Effect of treatment with Fufang Huangqi decoction (复方黄杞汤剂)on dose reductions and discontinuation of pyridostigmine bromide tablets,prednisone,and tacrolimus in patients with type I or II myasthenia gravis
2022-10-14SHANCaifengZHANGJingshengLINYiHUANGXueshiWANGZhanyouPANHaiouQIAOWenjun
SHAN Caifeng,ZHANG Jingsheng,LIN Yi,HUANG Xueshi,WANG Zhanyou,PAN Haiou,QIAO Wenjun
SHAN Caifeng,ZHANG Jingsheng,QIAO Wenjun,the Affiliated Hospital of Liaoning University of Traditional Chinese Medicine,Liaoning Provincial Key Laboratory for Diagnosis and Treatment of Myasthenia Gravis,Liaoning University of Traditional Chinese Medicine,Shenyang 110033,China
LIN Yi,Department of General Surgery,the First People’s Hospital of Shenyang,Shenyang 110091,China
HUANG Xuwshi,Institute of Microbial Pharmaceuticals,College of Life and Health Sciences,Northeastern University,Shenyang 110819,China
WANG Zhanyou,Institute of Health Sciences,Key Laboratory of Medical Cell Biology of Ministry of Education,China Medical University,Shenyang 110122,China
PAN Haiou,Liaoning University of Traditional Chinese Medicine,foreign language college,Liaoning University of Traditional Chinese Medicine,Shenyang 110033,China
Abstract OBJECTIVE: To investigate the clinical efficacy of Fufang Huangqi decoction (复方黄杞汤剂) in combination with pyridostigmine bromide tablets,prednisone,and tacrolimus in the treatment of type I and II myasthenia gravis (MG) through changes in the clinical symptom scores of 100 patients with type I and II MG.This study also aimed to examine dose reductions and discontinuation of these 3 Western medicines after administration of Fufang Huangqi decoction.METHODS: The clinical data on 100 patients with type I or II MG who were treated in the outpatient department of the Affiliated Hospital of Liaoning University of Traditional Chinese Medicine,China,between June 2017 and June 2020 were collected.The patients were divided into 4 groups based on whether they had taken pyridostigmine bromide tablets,prednisone,and/or tacrolimus at the time of their hospital visit: the Fufang Huangqi decoction group(group A),the pyridostigmine bromide tablets+Fufang Huangqi decoction group (group B),the pyridostigmine bromide tablets+prednisone+Fufang Huangqi decoction group (group C),and the pyridostigmine bromide tablets+tacrolimus+Fufang Huangqi decoction group (group D).The average treatment time was (15.6 ±11.5) months (range: 0.5-55 months).Changes in the clinical symptom scores of the 4 groups of patients after medication administration and dose reductions and discontinuation of the 3 Western medicines were analyzed.RESULTS: An overall effectiveness rate of 86.00% was achieved in the 100 patients after treatment for (15.6 ±11.5) months (range 0.5-55 months).The effectiveness rates were 85.71% in group A,88.24% in group B,76.92%in group C,and 80.00% in group D.The dosage of pyridostigmine bromide was reduced for 69.12% of the patients in group B for the first time after (4.2 ± 4.1)months,and 45.59% of the patients in group B discontinued pyridostigmine bromide after (8.8 ± 6.1)months.The dosage of pyridostigmine bromide was reduced for 46.15% of the patients in group C for the first time after (5.3 ± 3.4) months,and 23.08% of the patients in group C discontinued pyridostigmine bromide after(19.8 ± 11.0) months;76.92% reduced hormone dosage after (2.8 ± 1.9) months,and 23.08% discontinued hormone treatment after (6.7 ± 2.9) months.The dosage of pyridostigmine bromide was reduced for 1 patient in group D after 1 month;this patient discontinued pyridostigmine bromide after 3 months and reduced tacrolimus dosage after 5 months.One patient in group D discontinued pyridostigmine bromide and tacrolimus on his own initiative at 0.5 months and took Fufang Huangqidecoction for 2 months without discontinuing Western medicine.CONCLUSION: Fufang Huangqi decoction is effective for the treatment of type I and II MG and improves the associated clinical symptoms.Moreover,this agent is conducive to dose reductions and discontinuation of basic Western medicines,thereby reducing the side effects experienced by patients.
Keywords: myasthenia gravis;drug tapering;drug-related side effects and adverse reactions;Fufang Huangqi decoction
1.INTRODUCTION
Myasthenia gravis (MG) is an acquired autoimmune disease that results in disordered transmission at the neuromuscular junction.MG is mainly mediated by autoantibodies,such as antibodies against the acetylcholine receptor (AChR).MG can occur at any age.The common clinical symptoms of MG include ptosis and difficulty chewing and swallowing.In severe cases,lifethreatening myasthenic crisis occurs.The symptoms of MG often fluctuate.The global incidence of MG is 150-250 cases per million,and the estimated annual incidence is 4-10 cases per million.The incidence of MG in China is approximately 0.68/100 000.The incidence of MG is slightly higher in females,and the in-hospital mortality rate is 14.69%.1
The pathogenesis of MG remains unclear.However,accumulating evidence suggests that the occurrence and development of MG are related to activation of anti-AChR CD4+T cells and the production of high-affinity specific AChR antibodies through interactions with B cells.CD4+T cells can be classified into several subtypes according to the cytokines they secrete.T-helper 1 (Th1)cells mainly secrete interleukin 2 (IL-2),interferon-γ(IFN-γ),and tumor necrosis factor alpha (TNFα),all of which promote cellular immune responses.T-helper 2(Th2) cells promote humoral immune responses,while T-helper 3 (Th3) cells are mainly involved in immuneosuppressive mechanisms.CD4+T cells jointly affect the occurrence of MG.2Currently,the main treatments for MG include cholinesterase inhibitors,glucocorticoids,immunosuppressants,intravenous immunoglobulin,plasma exchange,and thymectomy.Long-term use of some of the drugs used to treat MG may cause diseases such as hypertension,hyperglycemia,obesity,osteoporosis,and child growth retardation and increase the burden on the liver and kidneys of patients.Therefore,patients who receive these drugs must undergo regular routine blood tests,liver function tests,and kidney function tests.An increasing number of studies have shown that traditional Chinese medicine can have a very good therapeutic effect on this disease.After more than 30 years of clinical observation,our team has shown that taking Fufang Huangqi decoction (复方黄杞汤剂) alone for 3-6 months can begin to have a therapeutic effect,and the symptoms can often be stabilized after it has been taken for over 1 year.Taking Fufang Huangqi decoction helps patients reduce and discontinue the use of Western medicines,and there are no obvious toxic side effects.3-5Thus,the adverse effects of Western medicines such as liver and kidney toxicity and recurrence of the disease after discontinuation of medication use can be effectively avoided.We conducted the present study to further evaluate the efficacy of Fufang Huangqi decoction.This study summarizes changes in the clinical symptom scores of patients with type I or II MG who received treatment with a combination of Fufang Huangqi decoction and Western medicines and who underwent dose reductions and discontinuation of pyridostigmine bromide tablets,prednisone,and/or tacrolimus and thereby provides a reference for the clinical treatment of MG.
2.MATERIAILS AND METHODS
2.1.Research subjects
We collected clinical data on 100 patients with type I or II MG who were treated in the outpatient department of the Affiliated Hospital of Liaoning University of Traditional Chinese Medicine between June 2017 and June 2020.At their initial hospital visits,14 patients had not taken pyridostigmine bromide tablets,prednisone,or tacrolimus,68 had taken only pyridostigmine bromide tablets,13 had taken pyridostigmine bromide tablets and prednisone,and 5 had taken pyridostigmine bromide tablets and tacrolimus.This study was approved by the ethics committee of the hospital,and all patients signed an informed consent form.
2.2.Inclusion criteria
The inclusion criteria were as follows: (a) MG patients who met the relevant diagnostic criteria used in traditional Chinese medicine and Western medicine and were diagnosed with type I or II MG according to the Osterman criteria;6,7(b) patients who gave informed consent to data collection,for whom researchers had detailed data and contact information,and who cooperated with the examination and completed the scoring form;and (c) patients with complete medical records.There was no limit on the patients’ age or sex.Patients who met all 3 criteria were included in the study.
2.3.Exclusion criteria
The exclusion criteria were as follows: (a) patients who did not meet the inclusion criteria;(b) patients with type III,type IV,or type V MG and patients experiencing MG crisis;(c) patients who had taken drugs other than pyridostigmine bromide tablets,prednisone,or tacrolimus to treat MG;(d) patients who were allergic to the ingredients of pyridostigmine bromide tablets,prednisone,tacrolimus,or Fufang Huangqi decoction;(e)patients who were unable to cooperate with the treatment due to the presence of concurrent primary diseases such as severe cardiocerebrovascular disease,liver or kidney dysfunction,hematopoietic dysfunction,or mental disease and patients with a history of malignant tumors,HIV infection,or severe immunodeficiency;(f) patients who had participated in clinical trials involving other drugs within 1 month of the beginning of this study;and(g) patients whose medical records were incomplete.Patients who met any of the above criteria were excluded from this study.
2.4.Treatment methods
2.4.1 Patient groups
In total,100 patients with type I or II MG who met the criteria were included in this study.The patients were divided into 4 groups based on their medication status at the time of admission: group A included 14 patients who were taking only Fufang Huangqi decoction;group B included 68 patients who were taking pyridostigmine bromide tablets+Fufang Huangqi decoction;group C included 13 patients who were taking pyridostigmine bromide tablets+prednisone+Fufang Huangqi decoction;and group D consisted of 5 patients who took pyridostigmine bromide tablets+tacrolimus+Fufang Huangqi decoction.The pyridostigmine bromide tablets(60 mg) were provided by Shanghai Zhongxi Sunve Pharmaceutical Co.,Ltd.(Shanghai,China);prednisone(prednisone acetate tablets,5 mg) was supplied by Tianjin Tianyao Pharmaceutical Co.,Ltd.(Tianjin,China);and tacrolimus (1.0 mg) was provided by Sinopharm Chuankang Pharmaceutical Co.,Ltd.,China(Chengdu,China).
2.4.2 Medication methods
(a) The 4 groups of patients were given 100 mL of Fufang Huangqi decoction provided by the Pharmacy of the Affiliated Hospital of Liaoning University of Traditional Chinese Medicine.The decoction,which was mainly composed of Huangqi (Radix Astragali Mongolici),Danggui (Radix Angelicae Sinensis),Gouqizi(Fructus Lycii),Yimucao (Herba Leonuri Japonici),Baizhu(Rhizoma Atractylodis Macrocephalae),and other constituents,was given orally twice a day (before breakfast and after dinner).The average treatment time was (15.6 ± 11.5) months (range: 0.5-55 months).(b) Patients who had taken pyridostigmine bromide tablets,prednisone,or tacrolimus before their initial clinical visits were instructed to take the medications at the original doses during the initial stage of the study.The dosages of the Western medicines were gradually reduced according to the patients’ remission conditions,and treatment with the Western drugs was eventually discontinued.The average duration of Western medicine use was (11.2 ± 9.5) months (range:0.5-36 months).
2.5.Evaluation of treatment efficacy
Treatment efficacy was evaluated objectively in the 100 patients using the clinical absolute and relative scoring system developed by Xianhao Xu as a reference.8 Treatment efficacy was scored once monthly during the first 3 months and once every 6 months thereafter as follows: relative score=[(absolute score before medication -absolute score after medication) ÷ absolute score before medication] × 100%.The therapeutic effect was evaluated by considering changes in the patients’scores.A relative score of 95% or greater indicated clinical recovery,a relative score between 80% and 95%indicated that the patient had basically recovered,a relative score between 50% and 80% indicated a significant effect of the treatment,a relative score between 25% and 50% indicated that the patient’s condition had improved,and a relative score less than 25%indicated that the treatment was ineffective.The effectiveness rate was calculated as follows:effectiveness rate=clinical recovery rate+basic recovery rate+significant effectiveness rate +improvement rate.The clinical absolute scoring system(60 points) evaluated the following 8 aspects.(a) To score ptosis,the patient was instructed to look straight ahead,and the extent of corneal coverage by the upper eyelid was observed and recorded as the clock position.The left and right eyes were scored separately,yielded a maximum score of 8 points for each patient,as follows:0 points,11 to 1 o’clock;1 point,10 to 2 o’clock;2 points:9 to 3 o’clock;3 points: 8 to 4 o’clock;and 4 points: 7 to 5 o’clock.(b) To perform the upper eyelid fatigue test,the patient was instructed to keep his or her eyes open and to look upward,and the time to ptosis induction (s)was recorded.The standard for defining ptosis was corneal coverage by the upper eyelid at the 9-3 o’clock horizontal position,and the left and right eyes were scored separately,yielding a maximum score of 8 points per patient,as follows: 0 points,> 60;1 point,31-60;2 points,16-30;3 points,6-15;and 4 points,≤ 5.(c) To score restricted eyeball horizontal movement,the patient was instructed to look to the left and to the right.The width of white coloration exposed in each eye was measured during abduction and adduction,and the values(in mm) obtained for each eye were summed.Again,the left and right eyes were scored separately (a total of 8 points per patient) as follows: 0 points if the white of the eye was exposed ≤ 2 mm during abduction and adduction (no diplopia),1 point if the white of the eye was exposed ≤ 4 mm during abduction and adduction(diplopia),2 points if the white of the eye was exposed >4 mm and ≤ 8 mm during abduction and adduction,3 points if the white of the eye was exposed > 8 mm and≤ 12 mm during abduction and adduction,and 4 points if the white of the eye was exposed > 12 mm during abduction and adduction.(d) To perform the upper limb fatigue test,the patient was instructed to stretch both arms horizontally,and the time (in seconds)until he or she experienced upper limb fatigue was recorded.The left and right arms were scored individually as follows,yielding a maximum score of 8 points per patient: 0 points,> 120 s;1 point,61-120 s;2 points,31-60 s;3 points,11-30 s;and 4 points,0-10 s.(e)To perform the lower limb fatigue test,the patient maintained a supine position and flexed the knees and hips of both lower limbs at a 90° angle.The time to the induction of lower limb fatigue (in seconds) was recorded.The left and right lower limbs were scored separately,yielding a maximum of 8 points per patient,as follows: 0 points,> 120 s;1 point,61-120 s;2 points,31-60 s;3 points,11-30 s;and 4 points,0-10 s.(f) Facial muscle weakness was evaluated using the following scoring system: 0 points,normal;1 point,slightly reduced eye closure strength with incompletely buried eyelashes;2 points,poor eye closure strength with the ability to close the eyelids with difficulty and exposed eyelashes;3 points,inability to close the eyelids or puff out the cheeks without leaking air;and 4 points,inability to pout lips while maintaining a mask-like face.(g)Chewing and swallowing functions were evaluated using the following scoring system: 0 points,ability to eat normally;2 points,fatigue after eating standard food and prolonged eating time but no effect on the amount of food consumed;4 points,fatigue after eating standard food,prolonged eating time,and decreased food intake;6 points,inability to eat standard food and consumption of semiliquid food only;and 8 points,feeding by nasogastric tube.(h) Respiratory muscle function was evaluated using the following scoring system: 0 points,normal function;2 points,shortness of breath during light activity;4 points,shortness of breath when walking on flat ground;6 points,shortness of breath when sitting still;and 8 points,assisted respiration.The relative score was calculated as follows: relative score=[(total score before treatment- total score after treatment)/ total score before treatment] × 100%.
2.6.Statistical methods
The data were entered into Microsoft Excel 2016 to establish the MG patient information database.Measurement data are expressed as mean ± standard deviation (±s).
3.RESULTS
3.1.General information
3.1.1 Age of onset,sex,and disease course
综上所述,段成式的交游范围十分广泛,涉及诗人、僧人、官员、仆人以及其他各色人等,但重点交游范围为喻凫、元繇与韦蟾等诗人,以及僧无可、僧栖简与僧靖奢等僧人。从时间上来看,前期与僧人往来较多,后期与诗友交往较多。从地点上来看,属襄阳一地交游范围广,次数频繁,段成式的存世诗歌很大一部分作于此地。段成式的交游既与他的宦海沉浮有重要影响(如李德裕、徐商等),也为他的诗文与笔记小说创作提供了丰厚的艺术土壤,最终成就了《酉阳杂俎》一书内容繁杂、五花八门、包罗万象的特点。
Among the 100 patients with type I or II MG,the youngest age of onset was 4 years,and the oldest age of onset was 81 years.The average age of onset was (52.6± 16.5) years.Overall,59 males and 41 females were included;the male-to-female ratio was 1.44:1.The shortest disease course was 1 month,and the longest disease course was 173 months.The average disease course was (39.7 ± 36.0) months.In group A,the maleto-female ratio was 5:9,with a mean age of (42.4 ± 19.7)years and a mean disease duration of (48.3 ± 45.9)months.In group B,the male-to-female ratio was 45:23,with a mean age of (54.1 ± 16.3) years and a mean disease duration of (38.6 ± 35.8) months.In group C,the male-to-female ratio was 5:8,with a mean age of (51.6 ±9.5) years and a mean disease duration of (42.8 ± 27.5).In group D,the male-to-female ratio was 4:1,the mean age was (63.8 ± 7.4) years,and the mean duration of illness was (23.4 ± 9.3) months.
3.1.2 Dosages of Western medicines
Of the 100 patients,86 had been taking various doses of pyridostigmine bromide (as tablets),prednisone,and/or tacrolimus prior to their initial hospital visits.The average duration of Western medicine use was (11.2 ±9.5) months (range: 0.5-36 months).In Group B,the average dosage of pyridostigmine bromide tablets at the initial hospital visit was (3.1 ± 1.7) tablets/d,and the average duration of administration was (10.8 ± 8.8)months.In Group C,the average dosage of pyridostigmine bromide tablets at the initial hospital visit was (3.3 ± 1.4) tablets/day,the average dosage of prednisone was (5.6 ± 3.3) tablets/d,and the average duration of administration of Western medicine was(10.8 ± 8.8) months.In group D,the average dosage of pyridostigmine bromide tablets at the initial hospital visit was (3.1 ± 1.0) tablets/d,the average dosage of tacrolimus was (2.4 ± 0.9) tablets/d,and the average duration of administration of Western drugs was (9.5 ±6.2) months.
3.2.Classification based on Western medical standards
Of the 100 patients,45 patients were diagnosed with type I MG (45/100,45%),19 patients were diagnosed with type IIa MG (19/100,19%),and 36 patients were diagnosed with type IIb MG (36/100,36%).Group A included 8 cases of type I MG (8/14,57.14%),2 cases of type IIa MG (2/14,14.29%),and 4 cases of type IIb MG(4/14,28.57%).Group B included 31 cases of type I MG(31/68,45.59%),13 cases of type IIa MG (13/68,19.12%),and 24 cases of type IIb MG (24/68,35.29%).Group C included 3 cases of type I MG (3/13,23.08%),4 cases of type IIa MG (4/13,30.77%),and 6 cases of type IIb MG (6/13,46.15%).Group D included 3 cases of type I MG (3/5,60.00%),0 cases of type IIa MG (0/5,0%),and 2 cases of type IIb MG (2/5,40.00%).
3.3.Outcomes
3.3.1 Overall outcomes of the 100 MG patients
Of the 100 patients,the condition of 19.00% improved,38.00% showed a significant effect,11.00% basically recovered,and 18.00% were clinically cured.The total effectiveness rate was 86.00%.Eighty-six MG patients had been taking various doses of Western medicine(pyridostigmine bromide tablets,prednisone,and/or tacrolimus) at the time of the initial hospital visit.Of these patients,70.93% began taking reduced doses of the Western medicines at some point during the study,and 43.02% stopped taking the Western medicines by the end of the study.
3.3.2 Outcomes of the patients in each group
The MG patients treated solely with Fufang Huangqi decoction (group A) exhibited an effectiveness rate of 85.71%.Of these patients,28.57% showed improvement in their condition,35.71% showed a significant effect,0%basically recovered,and 21.43% were clinically cured.The effectiveness rate in the pyridostigmine bromide tablets+Fufang Huangqi decoction group (group B) was 88.24%.Of the patients in group B,16.18% showed an improved condition,42.64% showed a significant effect,10.29% basically recovered,19.12% were clinically cured,69.12% began taking a reduced dose of the pyridostigmine bromide tablets,and 45.59% stopped taking the pyridostigmine bromide tablets.The effectiveness rate in the pyridostigmine bromide tablets+prednisone+Fufang Huangqi decoction group (group C) was 76.92%.Among the patients in group C,23.08%showed an improved condition,23.08% showed a significant effect,15.38% basically recovered,15.38%were clinically cured,46.15% began taking a reduced dose of pyridostigmine bromide tablets,23.08% stopped taking pyridostigmine bromide tablets,76.92% began taking a reduced dose of prednisone,and 23.08% stopped using prednisone.The effectiveness rate in the pyridostigmine bromide tablets+tacrolimus+Fufang Huangqi decoction group (group D) was 80.00%.Of the patients in group D,20.00% showed an improved condition,20.00% showed a significant effect,40.00%basically recovered,and 0% were clinically cured.In addition,1 patient in group D (1/5,20.00%) began to take a reduced dose of pyridostigmine bromide tablets after concurrent treatment with Fufang Huangqi decoction for 1 month.This patient stopped taking pyridostigmine bromide tablets after 3 months and began to take a reduced dose of tacrolimus after 5 months.Another patient (1/5,20.00%) began to take reduced doses of pyridostigmine bromide tablets and tacrolimus after treatment with Fufang Huangqi decoction for 0.5 months.At the end of the observation period,this patient had been taking Fufang Huangqi decoction for 2 months without discontinuing pyridostigmine bromide tablets or tacrolimus.The specific outcomes of the patients in each treatment group are shown in Tables 1-4.
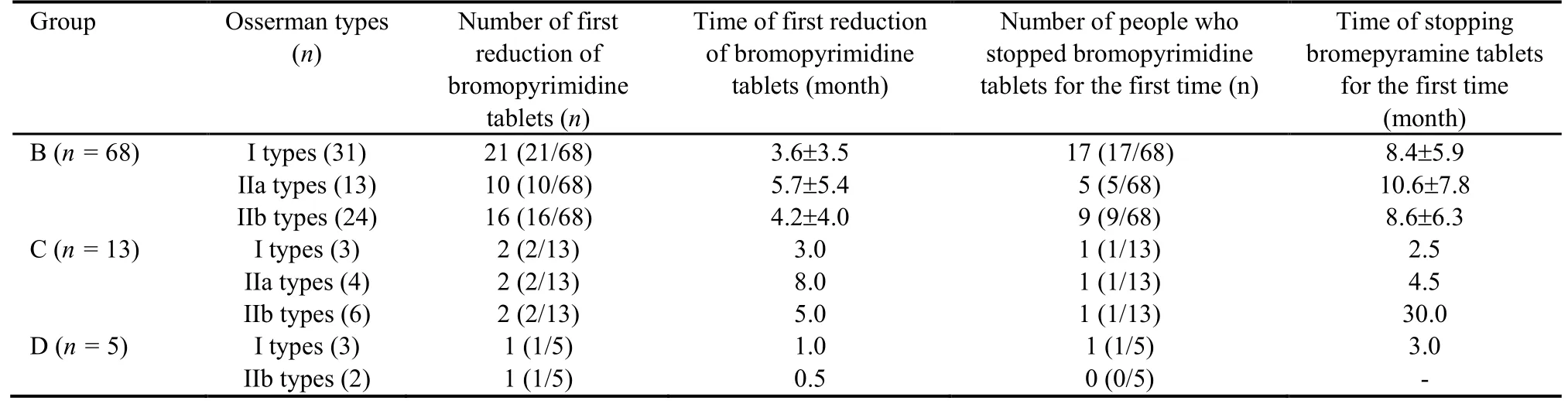
Table 1 Reduction and discontinuation of bromopyrimine tablets in 100 patients
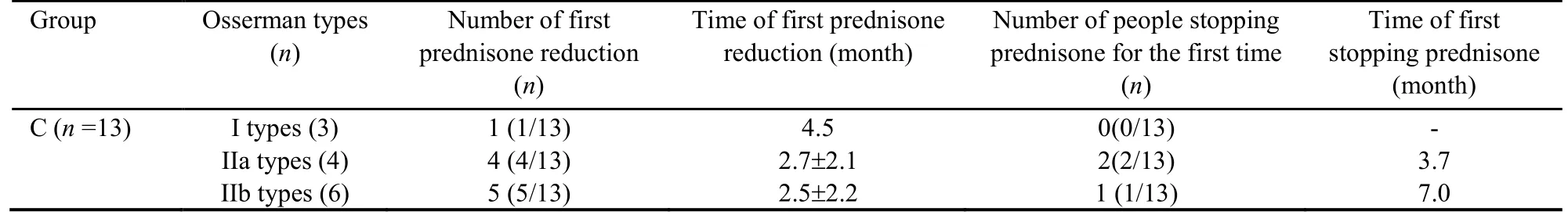
Table 2 Group C patient stopped prednisone
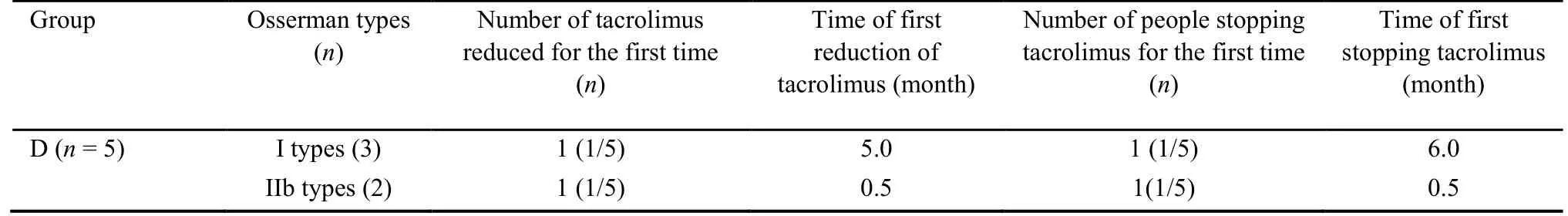
Table 3 Group D patients stopped tacrolimus
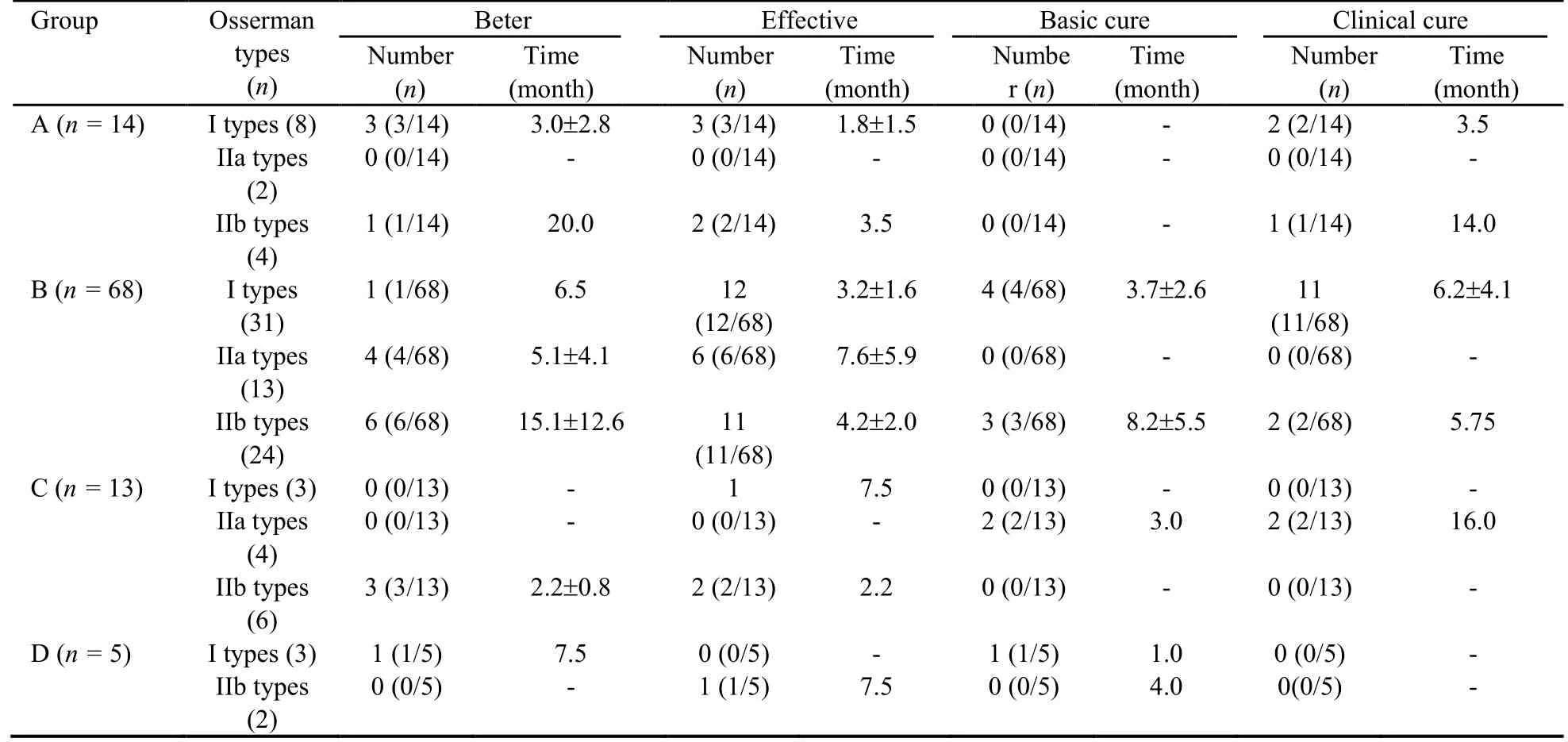
Table 4 Outcome of 100 patients
4.DISCUSSION
MG is a rare neuromuscular disorder.At the present time,most MG patients are treated with cholinesteraseinhibitors or immunosuppressants.Pyridostigmine bromide is a first-line drug that is used to achieve clinical improvement in MG symptoms,while glucocorticoids are currently the first-line drugs for the treatment of MG.Azathioprine is usually administered in combination with glucocorticoids.Tacrolimus is mainly used to treat patients who are intolerant to hormones or other immunosuppressants.Cyclosporine and cyclo-phosphamide are often not the first choice for treatment of MG in clinical practice.When myasthenic crisis occurs,patients are often treated using plasma exchange and intravenous injection of human immunoglobulin.In addition,surgery can be performed for the treatment of thymoma.Treatment of MG patients with Western medicines produces certain adverse reactions.Common adverse reactions to pyridostigmine bromide include nausea,salivation,abdominal pain,and diarrhea.Adverse reactions to glucocorticoids include weight gain,centripetal obesity,elevated blood pressure and blood sugar,osteoporosis,and femoral head necrosis.The adverse effects of azathioprine include bone marrow suppression and liver function damage.Adverse reactions to tacrolimus include elevated blood sugar and impaired liver/kidney function.Adverse reactions to cyclosporine and cyclophosphamide mainly include impairment of liver and kidney function.Plasma exchange and intravenous injection of human immunoglobulin are rather costly.1In recent years,Traditional Chinese Medicine has demonstrated its unique advantages,and its use has led to considerable progress in the treatment of MG.To further examine the therapeutic effect of Fufang Huangqi decoction,we conducted this study.
In this study,the ratio of male patients to female patients was 1.44∶1.The greater number of male than female patients in our study is unusual in that previous studies of MG have reported more female patients than male patients.The underlying reason for this difference remains unclear,but it may be related to the selection of only type I and II MG patients in this study or to the geographical distribution of the research subjects.In our 100 patients,the time required for improvement exceeded the time required to obtain a significant effect of treatment and the times required to achieve basic recovery and clinical recovery.Of the 19 patients whose conditions improved,8 (8/19,42.11%) with refractory MG achieved improvement after (16.2 ± 9.4) months,and 11 (11/19,57.89%) improved after (2.8 ± 2.7)months.No unified criteria are currently available for the diagnosis of refractory MG.This study adopted the generally recognized criteria of Drachman,9as follows:insensitivity to systemic therapies such as conventional cholinesterase inhibitors,glucocorticoids,and immunosuppressants (including ocular muscle-type MG and systemic MG);intolerance of the adverse reactions caused by routine systemic treatment;or an inability to tolerate reduction in the dosages of drugs used during conventional systemic treatment.MG was defined as refractory when one of the above scenarios occurred.
In this study,the effectiveness rates were 85.71% in group A,88.24% in group B,76.92% in group C,and 80.00% in group D.It is likely that the effectiveness rate in group C was lower because most of the patients in groups A,B,and D were type I MG patients,while those in group C were mainly type IIb MG patients.Studies have shown that treatment of MG patients with glucocorticoids for 8-48 weeks can achieve an effectiveness rate of 76.7%-85.0%,10-12consistent with our results for group C.Moreover,a follow-up study conducted over a period of 8 months to 17 years found symptoms to be relieved in 27.65% and significantly improved in 52.6% of MG patients treated with glucocorticoids.In this study,a significant effect was achieved in group D after treatment for 5.25 months.These results are similar to the results reported in previous studies.13,14The effectiveness rate in group D was slightly lower than the rate reported in a previous study (96.67%),11possibly related to the small sample size in group D.We aim to expand the patient sample size and conduct follow-up observations of the patients in group D to further explore the effectiveness rate in this group of patients and clarify the clinical efficacy of treatment with Fufang Huangqi decoction +pyridostigmine bromide tablets+tacrolimus.The effectiveness rate of glucocorticoids and tacrolimus in the treatment of MG is relatively high.However,the incidence of adverse reactions is also elevated.Studies have shown that the incidence of adverse reactions after treatment with prednisone for 6 to 24 months ranges from 39-88%.15,16Other studies have found that the incidence of adverse reactions in hospitalized MG patients receiving hormone treatment for (34.68 ± 1.49) d was 29.41% and that the incidence of adverse reactions after treatment with tacrolimus for (16.48 ± 17.93-26) months was 21.6%-56.3%.13,14,17,18In contrast,our study showed that Fufang Huangqi decoction did not cause adverse reactions during the observation period.Moreover,Fufang Huangqi decoction reduced the adverse reactions caused by Western medicines.
The primary etiology of MG is a decrease in the number of AChRs at the postsynaptic membrane of the neuromuscular junction;this decrease is caused by binding of autoantibodies to AChRs at the postsynaptic membrane,leading to insufficient endplate potentials and muscle transmission dysfunction.The concentration of antibodies may indicate the severity of the disease.Regulatory T cells play a crucial role in the production of autoantibodies and in the development of MG.CD4+T cells can be divided into subtypes according to the cytokines they secrete.Th1 cells mainly secrete interleukin 2 (IL-2),and interferon γ (IFN-γ) and thereby promote cellular immune responses.19,20Some studies have shown elevated IFN-γ or IL-4 expression in peripheral blood mononuclear cells of MG patients,21and IFN-γ expression at the neuromuscular junction can induce an MG-like syndrome.22IFN-γ is associated with AChR antibody levels and can induce chemokine expression in muscle,thymus,and lymph nodes of MG patients and EAMG rats.23It has also been shown that abnormal thymus cells in MG patients have miRNA and mRNA expression patterns that differ from those in the germinal centers of normal individuals and that these miRNAs and mRNAs are involved in the immune response process in MG.24B cells play a crucial role in MG that includes participation in autoantibody production,complement activation,and cytokine release.Studies have shown that autoantibody production by B cells and plasma cells is closely related to the pathogenesis of MG.25Fufang Huangqi decoction is mainly composed of Huangqi (Radix Astragali Mongolici),Danggui (Radix Angelicae Sinensis),Gouqizi(Fructus Lycii),Yimucao(Herba Leonuri Japonici),and Baizhu(RhizomaAtractylodis Macrocephalae).Modern pharmacology has shown that all of these ingredients exert immunomodulatory effects.Huangqi (Radix Astragali Mongolici) has good immunomodulatory ability and can regulate immune function by regulating T lymphocytes and B lymphocytes,26and the combined application of Huangqi(Radix Astragali Mongolici) and Danggui (Radix Angelicae Sinensis)can maintain immune homeostasis by regulating CD4+,CD8+,and IFN-γ.27Gouqizi(FructusLycii)extract can enhance cellular interventional immunity and humoral immune responses.28Yimucao(Herba Leonuri Japonici)can improve lymphocyte function,enhance systemic immunity,29promote the proliferation and differentiation of T and B lymphocytes,and improve the body’s cellular and humoral immune responses.30Studies have shown that treatment with Fufang Huangqi decoction not only reduces serum AChR antibody concentrations but also decreases the number of helper T cells,31increases the number of regulatory T cells,and decreases the CD4+/CD8+ratio;the abnormal immune response function of MG can thus be corrected.In this regard,the efficacy of Fufang Huangqi treatment does not differ from that of drugs in the glucocorticoid group.4Treatment with Fufang Huangqi decoction also reduces IFN-γ,mRNA,and AChR-Ab levels,32promotes the growth of new axons,increases the number of synaptic vesicles at the neuromuscular junction,alleviates synaptic damage in the EAMG rat model,and effectively regulates the levels of expression of several EAMGrelated proteins.The mechanism underlying Fufang Huangqi decoction action may involve selective targeting of lymphocyte subpopulations in which the decoction not only causes helper T cells to migrate to tissues other than blood,leading to a transient decrease in the number of circulating lymphocytes and interfering with cellular immunity,but also enhances the effects of regulatory T cells on B cells,inhibits the conversion of B cells to plasma cells,interferes with humoral immunity,improves the binding of AChRs to antibodies and to corresponding receptors,33and reduces the effect of serum antibodies against AChR on the immune system,thus correcting the immune imbalance of the body and significantly relieving the symptoms of muscle weakness.Fufang Huangqi decoction has been clinically shown to be effective in helping patients reduce and even discontinue the use of Western medicines and to alleviate the adverse effects of Western medicines and reduce relapse,thereby realizing clinical cure or symptom reduction.34
In summary,Fufang Huangqi decoction effectively alleviated the clinical symptoms of patients with type I and type II MG and enabled reductions in the doses of Western medicines (pyridostigmine bromide tablets,prednisone,and tacrolimus) used by these patients.Although the onset time of the clinical effect of Fufang Huangqi decoction exceeded that for Western medicines,the decoction improved the symptoms of most patients with type I or II MG,enabled reductions in the doses of Western medicines in 70.93% of the patients and discontinuation of Western medicines in 45.35% of the patients,reduced the adverse reactions caused by longterm use of Western medicines,and improved the quality of life of the patients without obvious toxic side effects.The results of this study demonstrate the curative effect of Fufang Huangqi decoction in the clinical treatment of patients with types I and II MG and the role of Fufang Huangqi decoction in dose reduction and discontinuation of pyridostigmine bromide tablets,prednisone,and tacrolimus in MG patients and thereby provide a new safe and effective method for the treatment of MG.The limitations of this study are mainly related to the nature of the study design and data collection and the small numbers of patients in certain subgroups.In future studies,we aim to include patients with all types of MG,increase the number of type I and type II MG patients,and extend the duration of the observations.
猜你喜欢
杂志排行
Journal of Traditional Chinese Medicine的其它文章
- Effectiveness and safety of tripterygium glycosides tablet (雷公藤多苷片) for lupus nephritis: a systematic review and Meta-analysis
- Efficacy of green tea extract on PC3 prostate cancer cells through upregulation of miR-195 expression and suppression of epithelial to mesenchymal transition
- Qilan preparation (芪蓝颗粒) inhibits proliferation and induces apoptosis by down-regulating microRNA-21 in human Tca8113 tongue squamous cell carcinoma cells
- Tenglong Buzhong granules (藤龙补中颗粒) inhibits the growth of SW620 human colon cancer in vivo
- Yajieshaba prevents lipopolysaccharide-induced intestinal barrier injury via anti-inflammatory and anti-apoptosis
- Antihepatofibrotic effect of Guizhifuling pill (桂枝茯苓丸) on carbon tetrachloride-induced liver fibrosis in mice
