vital status of the patients, and also slowed the rate of inclusion of new patients in the RICA.
For this reason, we decided to close the study in February 2021, having completed the predefined threemonth follow-up period of the patients included until November 2020, and we did not achieve the expected sample size, which may have detracted from the statistical power of the study.
However, we believe that our study also has strengths. To our knowledge, it is the largest study that compares both treatments in real life, both in number of patients and follow-up time, and also analyzes clinical effectiveness in readmissions and mortality. The study population consists of elderly patients with a high comorbidity burden, who are not represented in clinical trials, but who nevertheless reflect the real population admitted to internal medicine departments for HF. Furthermore, this is a multicenter study supported by the RICA, a registry that has given rise to several publications investigating various aspects of HF in Spain.[4,13-15]Therefore, we believe that our results can be generalized to the clinical practice of most hospitals in our country.
Real-life studies on the use of sacubitril-valsartan in the HF hospitalized patient are scarce. A systematic review published in 2021 by Chilbert,et al.[16]found four retrospective cohort studies, three performed in a single center, with 59, 21, and 143 patients, respectively, and one prospective and multicenter, with 100 patients.[17-20]These studies analyze achieved drug doses, discontinuation rates, and some side effects such as hypotension, and together they support the safety of the medication. However,none of them analyze clinical events or make a comparison with ACEI, as in our case.
The pivotal trial of sacubitril-valsartan, PARADIGM,was done in stable, non-hospitalized patients. The PIONEER-HF trial studied acutely ill patients, although the drug was introduced once the patient had stabilized.[10]Its primary objective was the reduction of NT-proBNP, although apost-hocanalysis found a decrease in mortality and early hospitalizations in the group treated with sacubitril-valsartan.[21]In this study, the patients had a mean age of 62 years, that is, 20 years younger than those in our study, and less comorbidity (lower frequency of atrial fibrillation, chronic renal failure, dyslipidemia,or diabetes mellitus).
A substudy of the PARADIGM trial analyzed the effect of sacubitril-valsartan in different age groups,finding that in those older than 75 years, the reduction of events associated with sacubitril-valsartan is lower and does not reach statistical significance.[22]In the LIFE clinical trial, in a population with advanced HF in functional class IV, treatment with sacubitril-valsartan compared with valsartan did not improve the combined clinical variable of survival days, days out of hospital, and freedom from HF decompensation. There were no serious side effects,but treatment had to be withdrawn due to intolerance in 20% of patients.[23]It could be that the drug does not retain its effect in the older and more frail population, or more probably that lower doses are used in this population, which may not be as effective.
In this regard, another important limitation of our study is that we do not know what doses of the drug the patients were given. However, it is likely that,as several studies have shown, many of them reached suboptimal doses. López-Azor,et al.,[20]studied a cohort of 100 patients who were started on sacubitril-valsartan in hospitalization and concluded that they reached lower doses than those patients who started treatment on an outpatient basis and also had a higher rate of treatment withdrawal.
Other studies also point out that the doses of sacubitril-valsartan used in real life are much lower than those that have shown benefit in PARADIGM.[24-26]Even in the TRANSITION trial, designed to measure drug tolerability in the setting of acute HF, only half of patients reached target doses in the first 10 weeks.[27]The study by Molina,et al.[28]highlights the high use in routine clinical practice of the sacubitril-valsartan presentation containing the lowest doses despite the fact that, according to the drug label, it is only indicated as a starting dose in specific situations, as well as the low percentage of patients treated with the target dose. The tendency to use lower doses than those in trials is common with other drugs that act on the renin-angiotensin system and can probably be explained by the fear of hypotension in an older and more complex population. It is questionable whether the efficacy described at higher doses will be maintained under these conditions.
In our study, patients treated with sacubitril-valsartan more frequently had comorbidity, specifically diabetes mellitus and chronic renal failure, two pathologies that worsen the prognosis of HF, so that from a theoretical point of view a higher readmission and mortality rate would have been expected in them than in the group treated with ACE inhibitors. However, we found a favorable trend in the group treated with sacubitril-valsartan. Several studies have shown that renal function deterioration is lower in patients treated with ARNI, and that the clinical benefits obtained with this treatment are maintained in diabetic patients.[29,30]
In our study, chronic renal failure did not modify the effect of sacubitril-valsartan, but diabetes mellitus did. Also in the sacubitril-valsartan group,ischemic etiology of HF was more frequent, and this variable modified the influence of the drug on events, so it is included in the final models. A post-hoc analysis of the PARADIGM-HF trial showed that the reduction in events in the sacubitril-valsartan arm was consistent regardless of ischemic or nonischemic etiology of HF.[31]It is also worth considering that these patients were more frequently treated with beta-blockers, MRA, and SGLT2. This may mean that the physicians more likely to use sacubitril-valsartan are more experienced or more involved in the management of HF, since sacubitrilvalsartan was chosen precisely in more severe patients or those who a priori could tolerate the drug less well, and treatment was better optimized. However, it could also be that, as some studies suggest,treatment with sacubitril-valsartan facilitates the introduction and tolerance of other HF-modifying drugs.[31]When we included treatment with these drugs in a multivariate regression model, treatment with beta-blockers and SGLT2 modified the readmission or mortality outcomes associated with sacubitril-valsartan.
Therefore, we believe that the recommendation to initiate treatment with sacubitril-valsartan on admission for acute HF should also be followed in elderly patients with high comorbidity, preferably under closer surveillance. In our opinion, and as the guidelines also recommend, these patients benefit from being included in specific HF units in which,by closely monitoring possible side effects and actively titrating, it is ensured that most of them reach the treatment doses that have been shown to be effective. Our results are consistent with what has been observed in older population with HF and comorbidities such as renal dysfunction, where pharmacological neurohormonal blockade with renin angiotensin ACE inhibitors and beta-blockers is also beneficial.[32,33,34,35]
In conclusion, treatment with sacubitril-valsartan since hospitalization for HF in elderly patients with high comorbidity was associated with a trend toward a reduction in readmissions and death due to HF compared to treatment with ACE inhibitors,which did not reach statistical significance either at 3 months or 1 year of follow-up.
杂志排行
Journal of Geriatric Cardiology的其它文章
- Screening for hypertension-mediated organ damage and aetiology: still of value after 65 years of age?
- Systemic inflammatory markers in elderly patients undergoing transcatheter aortic valve replacement
- Cost-utility analysis of transcatheter aortic valve implantation versus surgery in severe aortic stenosis patients with intermediate surgical risk in Thailand
- The relationship between serum miR-21 levels and left atrium dilation in elderly patients with essential hypertension
- Validating the accuracy of a multifunctional smartwatch sphygmomanometer to monitor blood pressure
- Small-molecule 7,8-dihydroxyflavone counteracts compensated and decompensated cardiac hypertrophy via AMPK activation
