Influence of buccal acupuncture on analgesic effect,immune indicators, and expression of Survivin and Livin proteins in patients with advanced-stage primary liver cancer
2022-10-26LINZhiguang林志光SUShengxian苏圣贤XIEXiaoli解小丽YANGYuanfeng杨源锋DONGQinglong董庆龙
LIN Zhiguang (林志光), SU Shengxian (苏圣贤), XIE Xiaoli (解小丽), YANG Yuanfeng (杨源锋), DONG Qinglong (董庆龙)
1 Department of Pain Medicine, Zhongshan People’s Hospital, Zhongshan 528400, China
2 Department of Anesthesiology, Graduate School of Guangzhou Medical University, Zhongshan 528400, China
3 Department of Anesthesia and Surgery, the First Affiliated Hospital of Guangzhou Medical University, Guangzhou 510120, China
Abstract
Keywords: Acupuncture Therapy; Buccal Acupuncture; Liver Neoplasms; Acupuncture Analgesia; Immune Function; Liver Function Tests; Biomarkers, Tumor; Survivin
Primary liver cancer (PLC) is a malignant tumor that occurs in hepatocytes or intrahepatic bile duct epithelial cells and is extremely malignant[1]. The early symptoms of this disease are not obvious, so most patients have already progressed to the advanced stage at the time of diagnosis, where surgery is not feasible, but only conservative treatment is indicated[2]. Transcatheter arterial chemoembolization (TACE) is an effective method for the treatment of advanced-stage PLC, but some studies have pointed out that TACE has a high recurrence rate after treatment, and patients need to receive multiple treatments, which largely increases their physical pain and economic pressure[3]. With the continuous development of traditional Chinese medicine (TCM), TCM treatment has gradually become an important part of comprehensive tumor treatment,and it has shown certain effectiveness in reducing adverse stimuli, improving the pain threshold, inhibiting tumor cells, and regulating the immune status[4]. Buccal acupuncture is a TCM method that acts as a treatment for systemic diseases through local acupuncture on the cheek[5]. This study investigated the clinical efficacy of buccal acupuncture in the treatment of advanced-stage PLC.
1 Clinical Materials
1.1 Diagnostic criteria
The Western medical diagnostic criteria referred to theGuidelines for Diagnosis and Treatment of Primary Liver Cancer (Version 2017)[6]. Diagnosis of PLC was confirmed by liver histology and extrahepatic histology;alpha-fetoprotein (AFP) ≥20 μg/L for more than one month and exclusion of patients with metastatic liver cancer, germline embryonic-derived tumors, active liver disease, and pregnancy; AFP <400 μg/L and at least two positive liver cancer markers and one imaging test, or at least two imaging examinations confirmed the diagnosis of liver cancer as an occupying lesion.
The TCM diagnostic criteria referred to theGuiding Principles for Clinical Study of New Chinese Medicines[7].Primary symptoms: pain in the rib cage, abdominal bloating, weakness, and poor appetite. Secondary symptoms: insomnia, acid vomiting, loose stools. A pale tongue with white coating and wiry pulse.
1.2 Inclusion criteria
Met the criteria of PLC diagnosis in TCM and Western medicine; international tumor node metastasis
The SPSS version 21.0 statistical software was used for data analysis. The measurement data that conformed to a normal distribution were expressed as mean ± standard deviation (±s) and checked using classification (TNM) stages Ⅲ-Ⅳ; expected survival>3 months; Karnofsky score (KPS) ≥60 points;measurable mass; no radiotherapy or surgery indications; tumor volume occupies less than 70% of the liver volume.
1.3 Exclusion criteria
Those with severe infections or other malignancies;secondary liver cancer; contraindications to TACE;patients with severe cardiovascular disease; coagulation or renal dysfunction; complete obstruction of the main portal vein; women during lactation or pregnancy.
1.4 Statistical methods
t-test; those that did not conform to a normal distribution were expressed as median and examined using a nonparametric test (Mann-WhitneyU-test). The enumeration data were expressed as percentages (%)and processed using the Chi-square test, and the rank data using the rank-sum test.P<0.05 indicated a statistically significant difference.
1.5 General data
Eighty patients with advanced-stage PLC visiting the Department of Oncology of Zhongshan People’s Hospital between January 2018 and August 2019 were selected as the study subjects. The patients were divided into a control group and a treatment group according to their treatment protocols, with 40 cases in each group. The study was approved by the Ethics Committee of Zhongshan People’s Hospital (Ethical Approval No. 0020164). The general data of patients in the two groups were statistically analyzed, and the differences were not statistically significant (P>0.05),(Table 1).
2 Treatment Methods
2.1 Control group
The control group received TACE treatment. Firstly, a Cobra catheter was inserted into the common hepatic artery via femoral artery puncture, and the location of the tumor and the blood supply artery were clarified by digital subtraction angiography (DSA), and 1.0 g of 5-fluorouracil [Specification: 50 mg (10 mL), State Food and Drug Administration (SFDA) Approval No.H22023469, Nantong Pharmaceutical General Factory,China], 150 mg of oxaliplatin [Specification: 50 mg(100 mL), SFDA Approval No. H20133247, Shandong New Times Pharmaceutical Co., Ltd., China], 0.3 g of calcium folinate [Specification: 100 mg (10 mL), SFDA Approval No. H20044158, Youcare Pharmaceutical Group Co., Ltd., China], and 20 mL of super-liquid iodized oil (Specification: 10 mL containing 40% iodine,SFDA Approval No. H20050307, Laboratorie Guerbet,France, the dosage depends on the patient’s blood supply and the size of the lesion) were mixed and slowly injected into the blood supply artery for hepatic artery embolization. After embolization, DSA was performed again to confirm the effect of treatment, and a liver function protection plan was formulated for the patient.After one-month treatment, the actual condition of the patient’s lesion will determine whether re-treatment is needed. Usually, 2-3 TACE treatments are needed for lesions over 5 cm, and 1 TACE treatment is needed for lesions smaller than 5 cm.
2.2 Treatment group
In the treatment group, buccal acupuncture was added to TACE. The points and positioning are as follows (Figure 1)[8].
Liver Area: The patient is in a sitting or supine position, and the Liver Area is drawn as a circle with a straight line from one-half of the eyebrows toward Taiyang (EX-HN5) as the diameter.
Neck: At the upper edge of the zygomatic arch root(the depression of the upper edge of the bone),corresponding to the C7spinous process.
Upper Neck: The lower edge of the bony cross-section vertical upward of the Neck point,corresponding to the C1spinous process.
Back: Inferior border of the zygomatic arch root under the temporomandibular joint capsule (there is a slip point under the joint capsule), corresponding to the T3spinous process.
Sacrum: 0.5 Cun anteriorly up the angle of the mandible (toward the tip of the nose, with the length of the second segment of the middle finger as 1 Cun),corresponding to the S3spinous process.
Lumbar: The midpoint of the line connecting the Back point and Sacrum point, corresponding to the L2spinous process.
Low Back: The middle and lower thirds of the line connecting the Lumbar and Sacrum points,corresponding to L5and S1.

Table 1. Baseline characteristics of the two groups

Figure 1. Diagram of buccal acupuncture points
Methods:The corresponding site of pain within the Liver Area was selected as the entry point according to the patient’s specific pain site. The midpoint between the Lumbar point and the Back point was selected as the entry point, and the tip of the needle was pointed upward into the Liver Area. After routine disinfection with medical alcohol, Hwato brand steel needles of 0.16 mm in diameter and 20 mm in length or 0.18 mm in diameter and 30 mm in length (Suzhou Medical Appliance Factory, China) were used and inserted obliquely for 10-20 mm. After the needle was inserted,the patient was asked about the pain level, and the direction of the needle tip and the depth of needling were adjusted appropriately. The depth of needling depends on the patient’s actual condition and personal constitution; shallow if the disease is mild and deep if it is severe. If the pain is relieved quickly, the patient is considered to have obtained Qi; if the pain is not relieved, the doctor should continue to adjust the direction of the needle tip and the depth of needling.After obtaining Qi, the needles were retained for 20-40 min, and the duration of needle retention should depend on the actual condition of the patient. During the needle retention period, the needles were adjusted and replenished according to the patient’s response.The treatment was given once every 3 d, and 5 times were taken as a course of treatment, and 2 courses were offered.
3 Observation of Therapeutic Efficacy
3.1 Observation indicators
3.1.1 Subjective indicators
Pain level assessment: The patients’ pain level was assessed by the numeric rating scale (NRS) and scored in the range of 0-10, in which 0 indicates no pain, 1-3 indicates mild pain, 4-6 indicates moderate pain, 7-10 indicates severe pain, and the higher the patient’s score,the more severe the pain. The onset time of analgesia and duration of analgesia were recorded and compared between the two groups.
3.1.2 Objective indicators
Liver function testing: Before and after treatment,4 mL of fasting venous blood was drawn from the patients, and the samples were tested by an automatic biochemical analyzer for glutathione aminotransferase(AST), alanine aminotransferase (ALT), and albumin/globulin (A/G).
Serum tumor marker levels were measured: The serum tumor markers AFP, α-L-fucosidase (AFU), and carcinoembryonic antigen (CEA) were detected by the electrochemiluminescence automatic immunoassay system and supporting kits before and after treatment,and the operation procedures were carried out strictly according to the kit instructions. Normal reference values of serum tumor markers: AFP <20 μg/L,AFU <40 U/L, and CEA <5 μg/L.
Survivin and Livin protein expression assay of liver cancer tissue: Liver cancer tissue was obtained by puncture before and after treatment, and the expression levels of Survivin and Livin proteins were detected by the Western blotting method.
T-lymphocyte subpopulation level assay: Patients’CD4+, CD8+, and CD4+/CD8+were detected by flow cytometry before and after treatment and analyzed for comparison.
3.1.3 Recent efficacy evaluation
CT was used to examine the patient’s lesions, and the patient’s recent efficacy was evaluated according to the objective tumor efficacy assessment criteria.
Complete relief (CR): Complete disappearance of tumor lesions, maintaining for at least 1 month.
Partial relief (PR): The product of the longest diameter and the shortest diameter of the tumor lesion was reduced by ≥50%, maintaining for at least one month.
Stable disease (SD): Reduction (or enlargement) of the product of the longest diameter and the shortest diameter of the tumor lesion by <25%.
Progressive disease (PD): Increase in the product of the longest diameter and the shortest diameter of the tumor lesion by ≥25% or the appearance of new lesions.
Objective response rate (ORR) = (CR + PR) ÷ Total number of cases × 100%.
Disease control rate (DCR) = (CR + PR + SD) ÷ Total number of cases × 100%.
3.2 Results
3.2.1 Comparison of the recent efficacy
The ORR and DCR of the treatment group were 37.5% and 77.5%, respectively, which were significantly higher than those of the control group, 22.5% and 52.5%, and the results of the rank-sum test suggested that the recent efficacy of the treatment group was better than that of the control group (P<0.05), (Table 2).control group (P<0.05). For details, see Table 3.

Table 2. Comparison of the recent efficacy between the two groups (case)
3.2.3 Comparison of the liver function
Before treatment, there were no statistical differences in AST, ALT, or A/G between the two groups(P>0.05). After treatment, AST, ALT, and A/G decreased in both groups compared with those before treatment(P<0.05), and all three items in the treatment group were significantly lower than those in the control group(P<0.05). For details, see Table 4-Table 6.
Table 3. Comparison of the pain level between the two groups ( ±s)
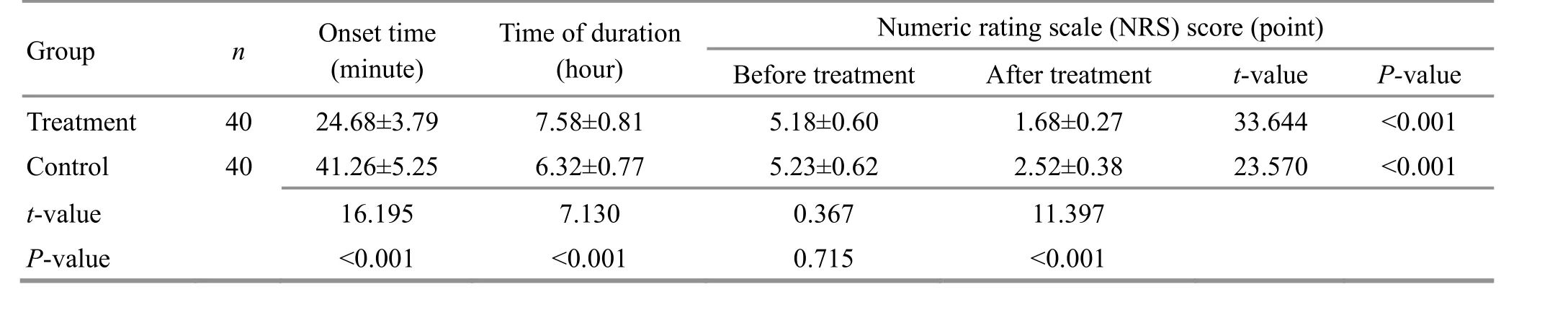
Table 3. Comparison of the pain level between the two groups ( ±s)
Group n Onset time(minute)Time of duration(hour)Numeric rating scale (NRS) score (point)Before treatment After treatment t-value P-value Treatment 40 24.68±3.79 7.58±0.81 5.18±0.60 1.68±0.27 33.644 <0.001 Control 40 41.26±5.25 6.32±0.77 5.23±0.62 2.52±0.38 23.570 <0.001 t-value 16.195 7.130 0.367 11.397 P-value <0.001 <0.001 0.715 <0.001
3.2.2 Comparison of the pain level
Before treatment, there was no statistical difference in the NRS score between the two groups (P>0.05).After treatment, the NRS scores in both groups were significantly lower than those before treatment (P<0.05);the NRS score in the treatment group was significantly lower than that in the control group (P<0.05), and the onset of analgesia was significantly faster than that in the control group (P<0.05), and the duration of analgesia was significantly longer than that in the
Table 4. Comparison of the aspartate aminotransferase (AST) level between the two groups ( ±s, U/L)

Table 4. Comparison of the aspartate aminotransferase (AST) level between the two groups ( ±s, U/L)
Group n Before treatment After treatment t-value P-value Treatment 40 99.63±16.84 74.67±15.22 6.955 <0.001 Control 40 97.68±17.62 86.77±16.35 2.871 0.005 t-value 0.506 3.426 P-value 0.614 0.001
Table 5. Comparison of the alanine aminotransferase (ALT) level between the two groups ( ±s, U/L)
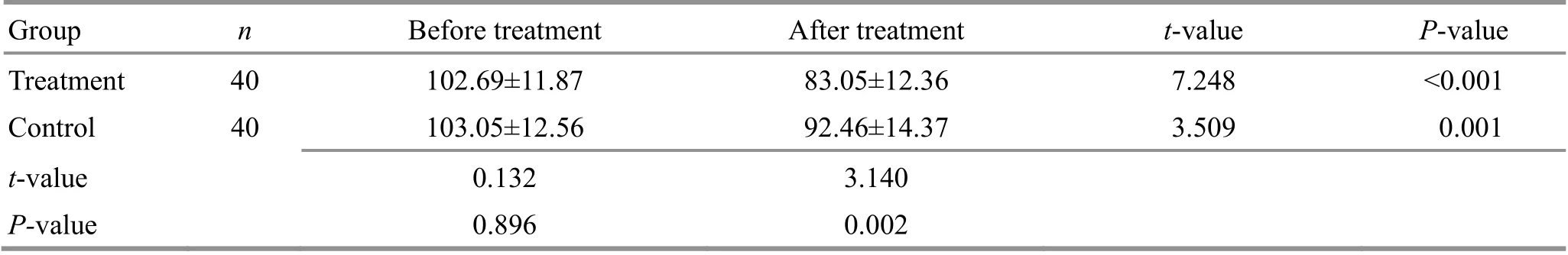
Table 5. Comparison of the alanine aminotransferase (ALT) level between the two groups ( ±s, U/L)
Group n Before treatment After treatment t-value P-value Treatment 40 102.69±11.87 83.05±12.36 7.248 <0.001 Control 40 103.05±12.56 92.46±14.37 3.509 0.001 t-value 0.132 3.140 P-value 0.896 0.002
Table 6. Comparison of the albumin/globulin (A/G) between the two groups ( ±s)

Table 6. Comparison of the albumin/globulin (A/G) between the two groups ( ±s)
Group n Before treatment After treatment t-value P-value Treatment 40images/BZ_62_723_2483_730_2487.png0.97±0.25 0.36±0.78 4.710 <0.001 Control 40 0.99±0.26 0.84±0.32 2.301 0.024 t-value 0.351 3.601 P-value 0.727 0.001
3.2.4 Comparison of the serum tumor marker levels
Before treatment, the levels of serum tumor markers AFP, AFU, and CEA were not statistically different between the two groups (P>0.05). After treatment, the levels of AFP, AFU, and CEA decreased in both groups compared with those before treatment (P<0.05), and the levels of all three items in the treatment group were significantly lower than those in the control group(P<0.05). For details, see Table 7-Table 9.
3.2.5 Comparison of the Survivin and Livin protein expression in liver cancer tissue
Before treatment, there were no statistical differences in the expression levels of Survivin or Livin proteins in liver cancer tissue between the two groups(P>0.05). After treatment, the expression levels of Survivin and Livin proteins in liver cancer tissue in both groups decreased compared with those before treatment (P<0.05), and the expression levels in the treatment group were significantly lower than those in the control group (P<0.05). For details, see Figure 2,Table 10, and Table 11.
3.2.6 Comparison of the T-lymphocyte subpopulation level
Before treatment, there were no statistical differences in the levels of T-lymphocyte subsets between the two groups (P>0.05). After treatment,CD8+decreased, and CD4+/CD8+increased in the treatment group, both statistically different from those before treatment (P<0.05); in the control group, only CD4+/CD8+was significantly lower than that before treatment (P<0.05). CD4+and CD4+/CD8+in the treatment group were significantly higher than those in the control group (P<0.05), and CD8+was significantly lower than that in the control group (P<0.05). For details, see Table 12-Table 14.
Table 7. Comparison of the serum alpha-fetoprotein (AFP) level between the two groups ( ±s, μg/L)

Table 7. Comparison of the serum alpha-fetoprotein (AFP) level between the two groups ( ±s, μg/L)
Group n Before treatment After treatment t-value P-value Treatment 40 353.29±43.96 211.85±35.78 15.782 <0.001 Control 40 354.48±45.72 256.74±36.82 10.530 <0.001 t-value 0.119 5.530 P-value 0.906 <0.001
Table 8. Comparison of the serum α-L-fucosidase (AFU) level between the two groups ( ±s, U/L)
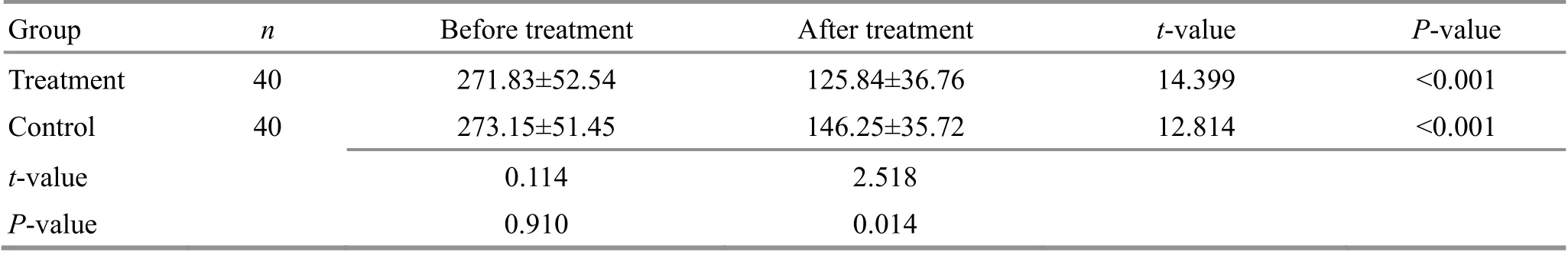
Table 8. Comparison of the serum α-L-fucosidase (AFU) level between the two groups ( ±s, U/L)
Group n Before treatment After treatment t-value P-value Treatment 40 271.83±52.54 125.84±36.76 14.399 <0.001 Control 40 273.15±51.45 146.25±35.72 12.814 <0.001 t-value 0.114 2.518 P-value 0.910 0.014
Table 9. Comparison of the serum carcinoembryonic antigen (CEA) level between the two groups ( ±s, U/L)
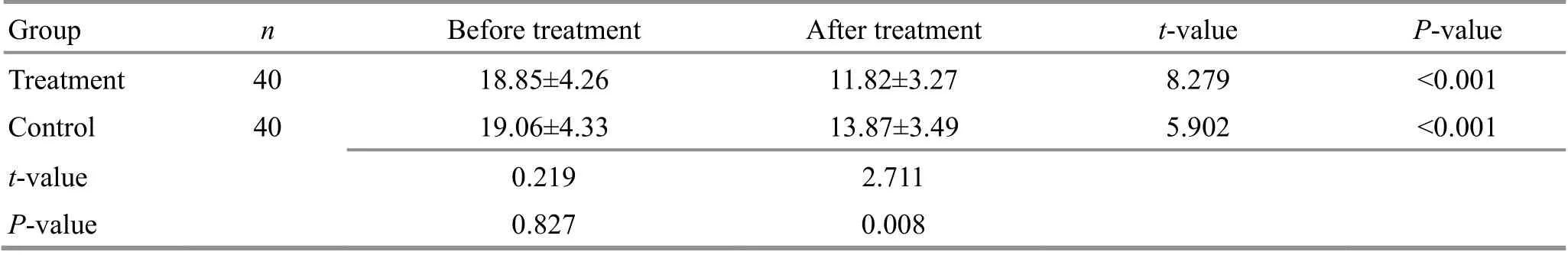
Table 9. Comparison of the serum carcinoembryonic antigen (CEA) level between the two groups ( ±s, U/L)
Group n Before treatment After treatment t-value P-value Treatment 40 18.85±4.26 11.82±3.27 8.279 <0.001 Control 40 19.06±4.33 13.87±3.49 5.902 <0.001 t-value 0.219 2.711 P-value 0.827 0.008

Figure 2. Survivin and Livin protein expression in liver cancer tissue (Western blotting)
Table 10. Comparison of the Survivin protein expression in liver cancer tissue between the two groups ( ±s)
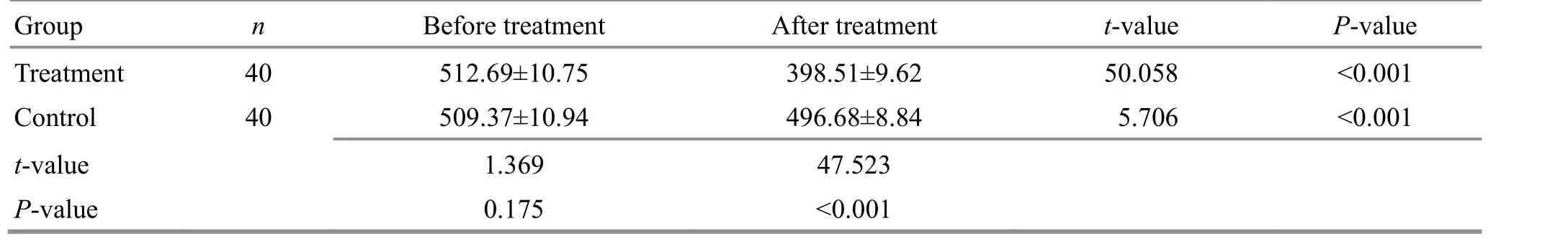
Table 10. Comparison of the Survivin protein expression in liver cancer tissue between the two groups ( ±s)
Group n Before treatment After treatment t-value P-value Treatment 40 512.69±10.75 398.51±9.62 50.058 <0.001 Control 40 509.37±10.94 496.68±8.84 5.706 <0.001 t-value 1.369 47.523 P-value 0.175 <0.001
Table 11. Comparison of the Livin protein expression in liver cancer tissue between the two groups ( ±s)

Table 11. Comparison of the Livin protein expression in liver cancer tissue between the two groups ( ±s)
Group n Before treatment After treatment t-value P-value Treatment 40 99.83±9.58 45.34±5.93 30.588 <0.001 Control 40 99.57±9.64 71.92±6.43 15.091 <0.001 t value 0.121 19.219 P value 0.904 0.000
Table 12. Comparison of the CD4+ level between the two groups ( ±s, %)

Table 12. Comparison of the CD4+ level between the two groups ( ±s, %)
t-value P-value 0.965 0.337 1.950 0.055
Table 13. Comparison of the CD8+ level between the two groups ( ±s, %)

Table 13. Comparison of the CD8+ level between the two groups ( ±s, %)
t-value P-value 4.029 <0.001 1.562 0.122
Table 14. Comparison of the CD4+/CD8+ level between the two groups ( ±s)

Table 14. Comparison of the CD4+/CD8+ level between the two groups ( ±s)
t-value P-value 2.289 0.025 2.235 0.028
4 Discussion
PLC is a common malignant tumor, and its incidence has been showing an increasing trend worldwide[9]. The early symptoms of this disease are atypical, and these symptoms are mostly gastrointestinal ones. When the disease progresses to the advanced stage, persistent pain in the liver area, ascites, poor nausea, jaundice,and abdominal masses will present. Radiotherapy is a common clinical treatment of malignant tumors, but the biological characteristics of PLC determine its low sensitivity to radiotherapy[10]. TACE has a high near-term remission rate and was once the preferred method for the treatment of advanced liver cancer. However, it relies on chemotherapeutic drugs injected through the blood supply vessels of the lesion and is less effective in lesions without blood supply vessels[11]. Combined with the cytotoxic effects of the drugs, patients may experience adverse effects such as liver function impairment, immune dysfunction, and gastrointestinal symptoms[12], resulting in the overall poor efficacy of TACE monotherapy.
Buccal acupuncture is a kind of holographic therapy.It usually selects points corresponding to the lesion on the same side, on the contralateral side, or on both sides, or selects points according to reining Zang-Fu organs of the disease, following the principles of left-right correspondence or cross correspondence[13].In the present study, the treatment group selected points according to the reining Zang-Fu organs of the disease[14]and used the Liver Area. To avoid too shallow the insertion, the oblique acupuncture method was used in this study. According to the anatomical structure,the Liver Area locally distributes mainly the facial nerve and trigeminal nerve, both of which are mixed nerves.PU R S,et al[15]found that buccal acupuncture therapy produced analgesia by transmitting acupuncture signals to higher central sites through the trigeminal nerve. In the present study, the onset of analgesia in the buccal acupuncture therapy was significantly faster than that in the control group (P<0.05), the duration of analgesia was significantly longer than that in the control group(P<0.05), and the NRS score in the treatment group was significantly lower than that in the control group after treatment (P<0.05), suggesting that buccal acupuncture therapy has a better analgesic effect.
AFP is tumor-specific and widely used in clinical practice[16]. AFU is a lysosomal acid hydrolase that can catalyze a variety of glycoproteins and oligosaccharides.The current study showed that the serum level of AFU was abnormally elevated in patients with liver cancer,suggesting that it may be involved in the process of fucosylation in carcinoma cells. Hyperfunction of glycosidases in cancer development leads to abnormal up-regulation of AFU levels[17]. CEA is a good tumor marker to determine cancer treatment efficacy, disease progression, and prognosis, but its specificity is not strong, sensitivity is not high, and thus it is usually not used for early diagnosis of tumors[18]. The present study showed that the serum AFP, AFU, and CEA levels in the treatment group were significantly lower than those in the control group (P<0.05), suggesting that buccal acupuncture therapy combined with TACE can effectively improve patients’ condition and delay the progression of advanced-stage PLC, and its specific mechanism of action may involve the regulation of Survivin and Livin proteins in liver cancer tissue. Survivin and Livin are the most important markers in aberrantly expressed apoptosis suppressor proteins in a variety of tumor tissues[19]. Both have direct or indirect inhibitory effects on caspase-9, the initiating signaling molecule of caspase apoptosis signaling, and caspase-3 and caspase-7, the effector molecules, thereby blocking the apoptotic process. The expression of Survivin protein in tumor tissue such as breast, colon, and gastric cancers is significantly up-regulated, which can promote the progression of the tumor and contribute to its distant spread[20]. The expression of Survivin protein is lower in the pericarcinomatous tissue, but it is higher in the cancer tissue. Therefore, it is significant for the diagnosis of liver cancer and judgment of its prognosis to measure the expression level of Survivin protein in liver cancer tissue[21]. Livin is a protein specifically expressed in human solid tumor cells, embryonic tissues, and placental tissues, but it is also expressed in normal human tissues, though its expression is low in lymphocytes, skeletal muscle, heart, brain, spleen, and lung. Clinical studies have indicated that Livin expression is abnormally elevated in tumor tissue such as melanoma, bladder cancer, and lung cancer. High Livin protein expression is closely associated with tumor differentiation, invasion, and metastasis[22]. The results of this study showed that the expression levels of Survivin and Livin proteins in liver cancer tissue in the treatment group were significantly lower than those in the control group (P<0.05), suggesting that buccal acupuncture therapy combined with TACE can effectively down-regulate Survivin and Livin protein expression levels in liver cancer tissue, thus inhibiting the process of liver cancer disease progression and tumor spread. However, there was no correlation between Survivin and Livin expression. The specific mechanism by which buccal acupuncture therapy combined with TACE acts on Survivin and Livin proteins in liver cancer tissue is still unclear and requires subsequent in-depth studies. Immune function plays an important role in disease treatment. Probably due to the toxic side effects of chemotherapy, patients treated with TACE alone usually suffer from liver impairment and decreased immune function, resulting in greater weakness. This significantly reduces treatment adherence. CD4+and CD4+/CD8+in the treated group were significantly higher (P<0.05), and CD8+was significantly lower (P<0.05) than those in the control group, indicating that TACE plus buccal acupuncture improves the immune function of patients and contributes to the clinical efficacy.
In conclusion, buccal acupuncture may delay disease progression by down-regulating Survivin and Livin protein expression in liver cancer tissue and modulating immunity. Buccal acupuncture combined with TACE has a better analgesic effect and is worthy of clinical promotion.
Conflict of Interest
The authors declare that there is no potential conflict of interest in this article.
Acknowledgments
This work was supported by Guangdong Provincial Medical Science and Technology Key Research Fund (广东省医学科学技术重点研究基金, No. 2018YS1548).
Statement of Informed Consent
Informed consent was obtained from all individual participants.
Received: 5 August 2020/Accepted: 8 January 2021
猜你喜欢
杂志排行
Journal of Acupuncture and Tuina Science的其它文章
- Efficacy of mild moxibustion combined with surgery for meniscal injury and its effect on TGF-β1 and PDGF levels in the fluid of knee joint
- Effects of acupuncture plus medication on hippocampus SIRT1 and FOXO3a expression, MDA content, and SOD activity of rats with Alzheimer disease
- Improvement effect of acupuncture on locomotor function in Parkinson disease via regulating gut microbiota and inhibiting inflammatory factor release
- Effects of Mo-Rubbing abdomen manipulation on glucose metabolism and inflammatory factors in rats with type 2 diabetes mellitus
- Observation on the therapeutic efficacy of Tuina plus “three-bridge” exercise for non-specific low back pain
- Influence of herbal cake-partitioned moxibustion on lumbar functions and inflammatory factors in patients with lumbar disc herniation due to kidney deficiency and blood stasis
