干扰lncRNA H19通过调控Notch信号通路增强耐顺铂乳腺癌细胞的药物敏感性*
2022-10-13施文标金晓燕庞文洋王远帆汪正一张强
施文标, 金晓燕, 庞文洋, 王远帆, 汪正一, 张强
干扰lncRNA H19通过调控Notch信号通路增强耐顺铂乳腺癌细胞的药物敏感性*
施文标, 金晓燕, 庞文洋, 王远帆, 汪正一, 张强△
(台州市立医院乳腺外科,浙江 台州 318000)
探究长链非编码RNA(long non-coding RNA, lncRNA) H19对乳腺癌细胞顺铂(cisplatin/cis-diamminodichloroplatinum, DDP)耐药性的作用及其分子机制。构建耐DDP人乳腺癌细胞株MCF7-Re;采用RT-qPCR检测lncRNA H19在人正常乳腺上皮细胞株MCF10A及人乳腺癌细胞株MCF7和MCF7-Re中的表达水平。构建lncRNA H19干扰质粒;采用CCK-8法检测DDP处理后MCF7-Re细胞的活力;采用TUNEL法检测细胞凋亡;Western blot检测Bcl-2、Bax、cleaved caspase-3、ATP结合盒蛋白B亚家族成员1(ATP-binding cassette protein subfamily B member 1, ABCB1)、Notch1和发状分裂相关增强子1(hairy and enhancer of split 1, Hes1)的表达;构建Notch1过表达质粒,进行逆转实验验证lncRNA H19的作用机制。lncRNA H19在MCF7-Re细胞中表达显著增加。干扰H19在DDP处理后进一步降低MCF7-Re细胞活力(<0.01),耐药蛋白ABCB1表达显著降低(<0.01),细胞凋亡率显著升高(<0.01),Bcl-2蛋白表达显著减少(<0.01),Bax和cleaved caspase-3蛋白表达显著增加(<0.01),并且Notch信号通路相关蛋白(Notch1和Hes1)的表达减少(<0.01)。转染Notch1过表达质粒后,干扰lncRNA H19表达的MCF7-Re细胞活力得到一定程度的上升(<0.01),耐药蛋白ABCB1表达显著增加(<0.01),细胞凋亡率显著降低(<0.01),Bcl-2蛋白表达显著增加(<0.01),Bax和cleaved caspase-3蛋白表达显著减少(<0.01)。干扰lncRNA H19通过抑制Notch信号通路抑制耐DDP乳腺癌细胞的增殖,并增强该细胞对DDP的敏感性。
长链非编码RNA H19;乳腺癌;顺铂;耐药性;Notch信号通路
乳腺癌是最常见的威胁生命的癌症,也是女性癌症相关死亡的主要原因[1]。近数十年间,随着对乳腺癌生物学的深入了解及现代医疗科技的进步,通过手术、放射和药物治疗可缓解乳腺癌症状和延长生存期[2]。然而,目前临床治疗中肿瘤细胞出现的耐药性大大降低了药效并导致药物治疗失败[3-4]。顺铂(cisplatin/cis-diamminodichloroplatinum, DDP)是一种广谱抗肿瘤药物,是晚期乳腺癌患者常用的化疗药物之一,对包含乳腺癌在内的多种癌症具有短期治疗效果[5],但是由于大多数乳腺癌对DDP存在的原发性耐药或获得性耐药限制了其在临床的运用[6]。因此,研究提高DDP化疗敏感性的机制对乳腺癌的治疗非常重要。
长链非编码RNA(long non-coding RNA, lncRNA)在癌症[7]、免疫[8]、炎症[9]等许多生物学过程中具有重要作用。有研究发现,lncRNA H19在上皮-间充质转化过程中介导卵巢癌细胞的DDP耐药和迁移[10]。lncRNA H19表达在耐多柔比星的乳腺癌细胞中显著上调[11]。但目前lncRNA H19对乳腺癌DDP耐药性的影响未见报道。据报道,在大鼠脊髓损伤模型中,电针通过下调H19/EZH2轴抑制Notch信号通路,从而促进大鼠恢复[12]。沉默人乳腺癌MCF7细胞可促进细胞凋亡[13]。沉默通过Notch/Snail通路可部分恢复结肠癌耐药细胞株LoVo/5-FU对化疗药物的敏感性[14]。
因此,本研究通过构建耐DDP的人乳腺癌细胞株MCF7-Re,初步探讨lncRNA H19对乳腺癌DDP敏感性的影响及其分子机制,以期为进一步研究对抗乳腺癌DDP耐药性的治疗手段提供理论参考。
材料和方法
1 主要仪器与试剂
人正常乳腺上皮细胞株MCF-10A(武汉普诺赛生命科技有限公司);人乳腺癌细胞株MCF7(武汉普诺赛生命科技有限公司);DDP(上海源叶生物科技有限公司;批号:S31072);TUNEL染色试剂盒(上海碧云天生物技术有限公司;批号:C1086);CCK-8试剂盒(Biosharp;批号:BS350A);TRIzol试剂和Lipofectamine™ 2000(Invitrogen);cDNA合成试剂盒(TaKaRa);引物序列及siRNA-H19和siRNA-NC质粒(上海吉康生物科技有限公司);Bcl-2兔多克隆抗体(批号:ab196495)、Bax兔单克隆抗体(批号:ab32503)、cleaved caspase-3兔多克隆抗体(批号:ab32042)、Notch1兔单克隆抗体(批号:ab52627)、发状分裂相关增强子1(hairy and enhancer of split 1, Hes1)兔多克隆抗体(批号:ab71559)、β-actin兔多克隆抗体(批号:ab8227)和辣根过氧化物酶(horseradish peroxidase, HRP)标记的山羊抗兔IgG Ⅱ抗(批号:ab6721)均购自Abcam;ATP结合盒蛋白B亚家族成员1(ATP-binding cassette protein subfamily B member 1, ABCB1)兔单克隆抗体(Cell Signaling Technology;批号:13342);十二烷基硫酸钠-聚丙烯酰胺凝胶电泳(sodium dodecyl sulfate-polyacrylamide gel electrophoresis, SDS-PAGE)及转移装置(上海优宁维生物科技股份有限公司);LAS400凝胶成像系统(GE)。
2 细胞培养、耐药细胞构建和转染
用含10%胎牛血清的RPMI-1640培养液在37 ℃、5% CO2的细胞培养箱中培养MCF-10A和MCF7细胞。通过使用DDP干预构建耐DDP的MCF7细胞(MCF7-Re细胞)。使用含1 μmol/L DDP的培养液来维持DDP抗性,当细胞处于对数生长期时,0.25%胰蛋白酶消化,调整细胞密度为5×107/L。取10 mL细胞悬液接种到培养瓶中培养24 h直至贴壁,首先加入0.5 μmol/L DDP孵育48 h,然后更换培养液。消化后,加入2.5 μmol/L DDP培养48 h。后续逐渐增加DDP浓度至5和10 μmol/L,得到耐受10 μmol/L DDP的MCF7-Re细胞。取对数生长期细胞,使用Lipofectamine™ 2000将siRNA-H19、siRNA-NC、Ov-NC或Ov-Notch1转染至MCF7-Re细胞。转染24 h后,更换培养液继续培养24 h,收集细胞,RT-qPCR检测质粒干扰水平。
3 主要方法
3.1RT-qPCR使用TRIzol试剂从各实验组MCF7-Re细胞中提取总RNA,使用cDNA合成试剂盒将RNA反转录为cDNA,使用MiniOpticon real-time PCR检测系统进行PCR,以GAPDH为内参照,检测Notch1和lncRNA H19表达水平。Notch1的正向引物序列为5'-AGGCTCTGCCGACATCA-3',反向引物序列为5'-AGGAAGGGGTGCTCTGG-3';lncRNA H19的正向引物序列为5′-ATCGGTGCCTCAGCGTTCGG-3′,反向引物序列为5′-CTGTCCTCGCCGTCACACCG-3′;内参照GAPDH的正向引物序列为5'-ACAACTTTGGTATCGTGGAAGG-3',反向引物序列为5'-GCCATCACGCCACAGTTTC-3'。
3.2CCK-8法检测细胞活力取对数生长期的MCF7及MCF7-Re细胞,接种于96孔板中,每孔1.0×104个。细胞贴壁后,以不同剂量(0、2.5、5、10和20 μmol/L)的DDP处理24 h后加入10% CCK-8试剂,在细胞培养箱中培养4 h,酶标仪检测450 nm处吸光度,计算细胞相对活力。同时,在使用siRNA-H19或siRNA-NC质粒转染MCF7-Re细胞后,以不同剂量(0、2.5、5、10和20 μmol/L)的DDP处理24 h,加入10% CCK-8试剂,在细胞培养箱中培养4 h,酶标仪检测450 nm处吸光度,计算细胞活力。根据CCK-8实验结果,使用20 μmol/L的DDP用于后续实验
3.3TUNEL法检测细胞凋亡取对数生长期的MCF7-Re细胞,分为对照组、siRNA-NC组、siRNA-H19组、DDP组、DDP+siRNA-NC组和DDP+siRNA-H19组,取各组细胞进行切片,使用二甲苯透明、梯度乙醇脱蜡,将各组细胞切片用蛋白酶处理20 min,之后用TUNEL反应混合液于37 ℃避光孵育1 h,凋亡细胞会被染成绿色,在荧光显微镜下观察(×400)。凋亡率(%)=阳性染色细胞数/总细胞数×100%
3.4Western blot取对数生长期的MCF7-Re细胞,分为对照组、siRNA-NC组、siRNA-H19组、DDP组、DDP+siRNA-NC组和DDP+siRNA-H19组。将接种细胞的6孔板置于冰上,PBS漂洗后加入裂解液提取总蛋白,测定蛋白浓度。煮沸变性蛋白样品,以每孔50 μg蛋白进行SDS-PAGE,70 V电压下跑至分界面时,变换电压为110 V,300 mA恒流转膜1 h,5%牛血清白蛋白封闭2 h,加入Bax、Bcl-2、cleaved caspase-3、ABCB1、Notch1、Hes1和β-actin抗体,4 ℃孵育过夜后加入Ⅱ抗,室温孵育1 h,取出条带,加入ECL显影液上机检测,结果用ImageJ软件对条带进行灰度值定量分析。
4 统计学处理
使用GraphPad Prism 8.1软件处理数据。结果以均数±标准差(mean±SD)表示。两组间均数比较采用检验;多组间均数比较采用单因素方差分析及LSD-检验。以<0.05表示差异有统计学意义。
结果
1 lncRNA H19在耐药的乳腺癌细胞株中表达增加
图1A显示不同剂量的DDP处理MCF7及MCF7-Re细胞后细胞活力的变化。随着DDP剂量的增加,MCF7及MCF7-Re细胞活力逐渐降低,MCF7细胞较MCF7-Re细胞活力下降更明显(<0.01),说明耐DDP乳腺癌细胞株MCF7-Re构建成功。图1B显示,与正常乳腺上皮细胞MCF-10A相比,乳腺癌细胞MCF7中lncRNA H19表达水平显著上调(<0.01),表明lncRNA H19在乳腺癌中的可能发挥促癌作用;与MCF-10A和MCF7细胞相比,lncRNA H19的表达水平在MCF7-Re细胞中显著增加(<0.01),说明lncRNA H19可能在乳腺癌耐药过程中发挥作用。
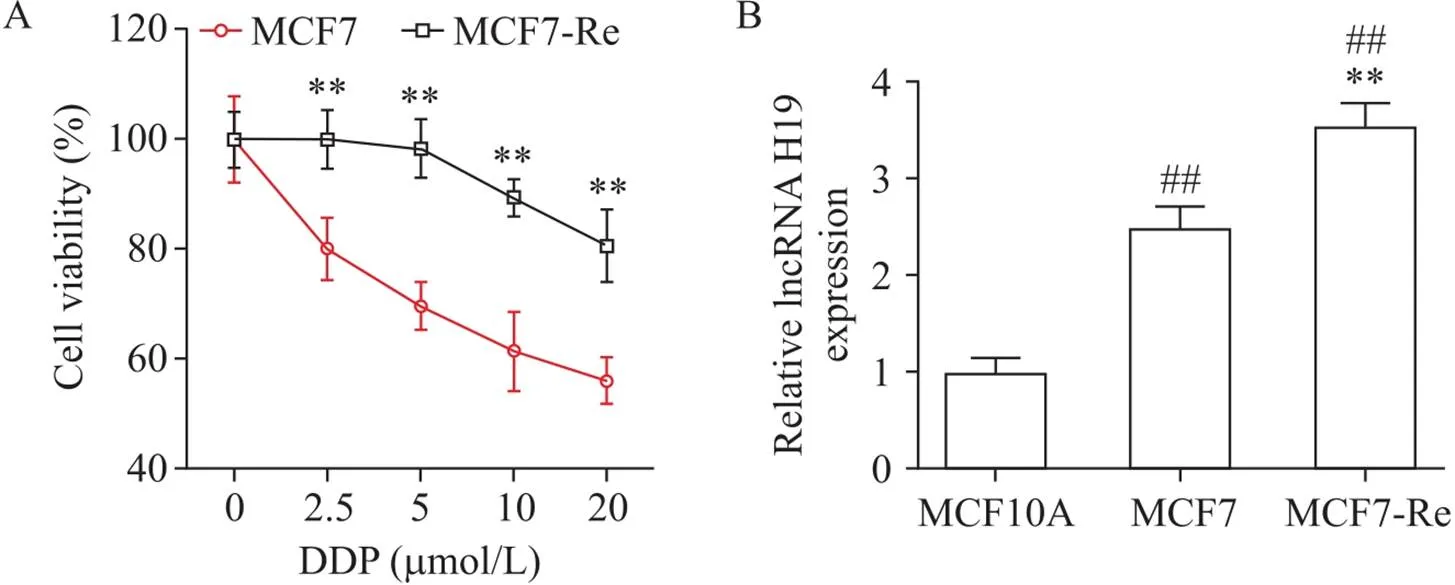
Figure 1. Expression of lncRNA H19 in cisplatin (DDP)-resistant breast cancer cells. A: the viability of MCF7 and MCF7-Re cells after DDP treatment was detected by CCK-8 assay; B: the expression of lncRNA H19 in MCF-10A, MCF7 and MCF7-Re cells was detected by RT-qPCR. Mean±SD. n=3. **P<0.01 vs MCF7; ##P<0.01 vs MCF10A.
2 干扰lncRNA H19可增强MCF7-Re细胞对DDP的敏感性
图2A显示,与siRNA-NC组相比,siRNA-H19组MCF7-Re细胞中H19的表达水平显著降低(<0.01),说明干扰质粒构建成功。图2B显示,不同剂量的DDP处理后,与siRNA-NC组相比,siRNA-H19组细胞活力进一步降低(<0.01)。图2C~E显示,与siRNA-NC相比,siRNA-H19组细胞凋亡率显著提高(<0.01),Bax和cleaved caspase-3表达显著增加,Bcl-2和ABCB1表达显著减少(<0.01);与DDP+siRNA-NC组相比,DDP+siRNA-H19组细胞凋亡率显著升高(P<0.001),Bax和cleaved caspase-3表达显著增加,Bcl-2和ABCB1表达显著减少(<0.01)。上述结果表明,干扰lncRNA H19可增强乳腺癌细胞对DDP的敏感性。

Figure 2. Effect of lncRNA H19 knockdown on the sensitivity of breast cancer cells to cisplatin (DDP). A: the knockdown effect of siRNA-H19 in MCF7-Re cells was detected by RT-qPCR; B: the viability of MCF7-Re cells after DDP treatment was detected by CCK-8 assay; C: TUNEL staining was used to detect cell apoptosis; D: apoptosis-related proteins were detected by Western blot; E: drug resistance protein ABCB1 was detected by Western blot. The concentration of DDP in C, D and E was 20 μmol/L. Mean±SD. n=3. **P<0.01 vs siRNA-NC group; ##P<0.01 vs DDP+siRNA-NC group.
3 干扰lncRNA H19可抑制MCF7-Re细胞中的Notch信号通路
图3显示,与siRNA-NC相比,siRNA-H19组MCF7-Re细胞中Notch1和Hes1蛋白表达水平均显著降低(<0.01),说明干扰H19抑制了Notch信号通路。
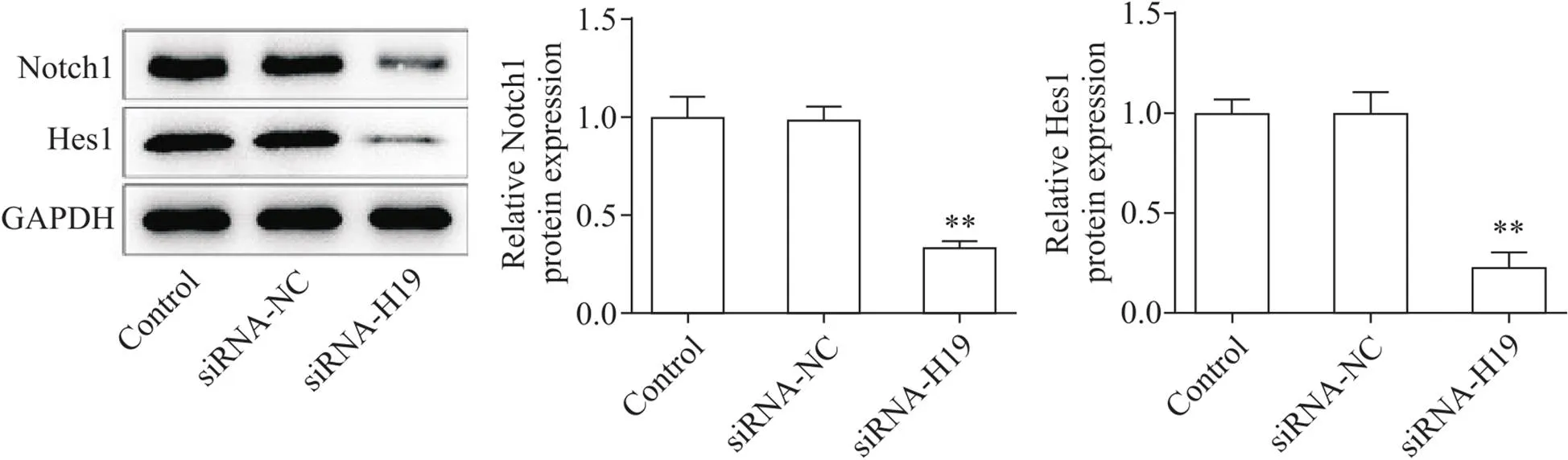
Figure 3. The expression levels of Notch signaling pathway-related proteins in breast cancer cells after lncRNA H19 knockdown were detected by Western blot. Mean±SD. n=3. **P<0.01 vs siRNA-NC group.
4 干扰lncRNA H19通过抑制Notch信号通路增强MCF7-Re细胞对DDP的敏感性
图4A、B显示,与Ov-NC组相比,Ov-Notch1组的Notch1表达增加,说明Notch1的过表达质粒构建成功。图4C显示,以不同剂量的DDP处理MCF7-Re细胞后,与siRNA-H19+Ov-NC相比,siRNA-H19+Ov-Notch1组细胞活力降低趋势减缓(<0.01)。图4D~F显示,以20 μmol/L DDP处理MCF7-Re细胞后,与DDP+siRNA-H19+Ov-NC相比,DDP+siRNA-H19+Ov-Notch1组细胞凋亡率显著降低(<0.01),Bax和cleaved caspase-3表达显著减少,Bcl-2和ABCB1表达显著增加(<0.01)。上述结果表明,干扰lncRNA H19可通过抑制Notch信号通路增强乳腺癌对DDP的敏感性。
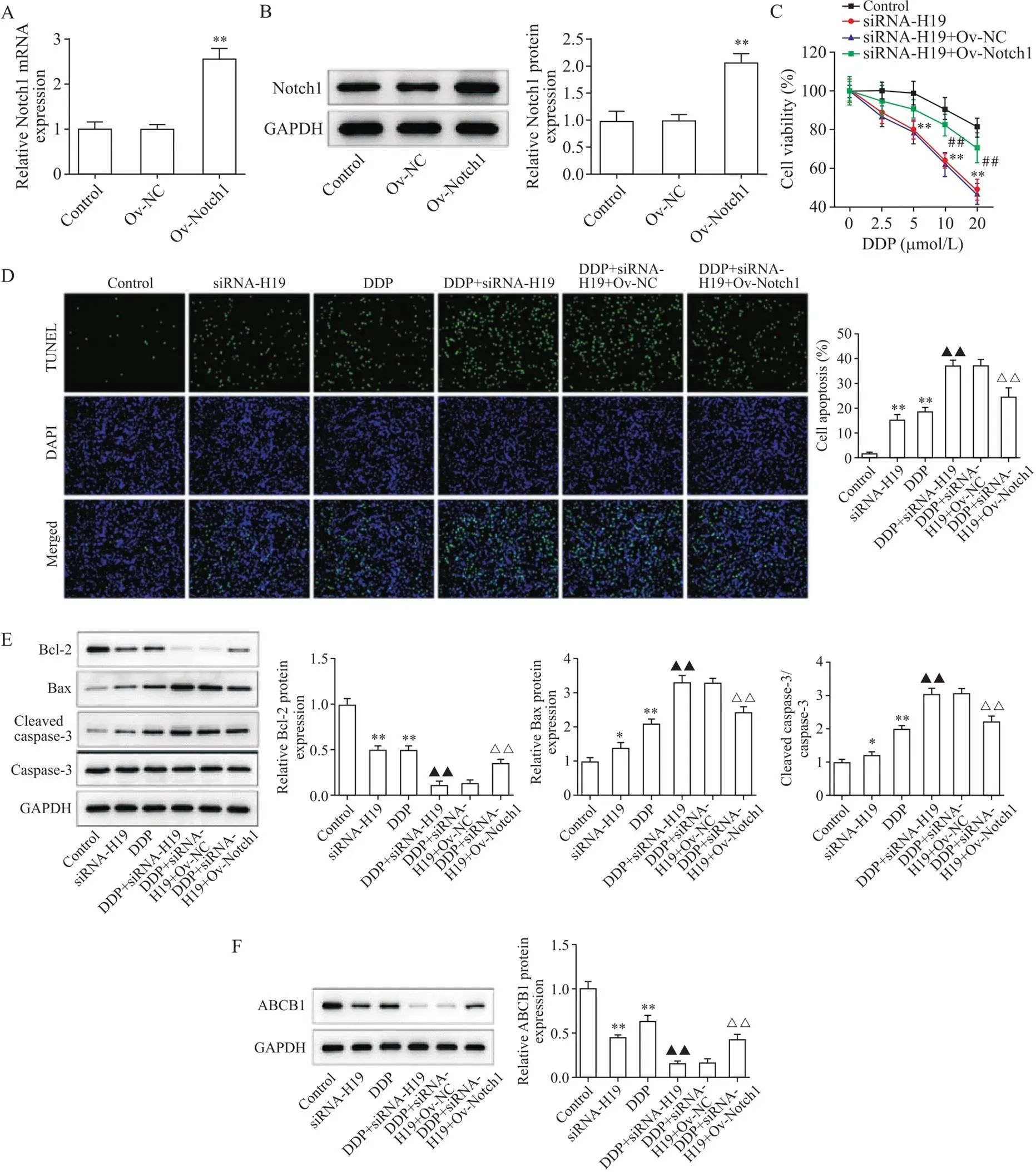
Figure 4. Knockdown of lncRNA H19 enhanced the sensitivity of breast cancer cells to cisplatin (DDP) by inhibiting Notch signaling pathway. A and B: RT-qPCR and Western blot were used to detect the overexpression of Notch1; C: the viability of MCF7-Re cells after DDP treatment was detected by CCK-8 assay; D: TUNEL staining was used to detect cell apoptosis; E: apoptosis-related proteins were detected by Western blot; F: drug resistance protein ABCB1 was detected by Western blot. The concentration of DDP in D, E and F was 20 μmol/L. Mean±SD. n=3. *P<0.05, **P<0.01 vs control or Ov-NC group; ##P<0.01 vs siRNA-H19+Ov-NC group; ▲▲P<0.01 vs DDP group; △△P<0.01 vs DDP+siRNA-H19+Ov-NC group.
讨论
乳腺癌作为对女性健康危害严重的疾病之一,目前化疗仍是治疗乳腺癌的最有效方法,但临床上经常发生肿瘤多药耐药[15]。DDP耐药是一个多因素的过程,乳腺癌患者的化疗耐药严重限制了治疗效果,因此研究耐药产生机制对乳腺癌的治疗具有深远意义[16]。lncRNA是一类长度大于200 nt的非编码RNA。lncRNA H19被认为是一种癌胚转录物,在几种肿瘤中表达失调,导致恶性组织中lncRNA H19异常上调[17]。研究发现,敲减lncRNA H19通过调节乳腺癌细胞中的miR-130a-3p/SATB1抑制细胞增殖并诱导细胞凋亡[18]。
在本研究中,我们首先构建了MCF7-Re耐药细胞株,RT-qPCR结果表明lncRNA H19在MCF7-Re细胞中表达增加。接着构建了lncRNA H19干扰质粒siRNA-H19,干扰H19表达可加速DDP处理后的MCF7-Re细胞活力的降低,促进了细胞凋亡。已知Bcl-2/Bax/cleaved caspase-3是凋亡相关蛋白,下调caspase-3和Bax以及上调Bcl-2可抑制细胞凋亡[19]。Western blot结果表明,siRNA-H19降低了DDP处理后MCF7-Re细胞中Bcl-2蛋白水平并提高了Bax和cleaved caspase-3蛋白水平,说明敲减H19可抑制DDP处理后的MCF7-Re细胞凋亡。ABCB1是一种经典的多药耐药蛋白,circRNA_103615沉默显著降低了DDP对A549细胞的IC50,并降低了ABCB1的表达水平[20]。本研究中,siRNA-H19降低了MCF7-Re细胞中耐药蛋白ABCB1的表达水平,表明敲减H19减轻了MCF7-Re细胞的DDP耐药性,提高了细胞对DDP的化疗敏感性。
Notch通路是一种高度保守的信号通路,在胚胎发育、器官成熟及肿瘤进展中发挥重要作用[21]。研究表明,Notch1的抑制显着下调MCAM表达,导致TNBC细胞对顺铂的化学耐药性的逆转[22]。Zhang等[23]报道,在乳腺癌细胞株MCF7中上调Notch1表达可诱导上皮-间充质转化,促进细胞侵袭和迁移。因此我们推测抑制Notch1信号可能会降低乳腺癌细胞的DDP耐药性。有研究表明,H19能够激活Notch信号蛋白的表达[12]。本研究中,Western blot结果表明,siRNA-H19降低了MCF7-Re细胞中Notch1和Hes1蛋白的表达,说明干扰H19抑制Notch1信号通路。同时,构建Notch1过表达质粒激活Notch1信号,减缓了DDP处理后MCF7-Re细胞活力降低的趋势,抑制了细胞凋亡,并且上调了耐药蛋白ABCB1的表达。这表明过表达Notch1逆转了干扰lncRNA H19对乳腺癌细胞耐药的抑制作用。
综上所述,本研究发现干扰lncRNA H19通过抑制Notch1信号通路进一步降低DDP处理后的MCF7-Re细胞活力,促进细胞凋亡,减少耐药蛋白ABCB1表达,进而增强乳腺癌细胞对DDP的敏感性。目前本研究只局限于体外细胞实验,而机体内作用机制复杂,往往能反映真实情况,后续将继续进行体内实验研究。
[1] Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2018, 68(6):394-424.
[2] Wörmann B. Breast cancer: basics, screening, diagnostics and treatment[J]. Med Monatsschr Pharm, 2017, 40(2):55-64.
[3] Radecka B, Litwiniuk M. Breast cancer in young women[J]. Ginekol Pol, 2016, 87(9):659-663.
[4] Maruthanila VL, Elancheran R, Kunnumakkara AB, et al. Recent development of targeted approaches for the treatment of breast cancer[J]. Breast Cancer, 2017, 24(2):191-219.
[5] Makovec T. Cisplatin and beyond: molecular mechanisms of action and drug resistance development in cancer chemotherapy[J]. Radiol Oncol, 2019, 53(2):148-158.
[6] Chen X, Lu P, Wu Y, et al. MiRNAs-mediated cisplatin resistance in breast cancer [J]. Tumour Biol, 2016, 37(10):12905-12913.
[7] Li J, Meng H, Bai Y, et al. Regulation of lncRNA and its role in cancer metastasis[J]. Oncol Res, 2016, 23(5):205-217.
[8] Robinson EK, Covarrubias S, Carpenter S. The how and why of lncRNA function: an innate immune perspective[J]. Biochim Biophys Acta Gene Regul Mech, 2020, 1863(4):194419.
[9] Liao K, Xu J, Yang W, et al. The research progress of LncRNA involved in the regulation of inflammatory diseases[J]. Mol Immunol, 2018, 101:182-188.
[10] Wu Y, Zhou Y, He J, et al. Long non-coding RNA H19 mediates ovarian cancer cell cisplatin-resistance and migration during EMT[J]. Int J Clin Exp Pathol, 2019, 12(7):2506-2515.
[11] Wang Y, Zhou P, Li P, et al. Long non-coding RNA H19 regulates proliferation and doxorubicin resistance in MCF-7 cells by targeting PARP1[J]. Bioengineered, 2020, 11(1):536-546.
[12] Geng X, Zou Y, Li S, et al. Electroacupuncture promotes the recovery of rats with spinal cord injury by suppressing the Notch signaling pathway via the H19/EZH2 axis[J]. Ann Transl Med, 2021, 9(10):844.
[13] 袁磊, 陈旭东, 范文娟, 等. 沉默基因促进人乳腺癌MCF-7细胞JNK1和p53磷酸化[J]. 中国病理生理杂志, 2013, 29(6):1014-1019.
Yuan L, Chen XD, Fan WJ, et al. Silencinggene promotes phosphorylation of JNK1 and p53 in human breast cancer MCF-7 cells[J]. Chin J Pathophysiol, 2013, 29(6):1014-1019.
[14] 李霞, 马超, 孔令伟, 等. EphA2调控结直肠癌细胞耐药的初步研究[J]. 中国病理生理杂志, 2017, 33(12):2188-2194.
Li X, Ma C, Kong LW, et al. A preliminary study on the regulation of drug resistance in colorectal cancer cells by EphA2[J]. Chin J Pathophysiol, 2017, 33(12):2188-2194.
[15] 黄果, 王佑权, 陈娟. miRNA-138-5p靶向抑制HIF-1α表达对乳腺癌MCF-7细胞顺铂耐药的逆转作用及其机制[J]. 吉林大学学报(医学版), 2021, 47(2):360-368.
Huang G, Wang YQ, Chen J. miRNA-138-5p targeted inhibition of HIF-1α expression reverses cisplatin resistance in breast cancer MCF-7 cells and its mechanism[J]. J Jilin Univ (Med Ed), 2021, 47(2):360-368.
[16] Mi H, Wang X, Wang F, et al. SNHG15 contributes to cisplatin resistance in breast cancer through sponging miR-381[J]. Onco Targets Ther, 2020, 13:657-666.
[17] Ghafouri-Fard S, Esmaeili M, Taheri M. H19 lncRNA: roles in tumorigenesis[J]. Biomed Pharmacother, 2020, 123:109774.
[18] Zhong G, Lin Y, Wang X, et al. H19 knockdown suppresses proliferation and induces apoptosis by regulating miR-130a-3p/SATB1 in breast cancer cells[J]. Onco Targets Ther, 2020, 13:12501-12513.
[19] Zheng Z, Xiao Z, He YL, et al. Heptapeptide isolated fromexhibited anti-photoaging potential via MAPK/AP-1/MMP pathway and anti-apoptosis in UVB-irradiated HaCaT cells[J]. Mar Drugs, 2021, 19(11):626.
[20] Liang H, Lin Z, Lin H, et al. circRNA_103615 contributes to tumor progression and cisplatin resistance in NSCLC by regulating ABCB1[J]. Exp Ther Med, 2021, 22(3):934.
[21] Krishna BM, Jana S, Singhal J, et al. Notch signaling in breast cancer: from pathway analysis to therapy[J]. Cancer Lett, 2019, 461:123-131.
[22] Zeng D, Liang YK, Xiao YS, et al. Inhibition of Notch1 reverses EMT and chemoresistance to cisplatin via direct downregulation of MCAM in triple-negative breast cancer cells[J]. Int J Cancer, 2020, 147(2):490-504.
[23] Zhang X, Zhao X, Shao S, et al. Notch1 induces epithelial-mesenchymal transition and the cancer stem cell phenotype in breast cancer cells and STAT3 plays a key role[J]. Int J Oncol, 2015, 46(3):1141-1148.
Knockdown of lncRNA H19 enhances drug sensitivity of cisplatin-resistant breast cancer cells through Notch signaling pathway
SHI Wen-biao, JIN Xiao-yan, PANG Wen-yang, WANG Yuan-fan, WANG Zheng-yi, ZHANG Qiang△
(,,318000,)
To investigate the effect of long non-coding RNA (lncRNA) H19 on cisplatin/cis-diamminodichloroplatinum (DDP) resistance in breast cancer cells and its molecular mechanism.A DDP-resistant human breast cancer cell line MCF7-Re was constructed. RT-qPCR was used to detect the expression levels of lncRNA H19 in human normal breast epithelial cell line MCF10A, and human breast cancer cell lines MCF7 and MCF7-Re. The lncRNA H19 interference plasmid was constructed, and CCK-8 assay was used to detect the viability of MCF7-Re cells after DDP treatment. The cell apoptosis was detected by TUNEL. The protein levels of Bcl-2, Bax, cleaved caspase-3, ATP-binding cassette protein subfamily B member 1 (ABCB1), Notch1, and hairy and enhancer of split 1 (Hes1) were detected by Western blot. The Notch1 overexpression plasmid was constructed, and then was used in reversal experiments.The expression of lncRNA H19 was increased in MCF7-Re cells (<0.01). Knockdown of H19 further reduced the cell viability and the ABCB1 expression, but increased the apoptosis rate of MCF7-Re cells after treated with DDP (<0.01). The expression of Bax and cleaved caspase-3 was increased (<0.01), while the expression of Bcl-2 and Notch signaling pathway-related proteins (Notch1 and Hes1) was inhibited (<0.01). Overexpression of Notch1 increased the cell viability and the ABCB1 expression, but decreased the apoptosis rate of MCF7-Re cells after knockdown of lncRNA H19 (<0.01). The protein expression of Bcl-2 was increased (<0.01), while the protein expression of Bax and cleaved caspase-3 was decreased (<0.01).Knockdown of lncRNA H19 inhibits the proliferation of DDP-resistant breast cancer cells and enhances their sensitivity to DDP by inhibiting Notch signaling pathway.
Long non-coding RNA H19; Breast cancer; Cisplatin; Drug resistance; Notch signaling pathway
1000-4718(2022)09-1585-07
2022-04-26
2022-07-14
15824092996; E-mail: zhangqq1975@163.com
R737.9; R363.2
A
10.3969/j.issn.1000-4718.2022.09.007
[基金项目]2021年台州市科技计划项目(No. 21ywa37)
(责任编辑:林白霜,罗森)
