两种铜配位聚合物的晶体结构和量子化学计算
2022-12-06付立海李秀梅
付立海 李秀梅*, 刘 博 周 实
(1通化师范学院化学学院,通化 134002)
(2吉林师范大学化学学院,四平 136000)
0 Introduction
Metallosupramolecular species built from transition metal ions and organic bridging ligands have been rapidly developed in recent years because of their fascinating structural diversities and potential applications as functional materials[1-2].The rational design and synthesis of coordination architectures(discrete and polymeric)with metal ions(or metal clusters)and organic ligands is still at an evolutionary stage with the current focus mainly on understanding the factors to determine the crystal packing[3].In addition to coordination bonding,some weak interactions,such as hydrogen bonding[4-8]and π…π stacking[9-11]interactions also greatly affect the structures of complexes,and they may link multinuclear discrete subunits or low-dimensional entities into high-dimensional supramolecular networks.In this regard,the skillful combination of bridging carboxylates,3-(2-pyridyl)pyrazole(HL)chelating/1,4-bis(imidazol-1-yl)-butane(bib)bridging ligands,and metal ions have generated many interesting coordination architectures.As bridging ligands,the geometries of different pendant carboxylate groups may play profound roles in adjusting the topologies of their transition metal complexes.Moreover,luminescent organic compounds and complexes have been an active research area for decades because of their various potential applications in material science,in which stable luminescent complexes that are useful in electroluminescent devices are still not common and still challenging to be prepared[12].
Considering all the aspects stated above,our idea is to prepare and tune these kinds of complexes by carefully selecting three kinds of ligands:(a)a chelating ligand HL,with an N—H entity as a hydrogenbonding donor;(b)two structurally related pendant carboxylate ligands 4-nitrophthalic acid(4-H2nph),and 2,2'-bipyridine-4,4'-dicarboxylic acid(H2bda)with different aromatic skeletons;and(c)one flexible bridging ligand bib.In this work,two new Cu(Ⅱ)complexes with these ligands,[Cu4(4-nph)(L)6]n(1)and{[Cu2(bda)2(bib)2(H2O)4]·4H2O}n(2),were synthesized,structurally determined by X-ray analysis and other characterization methods.
1 Experimental
1.1 Reagent and general procedures
All solvents and reagents were commercially available and used as received,or prepared by reported procedures[13].IR spectra were measured on a Tensor 27 OPUS(Bruker)FT-IR spectrometer with KBr pellets.Thermogravimetric(TG)studies were carried out on a STA7300 analyzer under nitrogen at a heating rate of 10℃·min-1.Powder X-ray diffraction(PXRD)and elemental analyses for C,N,and H were performed with a Bruker D8 Advance X-ray diffractometer(radiation source:Cu Kα,0.154 184 nm,voltage:40 kV,current:40 mA,scan range:5°-90°)and a PE 2400C elemental analyzer,respectively.
1.2 Synthesis
Complex 1 was obtained by the reaction of Cu(OAc)2·H2O,4-H2nph,HL,and NaOH in a molar ratio of 1∶1∶1∶2 mixed with 12 mL of water under hydrothermal conditions(at 150℃for 7 d and cooled to room temperature with a 5 rate of℃·h-1).The blue cubic crystals were washed with water and dried in air.Yield:31%.Anal.Calcd.for C56H38Cu4N19O6(%):C,50.68,H,2.89,N,20.05.Found(%):C,49.65,H,2.27,N,19.65.IR(KBr,cm-1):3 422w,3 092w,1 610s,1 597 s,1 568w,1 519w,1 456m,1 434w,1 400w,1 381w,1 360m,1341m,1284w,1153 s,1078w,1017w,979w,951w,918w,828w,783w,758 m,723w,687w,643w,504w,483w.
Complex 2 was obtained by the reaction of Cu(OAc)2·H2O,H2bda,bib,and NaOH in a molar ratio of 1∶1∶1∶2 mixed with 12 mL of water under hydrothermal conditions(at 140℃for 7 d and cooled to room temperature with a rate of 5 ℃·h-1).The blue cubic crystals were washed with water and dried in air.Yield:25%.Anal.Calcd.for C44H56Cu2N12O16(%):C,46.52,H,4.97,N,14.79.Found(%):C,46.25,H,4.51,N,14.55.IR(KBr,cm-1):3 385m,3 316w,1 617s,1554s,1534w,1451w,1397w,1365s,1293w,1239m,1 116w,1 099m,1 032w,848w,787m,723w,700m,668w,626w.
1.3 Structure determination
Two single crystals with dimensions of 0.12 mm×0.10 mm×0.08 mm(1)and 0.15 mm×0.13 mm×0.10 mm(2)were collected at 296(2)K(1)and 293(2)K(2)on a Bruker SMART APEX Ⅱ CCD diffractometer with Mo Kα (λ=0.071 073 nm).The structures were solved by direct methods and refined by full-matrix least-squares on F2using the SHELXTL-2014 program.All non-hydrogen atoms were refined anisotropically.All the hydrogen atoms were positioned geometrically and refined using a riding model.A summary of the crystallography data and structure refinements for 1 and 2 is given in Table 1.The selected bond lengths and angles for complexes 1 and 2 are listed in Table 2.Hydrogen bond parameters of complexes 1 and 2 are given in Table 3.

Table 1 Crystal data for complexes 1 and 2

Table 2 Selected bond distances(nm)and angles(°)for 1 and 2

Continued Table 2

Table 3 Hydrogen bond parameters for 1 and 2
CCDC:2130919,1;2097002,2.
2 Results and discussion
2.1 Structural description
The single-crystal X-ray study reveals that complex 1 consists of four divalent Cu2+ion,one 4-nph2-anion,and six L-anions,as shown in Fig.1,which also exhibits the coordination environments of Cu(Ⅱ)ions and the ligands.The Cu1 ion in 1 is five-coordinated by five N atoms from three L-anions,and the Cu2 ion is also five-coordinated by four N atoms from three L-anions and one oxygen atom from bridging 4-nph2-anions.The Cu—N bond lengths are in a range of 0.195 2(3)-0.207 9(3)nm and the Cu2—O bond distance is 0.205 2(3)nm,which falls in the normal range.The coordination angles around the Cu(Ⅱ)ion vary from 75.65(10)°to 176.95(10)°[14].The Cu1 center displays a slightly distorted square-pyramidal coordination architecture,while the Cu2 center shows a distorted trigonal bipyramidal configuration.
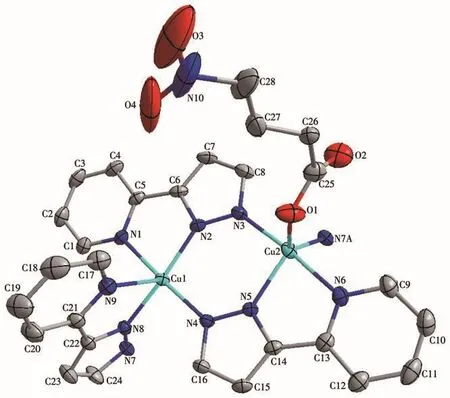
Fig.1 Drawing of the asymmetric unit of complex 1 with a 30% probability of thermal ellipsoids
In complex 1,each L-anion adopts the μ3coordination mode,chelating and bridging Cu(Ⅱ)ions to form a tetranuclear subunit.Meanwhile,each 4-nph2-anion adopts the µ2coordination mode,acting as a μ2-bridge to link two tetranuclear subunits,thus forming a 1D structure(Fig.2).Additionally,there are C—H…O and C—H…N hydrogen bonds derived from the carbon atoms,the nitrogen atom of L-and the carboxyl oxygen atom of 4-nph2-.The hydrogen-bonding parameters of 1 are listed in Table 3.Moreover,there exist π-π interactions between the pyridine ring and the pyrazole ring of L-.The centroid-to-centroid distances between adjacent rings are 0.360 4(2)nm for N2-N3-C8-C7-C6 and N4-N5-C14-C15-C16(Symmetry code:1/2-x,1/2-y,1-z)pyrazole rings and 0.360 3(2)nm for N1-C1-C2-C3-C4-C5 and N6-C9-C10-C11-C12-C13(Symmetry code:1/2-x,1/2-y,1-z)pyridine rings.The perpendicular distances are 0.336 88(14)and 0.327 07(14)nm and the dihedral angle are 1.9(2)°and 0.17(18)°,respectively.The related parameters are indicating the existence of π-π interaction.The complex extends into a 3D supramolecule(Fig.3)so that it is more stable.It should be noted that nitro-nitrogen atoms in 4-nph2-anion are disordered,which is proved by the crystal analysis.

Fig.2 View of the 1D structure of complex 1 along the a-axis

Fig.3 View of the 3D supramolecular architecture of complex 1
Complex 2 has a centrosymmetric dinuclear structure(Fig.4)with the Cu(Ⅱ)ion six-coordinated by two N atoms from one chelating bda2-anion,two N atoms from two bridging bib molecules,and two coordinated water molecules.H2bda acts as a typical chelating ligand coordinating to the Cu(Ⅱ) ion with the Cu—N bond distances ranging from 0.201 5(5)to 0.203 2(5)nm and the N—Cu—N angle being 80.6(2)°.For bib,there exist bridging coordination modes with Cu(Ⅱ)ion.Two N atoms(N3,N4)adopt the μ2bridging coordination mode to connect two Cu(Ⅱ)ions,i.e.,Cu1 and Cu1A,to form a dinuclear structure(Cu1…Cu1A 0.993 2 nm)with 26-member ring.The Cu—N distances(0.198 2(5)-0.198 8(5)nm)are quite similar to normal Cu—N bond distances(0.197 8(2)-0.211 3(3)nm)[15].The Cu—O bond distances are 0.241 6(5)-0.249 7(5)nm.All these Cu—N and Cu—O bond distances and the bond angles(Table 2)around each Cu(Ⅱ)center are in the normal range expected for such complexes.It is worth noting that the carboxyl oxygen atoms of bda2-are not coordinated to Cu(Ⅱ)ions,which presents strong intermolecular hydrogen-bonding interactions with crystalline water molecules(Table 3).Moreover,the adjacent dinuclear[Cu2(bda)2(bib)2(H2O)4]molecules are arranged into a 1D chain by the intermolecular O—H…O hydrogen-bonding interactions between carboxyl oxygen atoms and crystalline water molecules(Fig.5).
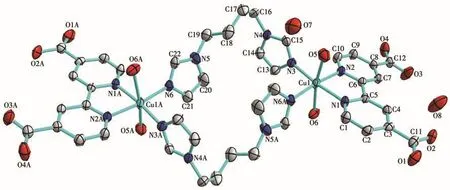
Fig.4 Drawing of the asymmetric unit of complex 2 with a 30% probability of thermal ellipsoids

Fig.5 View of the 1D chain of complex 2 by the intermolecular O—H…O hydrogen-bonding
Additionally,the adjacent 1D chain is extended into a 3D supramolecule by the intermolecular O—H…O hydrogen-bonding interactions between carboxyl oxygen atoms and coordinated water molecules.From the stacking diagram,there is π…π interactions between pyridyl rings from adjacent bda2-anions with the centroid-centroid separation of 0.384 6(4)nm for N2-C6-C7-C8-C9-C10 and N2B-C6B-C7B-C8B-C9B-C10B pyridyl rings(Symmetry code:1-x,1-y,1-z),interplanar separation of 0.343 7(3)nm for N2-C6-C7-C8-C9-C10 and N2B-C6B-C7B-C8B-C9B-C10B pyridyl rings(Symmetry code:1-x,1-y,1-z),and dihedral angle of 0.0(3)°,which further stabilize the structure of 2(Fig.6).

Fig.6 π-π interaction of complex 2
2.2 IR analysis
IR spectra of 1 and 2 are shown in a range of 400 to 4 000 cm-1(Fig.S1 and S2,Supporting information).The strong bands at about 1 610 and 1 597 cm-1for 1 are identified for νC—Ovibration of the 4-nph2-groups[16].The peak located at 1 456 cm-1for 1 is attributed to the stretching vibration of carboxylic groups[17].The strong bands in the region of 687-723 cm-1can be assigned to the νC—Nvibration of the N-heterocyclic rings of the ligands[18].
The feature peak of—OH groups from water molecules of 2 was observed at about 3 385 cm-1[19].The strong bands at about 1 617 cm-1for 2 are identified for νC—Ovibration of the bda2-groups[16].The peak located at 1 397 cm-1for 2 is attributed to the stretching vibration of carboxylic groups[17].The strong bands in the region of 668-723 cm-1can be assigned to the νC—Nvibration of the N-heterocyclic rings of the ligands[18].
2.3 PXRD and thermal stability analyses
To substantiate the phase purity of the assynthesized products 1 and 2,their PXRD patterns were measured.The experimental PXRD patterns are in good agreement with the corresponding simulated ones(Fig.7)except for the relative intensity variation because of the preferred orientations of the crystals.

Fig.7 PXRD patterns of 1 and 2:simulated(bottom)and experimental(top)
Complexes 1 and 2 were stable at ambient conditions,and TG experiments were performed to explore their thermal stabilities.For 1,there were two separate weight loss steps.The first weight loss of 15.12% occurred from 149 to 281℃,which is attributed to the loss of coordinated 4-nph2-(Calcd.16.56%).The second weight loss of 64.88% from 360 to 726℃corresponds to the loss of all the remaining L-anions(Calcd.64.49%),as shown in Fig.S3.For 2,the first weight loss of 13.08%(Calcd.12.68%)from 88 to 185℃corresponds to the release of water molecules.The following weight loss of about 75.82%(Calcd.76.13%)between 185 and 425℃corresponds to other coordinated bda2-anions and bib molecules,as shown in Fig.S4.
2.4 Quantum chemistry calculations
All calculations were fulfilled with the Gaussian16 program.The parameters of the molecular fragments for calculation were taken from the crystal structure of the title complex.The natural bond orbital(NBO)analysis was carried out by density functional theory(DFT)[20]with the PBE0[21-24]hybrid functional and the LANL2DZ basis set[25].
The selected natural atomic charges,natural electron configuration,Wiberg bond,and NBO bond orders(a.u.)for complex 1 are shown in Table 4.It is indicated that the electronic configurations of Cu1 and Cu2 ions,N and O atoms are 4s0.313d9.234p040,4s0.293d9.304p0.48,2s1.31~1.382p3.93~4.15and 2s1.672p5.03,respectively.Based on the above results,one can conclude that all Cu(Ⅱ)ion coordination with N and O atoms is mainly on 3d,4s,and 4p orbitals.All N atoms form coordination bonds with Cu(Ⅱ)ion using 2s and 2p orbitals.All O atoms supply electrons of 2s and 2p to Cu(Ⅱ)ion and form the coordination bonds.Therefore,the Cu1 ion obtained some electrons from five N atoms of L-,while the Cu2 ion obtained some electrons from four N atoms of L-and one O atom of 4-nph2-[26].Thus,according to valence-bond theory,the atomic net charge distribution and the NBO bond orders of complex 1(Table 4)shows the obvious covalent interaction between the coordinated atoms and Cu(Ⅱ)ion.The differences in the NBO bond orders for Cu—O and Cu—N bonds make their bond lengths different[27],which is in good agreement with the X-ray crystal structure data of compound 1.

Table 4 Selected atom net charges,electron configurations,Wiberg bond indexes,and NBO bond orders of complex 1
As can be seen from Fig.8,LUMO is mainly composed of L-,whereas HOMO mainly consists of L-,too.So,the charge transfer from ligand to ligand may be inferred from some contours of the molecular orbitals of complex 1.

Fig.8 Frontier molecular orbitals of complex 1
3 Conclusions
In summary,we have prepared two new copper complexes by employing 4-nitrophthalic acid (4-H2nph)/2,2'-bipyridine-4,4'-dicarboxylic acid(H2bda)as the primary ligand and 3-(2-pyridyl)pyrazole(HL)/1,4-bis(imidazol-1-yl)-butane(bib)as the ancillary ligand.In complex 1,the carboxyl ligand 4-nph2-bridges the metal centers via monodentate modes to form a 1D structure.In complex 2,the flexible bib linked metal centers to form a dinuclear structure with a 26-member ring.In addition,the 3D supramolecular architectures are formed by hydrogen bonding and π -π interactions,which play an important role in stabilizing the crystal structures of complexes 1 and 2.
Supporting information is available at http://www.wjhxxb.cn
