Protective effects and mechanisms of Lizhong decoction (理中汤)against non-alcoholic fatty liver disease in a rat model
2022-10-14YANGJiayaoTAODongqingMAWeiLIUSongLIAOYanSHULeiZHANGShuLIChenyuDUNianlong
YANG Jiayao,TAO Dongqing,MA Wei,LIU Song,LIAO Yan,SHU Lei,ZHANG Shu,LI Chenyu,DU Nianlong
YANG Jiayao,LIU Song,LIAO Yan,SHU Lei,ZHANG Shu,DU Nianlong,Department of Gastroenterology,Wuhan Integrated TCM and Western Medicine Hospital,Wuhan 430022,China
TAO Dongqing,Department of Endocrinology,The Third People’s Hospital of Hubei Province,Wuhan 430033,China
MA Wei,Department of Center Laboratory,Wuhan Integrated TCM and Western Medicine Hospital,Wuhan 430022,China
LI Chenyu,Hubei University of Traditional Chinese Medicine,Wuhan 430065,China
Abstract OBJECTIVE: To investigate the protective effects and molecular mechanisms of Lizhong decoction (理中汤,LZD) against non-alcoholic fatty liver disease (NAFLD).METHODS: Male Wistar rats were fed a high-fat diet for four weeks to induce NAFLD,and were administered LZD by gavage for four weeks.Potential therapeutic targets for NAFLD were analyzed using network pharmacology.Liver pathology was evaluated using Oil Red O and hematoxylin-eosin staining.Furthermore,mitochondrial function,lipid metabolism,oxidative stress,and inflammatory response were examined.RESULTS: Rats with NAFLD exhibited high levels of hepatic damage and cholesterol deposition.Moreover,apoptosis was increased,superoxide dismutase and glutathione content were reduced,malondialdehyde content was increased,and the protein expression of inflammatory cytokines and p-c-Jun N-terminal kinase was increased.The LZD treatment ameliorated mitochondrial dysfunction,reduced liver damage,inhibited oxidative stress and inflammatory response,upregulated peroxisome proliferator-activated receptor(PPAR)-γ expression,and suppressed dipeptidyl peptidase 4 (DPP4) expression in the liver.CONCLUSION: It was found that LZD alleviates NAFLD by activating PPAR-γ and inhibiting DPP4.
Keywords: non-alcoholic fatty liver disease;PPAR gamma;dipeptidyl peptidase 4;Lizhong decoction
1.INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) is a common cause of chronic liver disease1and is characterized by chronic systemic low-grade inflammation,oxidative stress,insulin resistance,and lipid accumulation in the liver without significant alcohol consumption2.Nonalcoholic fatty liver disease gradually progresses into increasingly severe hepatic disorders,such as nonalcoholic steatohepatitis,hepatic fibrosis,cirrhosis,and hepatocellular carcinoma.3,4Patients with NAFLD have a higher risk of cardiometabolic complications,such as cardiovascular disease and type 2 diabetes.5The two-hit hypothesis is widely used to elucidate the pathogenesis of NAFLD.6Obesity-related hyperinsulinemia and insulin resistance can lead to liver steatosis and promote the hepatic uptake of absolute non-esterified fatty acids,that are further esterified to form triacylglycerols,representing the first hit.7The second hit involves mitochondrial dysfunction,oxidative stress,and an inflammatory response,that ultimately leads to liver damage.8
Lizhong decoction (理中汤,LZD) is a well-known traditional Chinese herbal medicine formulation.Based on the theory of Chinese herbal medicine,LZD replenishes the spleen and warms the energy of Yang.Previous studies have demonstrated that LZD protects against inflammatory reactions,lipid peroxidation,and liver fibrosis.The major components are Dangshen(Radix Codonopsis),Baizhu (Rhizoma Atractylodis Macrocephalae),and Zhigancao (Radix Glycyrrhizae Preparata),which exert protective effects on the liver.However,the molecular mechanisms underlying the action of LZD in NAFLD treatment remain unclear.In this study,we induced a rat model of NAFLD using a high-fat diet and administered LZD as treatment.Therefore,we aimed to explore the effects of LZD on lipid metabolism,oxidative stress,and inflammatory responses in NAFLD-induced rats.
2.MATERIALS AND METHODS
2.1.Preparation of LZD
The chemical profile of LZD is provided in Supplementary Material 1.LZD is composed of Dangshen (Radix Codonopsis) (9 g),Baizhu (Rhizoma Atractylodis Macrocephalae) (9 g),Zhigancao (Radix Glycyrrhizae Preparata) (6 g),and Shengjiang(Rhizoma Zingiberis Recens) (9 g).All herbs were purchased from Hubei Tianji Chinese Herbal Sliced Medicine Co.,Ltd.A water-based decoction was prepared according to a traditional formulation.Aconite was soaked in 10-fold volume of distilled water for 30 min and decocted by boiling at 100°C.The temperature was lowered to 70 °C,and the decoction process was continued for 20 min.Simultaneously,the four other medicinal herbs were soaked in 10-fold volume of distilled water for 30 min.The two abovementioned mixtures were merged and decocted by boiling at 100 °C.The temperature was lowered to 70 °C,and the decoction process was continued for 30 min.Thereafter,double-layer gauze filtration was performed and the filtrate was retained.The residue was added to an 8-fold volume of distilled water and decocted at 100 °C.The temperature was lowered to 70 °C,and the decoction process was continued for 30 min.Double-layer gauze filtration was performed,and the filtrate was retained.The two filtrates were merged,concentrated,and stored.
2.2.Ultra-high-performance liquid chromatographyquadrupole time-of-flight mass spectrometry (UHPLCQTOF-MS)
A UHPLC apparatus was used to perform UHPLCQTOF-MS and a Triple TOF 5600 high-resolution mass spectrometer (AB Sciex,MA,USA).Metabolites were extracted by adding 300 μL of extract solution(containing 1 μg/mL internal standard in methanol) to 100 μL of the sample.The mixture was ultrasonicated in a water bath for 1 h,and the ultracentrifuged at 4°C and 12 000 rpm for 10 min.Next,UHPLC was performed on the supernatant using a UPLC BEH C18 column (1.7 μm× 2.1 × 100 mm;Waters,MA,UK).The mobile phase was water/0.1% formic acid (solvent A) and acetonitrile/0.1% formic acid (solvent B) at a gradient of 95/85/70/70/30/30/0/0% solvent A for 0/3.5/6/6.5/12/12.5/18/22 min,respectively,at a flow rate of 400 μL/min.The injection volume was 5 μL.The AB 5600 Triple TOF mass spectrometer is capable of performing primary and secondary mass spectrometry data acquisition based on the IDA function in the control software (Analyst TF 1.7,AB Sciex,MA,USA).In each data acquisition cycle,the most intense molecular ions (>100) were selected for the corresponding acquisition of secondary mass spectral data.The bombardment energy was 40 eV,the collision energy difference was 20 V,and 15 secondary spectra were acquired every 50 ms.The electrospray ionization parameters were as follows:atomization pressure (GS1),55 psi;auxiliary pressure,55 psi;air curtain pressure,35 psi;temperature,550°C;spray voltage,5500 V (positive ion mode) or -4000 V(negative ion mode).Data were analyzed using the Progenesis QI software (Waters,MA,USA).
2.3.Predicting targets of active substance and gene targets of NAFLD
The protein targets of the active substances in LZD were obtained from the Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform(TCMSP).Human genes associated with NAFLD were identified from the DiSGeNET and CooLGeN databases.The NAFLD targets were mapped using the Pharmacogenetics and Pharmacogenomics Knowledge Base (PharmGKB),Therapeutic Target Database (TTD),and Online Mendelian Inheritance in Man (OMIM)databases.
2.4.Animals and treatments
Specific-pathogen-free male Wistar rats (8 weeks old,180–200 g) were obtained from the Animal Center of Hubei Province (Wuhan,China).All experimental procedures were approved by the Animal Ethics Committee of Wuhan Integrated TCM and Western Medicine Hospital (certificate No.42000600013948).The rats were maintained at constant temperature (21 ±1) °C and humidity (50% ± 15%) under a 12/12 h light/dark cycle,with free access to deionized water and food disinfected though irradiated.After one week of adaptive feeding,the rats were fed a standard chow diet(n=8) as the negative control (NC) or a high-fat diet for four weeks to induce NAFLD (n=32).The high-fat diet was composed of solution A and solution B.Solution A was prepared by dissolving 25 g of axungia through heating.After stirring,10 g of cholesterol and 1 g of propylthiouracil tablet were added and thoroughly mixed.Next,25 mL of Tween 80 was added and mixed.Solution B was prepared by adding 20 mL of propylene glycol to 30 mL of water and heating to 60°C.Sodium deoxycholate (2 g) was added and mixed.Solutions A and B were mixed,and water was added the final volume was made up to 100 mL.The rats were administered the same dose of standard chow diet and high-fat diet daily by gavage at 10:00 a.m.NAFLD-induced rats were randomly divided into five groups (n=8 per group):model (MOD),high-dose LZD (HIG),medium-dose LZD (MID),low-dose LZD (LOW),and positive control(PC).The rats in the HIG,MID,and LOW groups received LZD treatment at 16,8,and 4 g·kg-1·d-1,respectively,and the rats in the PC group were treated with 250 mg·kg-1·d-1vitamin E.Rats in the NC and MOD groups were administered the same volume of normal saline by gavage for four weeks.
2.5.Biochemical analysis
At the end of the treatment period,the rats were anesthetized using 40 mg/kg pentobarbital after 12 h of fasting,and liver samples were collected from the rats.Triglyceride (TG),total cholesterol (TC),low-density lipoprotein cholesterol (LDL-C),high-density lipoprotein cholesterol (HDL-C),alanine aminotransferase(ALT),superoxide dismutase (SOD),malondialdehyde(MDA),and glutathione (GSH) levels in the liver were determined using commercial kits (Nanjing Jiancheng Bioengineering Institute,Nanjing,China) according to the manufacturer’s instructions.
2.6.Histopathology
Fresh liver tissue was stored at -80 °C for subsequent histopathological and molecular assays.Frozen sections were stained with Oil Red O and hematoxylin-eosin (HE)to investigate the architecture of the hepatic lipid droplets.The stained slides were visualized using an Olympus microscope (Tokyo,Japan),and images were captured using an Olympus digital camera.
2.7.Flow cytometry
Cells were resuspended in cell culture medium (0.5 mL)and JC-1 staining working solution (0.5 mL) was added.Following 20 min of incubation at 37 °C,the cells were centrifuged for 3 min at 400 ×g.The supernatant was discarded and the cells were washed twice with JC-1 dye buffer.Subsequently,400 μL of JC-1 staining buffer was added to resuspend the cells,and flow cytometry was performed (Beckman,State of California,CA,USA).
2.8.Western blot
Proteins in liver tissue homogenates were extracted using ice-cold lysis buffer.Protein concentrations were determined using a bicinchoninic acid protein assay kit(Bioswamp Life Science Lab,Wuhan,China).Proteins were loaded at 30 μg per lane and separated using 10%sodium dodecyl sulfate-polyacrylamide gel electrophoresis.After electrophoresis,the proteins were transferred to polyvinylidene fluoride membranes,blocked in 5% non-fat milk for 1 h,and incubated at 4 °C overnight with primary antibodies against peroxisome proliferator-activated receptor (PPAR)-γ (PAB30089,1∶1000;Bioswamp,Wuhan,China);dipeptidyl peptidase 4(DPP4,PAB32046,1 ∶1000;Bioswamp,Wuhan,China);tumor necrosis factor (TNF)-α (ab6671,1∶1000;Abcam,Cambridge,UK);interleukin (IL)-1β (ab2105,1∶1000,Abcam,Cambridge,UK);IL-6(ab9324,1∶5000,Abcam,Cambridge,UK);andp-c-Jun N-terminal kinase(p-JNK,MAB43555-p,1∶1000,Bioswamp,Wuhan,China).The membranes were then incubated with goat anti-rabbit secondary antibodies (Bioswamp,Wuhan,China),and the protein bands were visualized using an enhanced chemiluminescence system (GE Healthcare,MA,USA) and quantified using Quantity One software(Bio-Rad,CA,USA).
2.9.Statistical analysis
All data are expressed as the mean ± standard deviation.Statistical differences were determined by one-way analysis of variance and post hoc tests.Statistical significance was set atP < 0.05.Statistical analyses were performed using the statistical software SPSS 23.0 (IBM,Armonk,NY,USA).
3.RESULTS
3.1.Identification of active ingredients in LZD
According to the UHPLC-QTOF-MS results(Supplementary Material 1),seven compounds were identified as the active ingredients of LZD (LQ.POS.B >10000): (S)-6-gingerol,(S)-10-gingerol,codonopsine,ononin,licoisoflavanone,glyasperin C,and gancaonin B(Table 1).
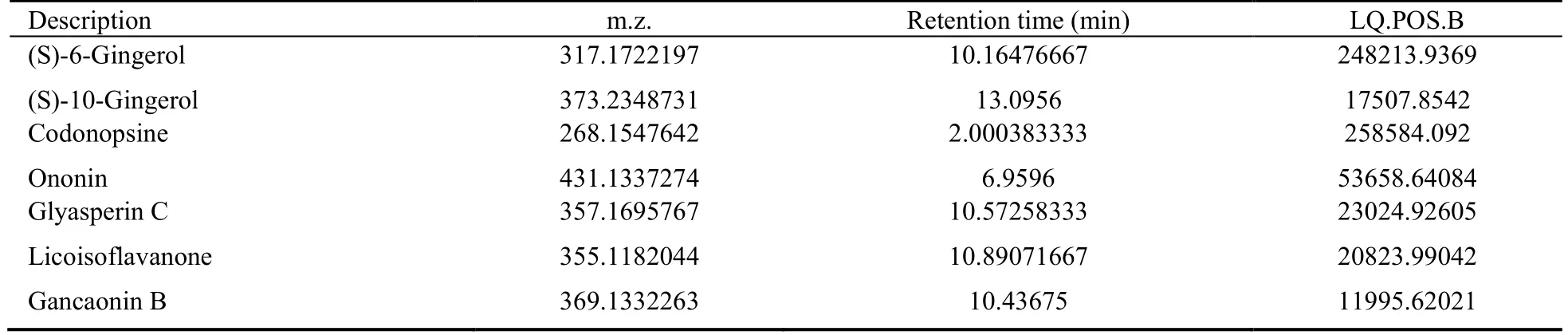
Table 1 Candidate active substances of LZD
3.2.Identification of targets of LZD in NAFLD
We obtained 64 protein targets from the TCMSP database (Supplementary Material 2) based on the seven candidate active components.Human genes related to NAFLD were screened based on the DiSGeNET and CooLGeN databases and mapped using the PharmGKB,TTD,and OMIM databases (Supplementary Material 3).In total,76 NAFLD-related human genes were identified.We found that four targets (TP53,JUN,DPP4,and PPAR-γ) among the seven candidate active components in LZD were related to NAFLD,suggesting that LZD may play a therapeutic role in NAFLD by regulatingTP53,JUN,DPP4,and PPAR-γ.The effect of LZD on NAFLD and its regulatory effects on PPAR-γ and DPP4 were subsequently investigated.
3.3.Effect of LZD on serological examination and histological changes in NAFLD-induced rats
As shown in Figure 1A-1E,the NAFLD (MOD group)showed significant increases the levels of TC,TG,LDLC,and ALT and decreases in HDL-C in liver tissues (P< 0.05) when compared with the NC group.After LZD treatment,the levels of hepatic TC,TG,LDL-C,and ALT decreased,and those of hepatic HDL-C increased(P< 0.05).Lipid accumulation in the liver samples was examined by ORO (Oil Red O) staining (Figure 1F-1K).A few lipid droplets were detected in the liver of the NC group rats,whereas NAFLD-induced rats showed extensive lipid deposition in the liver.Treatment with LZD remarkably reduced lipid accumulation in NAFLD rats.In addition,HE (hematoxylin-eosin) staining was performed to observe pathological changes in the rat livers (Figure 1L-1Q).The NC group showed clear hepatic lobular structures,and the liver cells were arranged radially around the central vein.In the MOD group,the hepatic lobules were incomplete,liver cells were disorderly arranged,many fat vacuoles appeared in the cells,and inflammatory cell infiltration and focal necrosis were observed in certain areas.Compared with that of the MOD group,the number of fat vacuoles was reduced and inflammation was alleviated in the LZDtreated rats.
3.4.Effect of LZD on mitochondrial function in NAFLD-induced rats
As shown in Figure 2,the green fluorescence flow cytometry signal in the MOD group is significantly increased compared with that in the NC group (P< 0.05),indicating a decrease in mitochondrial membrane potential.However,the mitochondrial membrane potential was remarkably recovered by LZD treatment in a dose-dependent manner (P< 0.05).These results indicate that LZD can counteract mitochondrial dysfunction in NAFLD-induced rats.
3.5.Effect of LZD on oxidative stress in NAFLDinduced rats
The levels of SOD,MDA,and GSH were measured to evaluate the effects of LZD on oxidative stress in NAFLD-induced rats (Figure 3).Compared with that of the NC group,SOD and GSH activities were significantly reduced and MDA activity was significantly increased in the MOD group (P< 0.05).Thus,LZD treatment significantly reversed these effects.
3.6.Effect of LZD on PPAR-γ and DPP4 expression in NAFLD-induced rats
The protein expression of PPAR-γ and DPP4 was detected in liver samples from rats treated with various doses of LZD (Figures 4A,4B,4H).Evidently,PPAR-γ expression was significantly downregulated in the livers of NAFLD-induced rats compared with those in the NC group,while DPP4 expression was significantly upregulated (P< 0.05).The protein expression levels of PPAR-γ and DPP4 were reversed by LZD treatment (P<0.05).
3.7.Effect of LZD on inflammatory response in NAFLD-induced rats
As shown in Figure 4C-4F,Figure 4I,the levels of TNFα,IL-1β,IL-6,and p-JNK in the MOD group are markedly decreased compared with those in NC rats,however,they were subsequently increased by LZD.
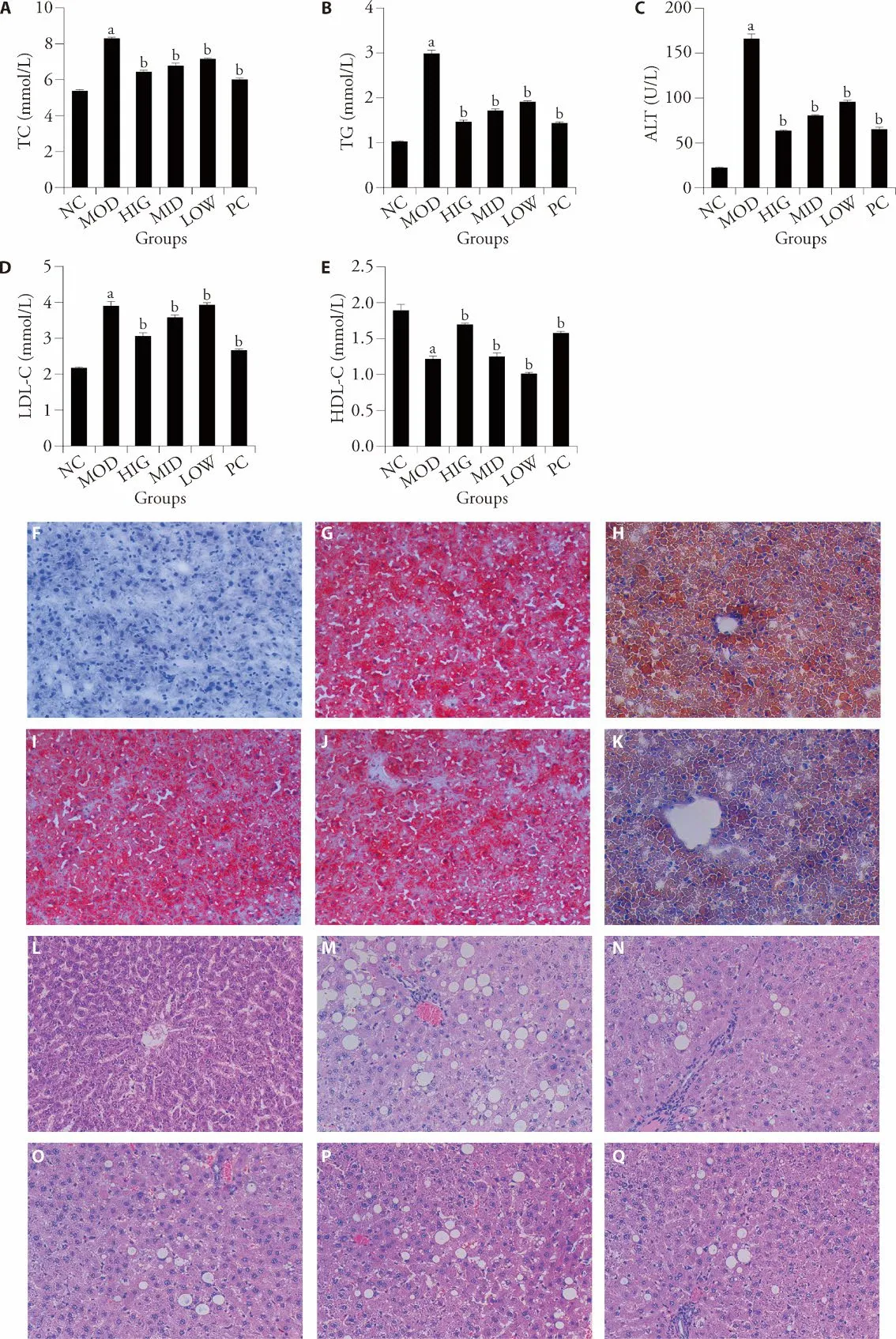
Figure 1 LZD alleviates NAFLD symptoms
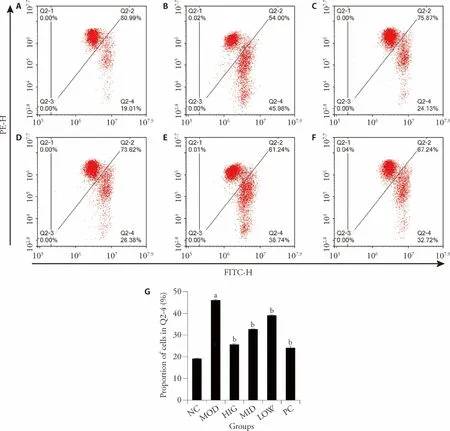
Figure 2 Effect of LZD treatment on mitochondrial function in NAFLD-induced rats
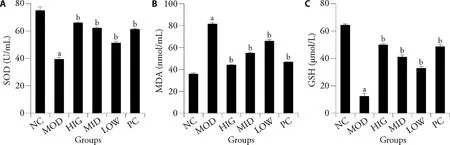
Figure 3 LZD reduces oxidative stress in liver of rats fed a high-fat diet
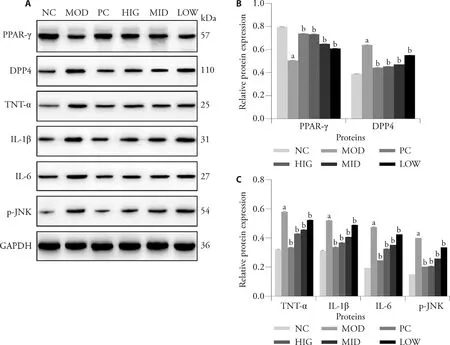
Figure 4 LZD inhibits the inflammatory response in the livers of rats fed a high-fat diet
4.DISCUSSION
The liver is an important hub for maintaining the lipid balance in the body.9Hepatic steatosis caused by various factors can disrupt lipid metabolism in the liver.In this study,we established a high-fat diet-induced NAFLD rat model and explored the protective mechanism of LZD against NAFLD.Severe fatty liver degeneration was observed in NAFLD rats using H&E and ORO staining.Compared with those of the NC group,the changes in the serum levels of TG,TC,LDL-C,and HDL-C in the MOD group indicated that lipid metabolism was disrupted.Notably,LZD significantly increased the HDL-C level and decreased the TG,TC,and LDL-C levels,revealing its role in improving dyslipidemia.ALT is one of the most sensitive indicators of liver function.10,11Long-term high-fat diets can cause severe liver damage,leading to elevated serum ALT levels.Our results showed that LZD inhibited changes in serum ALT in NAFLD-induced rats,further confirming the liverprotective effect of LZD.
Mitochondria are important organelles for intracellular ATP production and oxidative phosphorylation that maintain the dynamic balance between division and fusion and provide energy for complex and orderly biological activities.12Mitochondrial apoptosis reportedly occurs through the endogenous pathway and is primarily caused by changes in mitochondrial membrane potential.13Our flow cytometry analyses revealed that the mitochondrial membrane potential of NAFLD-induced rats was remarkably recovered by LZD in a dose-dependent manner.These results revealed that LZD treatment improved mitochondrial function.
Oxidative stress plays an important role in regulating NAFLD from steatosis to non-alcoholic steatohepatitis,cirrhosis,and fibrosis.14,15SOD eliminates free radicals generated during normal oxygen metabolism,and significantly reduces cellular damage.16GSH participates in the redox process and resists free radical damage to vital organs.17MDA is the toxic end product of lipid peroxides induced by oxygen free radicals,which indirectly reflects the ability to eliminate free radicals.18In this study,the levels of SOD and GSH in the MOD group were significantly lower than those in the NC group.In turn,LZD inhibited oxidative stress by increasing SOD and GSH activity and reducing MDA levels in the livers of NAFLD-induced rats.
As previously reported,the inflammatory response is a crucial mechanism for the occurrence and development of NAFLD,and pro-inflammatory cytokines such as IL-1β,TNF-α,and IL-6 play important roles in the pathophysiological process of NAFLD.19,20The JNK signaling pathway,which is closely related to the pathogenesis of NAFLD,can be activated by inflammatory factors and free fatty acids.21From the experimental results,we found that the levels of inflammatory factors and JNK were upregulated in rats fed a high-fat diet.Interestingly,LZD remarkably reduced the levels of these proteins,contributing to the alleviation of NAFLD.
A ligand-activated nuclear transcription factor,PPAR-γ,plays an important role in inhibiting the production of inflammatory factors.22DPP4 has been confirmed to be highly expressed in the liver and is involved in the development of chronic liver disease.23Notably,we found through network pharmacological analysis that the effect of LZD on NAFLD was related to PPAR-γ and DPP4,as confirmed using basic experiments.
In conclusion,LZD regulates lipid metabolism,oxidative stress,and inflammatory response in NAFLD-induced rats by acting on PPAR-γ and DPP4,ultimately exerting its therapeutic effects.
杂志排行
Journal of Traditional Chinese Medicine的其它文章
- Effectiveness and safety of tripterygium glycosides tablet (雷公藤多苷片) for lupus nephritis: a systematic review and Meta-analysis
- Efficacy of green tea extract on PC3 prostate cancer cells through upregulation of miR-195 expression and suppression of epithelial to mesenchymal transition
- Qilan preparation (芪蓝颗粒) inhibits proliferation and induces apoptosis by down-regulating microRNA-21 in human Tca8113 tongue squamous cell carcinoma cells
- Tenglong Buzhong granules (藤龙补中颗粒) inhibits the growth of SW620 human colon cancer in vivo
- Yajieshaba prevents lipopolysaccharide-induced intestinal barrier injury via anti-inflammatory and anti-apoptosis
- Antihepatofibrotic effect of Guizhifuling pill (桂枝茯苓丸) on carbon tetrachloride-induced liver fibrosis in mice
