Differential diagnosis of different types of solid focal liver lesions using two-dimensional shear wave elastography
2022-09-14JiaGuoDongJiangYiQianJiaoYuYiJunGuYuQingZhouHuiPingZhang
Jia Guo, Dong Jiang, Yi Qian, Jiao Yu, Yi-Jun Gu, Yu-Qing Zhou, Hui-Ping Zhang
Abstract
Key Words: Focal liver lesions; Conventional ultrasound; Two-dimensional shear wave elastography;Differential diagnosis
lNTRODUCTlON
The clinical management and prognosis differ between benign and malignant solid focal liver lesions(FLLs), and among different pathological types of malignant FLLs[1-3]. Accurate diagnosis of solid FLLs, not only as benign or malignant FLLs, but also the possible types of malignant FLLs, is important[4-6].
Ultrasound elastography, especially two-dimensional shear wave elastography (2D-SWE) which provides quantitative information about tissue stiffness, is a useful tool to differentiate malignant from benign FLLs[7,8]. Our previous study confirmed the value of SWE in the differential diagnosis between benign and malignant FLLs[9].
However, the value of SWE in the differential diagnosis among different pathological types of malignant FLLs has not been proved. Parket al[10] showed that cholangiocellular carcinoma (CCC) had the lowest shear wave velocity, followed by liver metastases, and hepatocellular carcinoma (HCC) had the highest shear wave velocity; whereas Gerberet al[11] showed that CCC had the highest stiffness and liver metastases had the lowest.
Considering the inhomogeneity of tumor stiffness (especially malignant tumors), we used maximal elasticity (Emax) as the parameter to evaluate the stiffness of FLLs and obtained promising results[9]. In the present study, we used 2D-SWE with Emax as the parameter to measure the stiffness of FLLs and to explore the value of SWE with Emax in the differential diagnosis of FLLs, especially among different pathological types of malignant FLLs.
MATERlALS AND METHODS
Study design
This was a prospective study that was approved by the institutional ethical review board of our hospital. All patients provided written informed consent before ultrasound examination.
Patients
Inpatients in the department of Hepatobiliary Surgery between November 2021 and January 2022 who met the following criteria were included:(1)One or more solid FLLs shown on conventional ultrasound with a maximal lesion depth < 8 cm; and (2) The targeted FLL showed as green on quality mode,indicating good quality of SWE. The exclusion criteria were: (1) Patients refused to join the study; and(2) No definite final diagnosis; malignant tumors except for liver metastases had to be diagnosed pathologically after surgery; benign tumors or liver metastases could be diagnosed pathologically or by contrast-enhanced ultrasound (CEUS) together with contrast-enhanced computed tomography (CT) or contrast-enhanced magnetic resonance imaging (MRI) 1 wk before or after 2D-SWE. We included 132 solid FLLs in 127 patients. The gender, age and history of malignancy were recorded.
Conventional ultrasound examination
An Acuson Sequoia diagnostic ultrasound machine (Siemens Medical Solutions, Mountain View, CA,United States) and a transabdominal 5C1 probe were used for conventional ultrasound examination.Before the examinations, all patients fasted for at least 8 h.
One ultrasound physician with > 15 years’ experience in conventional ultrasound performed the hepatic ultrasound scan to detect FLLs. The length and width of every lesion were measured to calculate the volume according to the formula[12]: V = 0.5 LW2(V: Volume; L: Length; W: Width). A diagnosis as benign, malignant or undetermined was made for each lesion by the ultrasound physician according to the ultrasound features including the number, size, shape, boundary, echogenicity and blood flow of the lesion and surrounding hepatic echogenicity.
2D-SWE examination
After conventional ultrasound examination, another ultrasound physician who was well trained in ultrasound elastography performed all the 2D-SWE examinations using the same ultrasound equipment and probe, putting no pressure on the skin through the probe. The patients were asked to lie flat on the examination bed. The targeted FLL was shown clearly on the screen and the 2D-SWE mode was activated. A region of interest (ROI) was drawn including the whole or partial tumor and some surrounding liver tissue. If the tumor showed green on quality mode (Figure 1A), it meant that the quality of the 2D-SWE image was up to standard. In velocity mode, we set the speed bar at 0.5-4.0 m/s(Figure 1B); drew two circular ROIs (with 3 mm diameter) to be placed at the stiffest site in the tumor and at the periphery of the tumor (Figure 1C). The maximum values of the two ROIs were recorded(Figure 1D) as Emax for further analysis.
Statistical analysis
SPSS version 13.0 (IBM Corporation, Chicago, IL, United States) was used for statistical analysis.P<0.05 was considered statistically significant. Normal distribution using the Kolmogorov-Smirnov test was used first for measurement data. Independent samplettest, one way analysis of variance and least significant difference were used for data with normal distribution and the data were reported as mean ±SD. Otherwise, the Mann-Whitney or Kruskal-Wallis test was used and the data were reported as interquartile range. The cut-off point of Emax was calculated by a receiver operating characteristic curve. The enumerative data were presented as numbers and percentage and Pearson’s chi-square test and Fisher’s exact test were used for statistical analysis.
RESULTS
Patients and final diagnosis
There were 132 solid FLLs in 127 patients (80 men and 47 women; aged 24-77 years; mean age 54.23 ±12.04 years) in this study, including 32 benign FLLs in 31 patients (6 focal nodular hyperplasia, 15 hemangioma and 11 diagnosed definitely as benign but without pathological results) and 100 malignant FLLs in 96 patients (16 CCCs, 72 HCCs and 12 liver metastases). The differences in age, gender and history of malignancy between the malignant and benign groups are shown in Table 1. Compared with patients with benign FLLs, patients with malignant FLLs were older and more often male. The history of malignancy had no significant difference between the two groups. The differences in age, gender and history of malignancy among three types of malignancy are shown in Table 2. The age and gender had no significant differences among the three groups, although the age of patients with liver metastases was significantly higher than that of patients with HCC. Patients with liver metastases were more often
had a history of malignancy, compared with patients with primary liver cancer, including HCC and CCC.
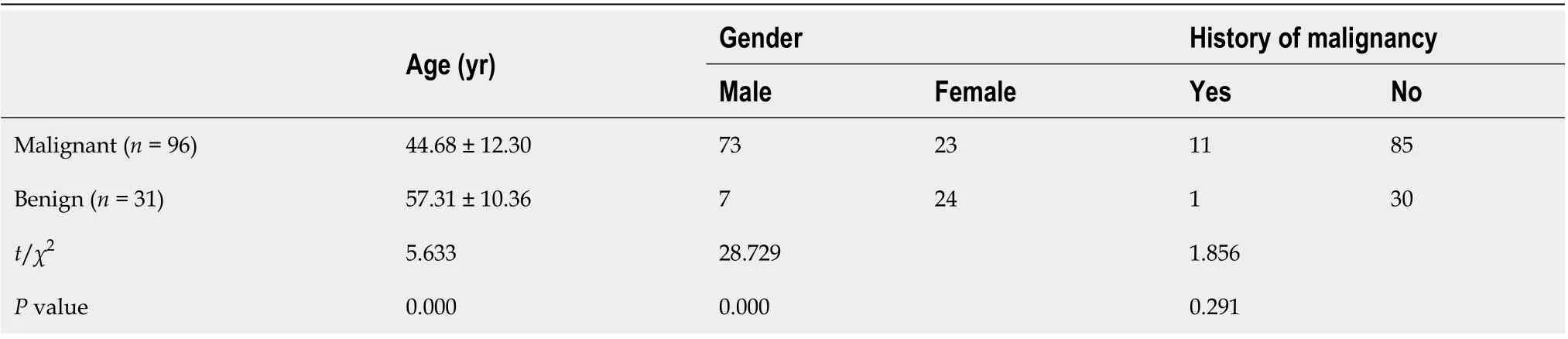
Table 1 Differences in age, gender and history of malignancy between malignant and benign groups

Table 2 Differences in age, gender and history of malignancy among three types of malignancy
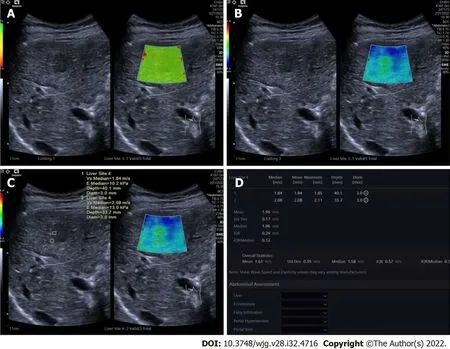
Figure 1 Two-dimensional shear wave elastography images in patients with focal liver lesions. A: Tumor shown on quality mode as green indicated that the quality of two-dimensional shear wave elastography image was up to standard; B: Tumor shown in velocity mode, speed bar set at 0.5-4.0 m/s; C:Two circular regions of interest (ROIs, with 3 mm diameter) placed at the stiffest site in the tumor and at the periphery of tumor; D: Maximum values of the two ROIs recorded as maximal elasticity.
Conventional ultrasound results
Volume of benign FLLs (20.73-113.69 cm3) and malignant FLLs (30.56-79.62 cm3) had no significant difference (Z= 0.263,P= 0.793). Volume of CCCs (39.58-120.22 cm3), HCCs (26.34-75.69 cm3) and liver metastases (32.76-41.67 cm3) had no significant difference (χ2= 1.869,P= 0.393). For 32 benign FLLs, 12 were diagnosed as benign, 18 as undetermined and two were misdiagnosed as malignant. For 100 malignant FLLs, 81 (12 CCCs, 59 HCCs and 10 metastases) were diagnosed correctly as malignant, 17 were undetermined (4 CCCs, 11 HCCs and 2 liver metastases) and 2 were misdiagnosed as benign (both HCCs). The undetermined rate of conventional ultrasound was 26.52% (35/132). For the FLLs with definite diagnosis by conventional ultrasound, the sensitivity, specificity and accuracy were 85.71%,97.59% and 95.88%, respectively.
2D SWE results
Comparisons between benign and malignant FLLs:The differences in 2D-SWE results between benign and malignant FLLs are shown in Table 3. Emax of malignant FLLs were significantly higher than those of benign FLLs. Emax of the periphery of malignant FLLs were significantly higher than those of benign FLLs. The cut-off point of Emax of the tumors was 1.905 with AUC 0.843 (Figure 2). The sensitivity,specificity and accuracy were 71.00%, 84.38% and 74.24% respectively using Emax > 1.905 m/s for diagnosis as malignant (Figure 3). For 35 FLLs with undetermined diagnosis by conventional ultrasound, 23 (65.71%) FLLs including 14 of 18 benign and nine of 17 malignant FLLs were diagnosed correctly.
Comparisons among different types of malignant FLLs: The differences in 2D-SWE results among different types of malignant FLLs are shown in Table 4. There were significant differences among Emax of CCCs, HCCs and liver metastases. Emax of liver metastases was significantly higher than that of CCCs and HCCs. There were no significant differences among Emax values of the periphery of CCCs,HCCs and liver metastases.
DlSCUSSlON
In this study, we explored the value of 2D-SWE using Emax as the quantitative parameter in the differential diagnosis of FLLs, especially among different pathological types of malignant FLLs. Our results showed that malignant FLLs were stiffer than benign ones and liver metastases were stiffer than primary liver carcinomas. 2D-SWE with Emax was a useful complement to conventional ultrasound for the differential diagnosis of FLLs.
The comparison of patients’ basic information showed that compared with patients with benign FLLs,patients with malignant FLLs tended to be older, male and have a history of malignancy. Among three different types of malignant FLLs, the distribution of patients’ age and gender had no significant difference, while patients with liver metastases were more likely to have a history of malignancy. These results were similar to those in previous studies[13].
Conventional ultrasound is well recognized as the first imaging choice for the screening and diagnosis of FLLs[14]. However, the diagnostic efficiency of conventional ultrasound for the differential diagnosis of benign and malignant FLLs or among different pathological types of malignant FLLs was not good enough. For some FLLs, the diagnosis using conventional ultrasound alone is difficult[15].That is one of the reasons for the development of new ultrasound technologies, including CEUS and ultrasound elastography. In this study, we diagnosed FLLs as benign, malignant and undetermined by conventional ultrasound and 35 of 132 (26.52%) were diagnosed as undetermined. Further diagnosis using other imaging methods was necessary.
CEUS could provide similar or better diagnostic performance for the differential diagnosis of FLLs without radiation and iodine allergy, compared with contrast enhanced CT[16]. CEUS has particularly high value for the differential diagnosis of complex cystic and cysticlike FLLs[17,18]. In this study, for some benign tumors or liver metastases without pathological diagnosis, the combined results of CEUS together with contrast enhanced CT or contrast enhanced MRI were as final. Compared with CEUS,ultrasound elastography has the advantages of being less expensive and less time-consuming[19].
Our previous study using virtual touch tissue quantification imaging with Emax has proven its value in the differential diagnosis between benign and malignant FLLs[9]. 2D-SWE is one of the most advanced elastography techniques and could provide overall elastic information in tumors and locate the stiffest part[20]. In this study, the cutoff point of Emax was 1.905, which was similar to our previous result[9]. Twenty-three of 35 (65.71%) FLLs with undetermined diagnosis by conventional ultrasound were diagnosed accurately using 2D-SWE with Emax. If FLLs could be diagnosed confidently using conventional ultrasound, no further examination was needed; otherwise, 2D-SWE with Emax could be a useful complement to conventional ultrasound and improve the diagnostic efficiency.

Table 3 Differences in two-dimensional shear wave elastography results between benign and malignant focal liver lesions

Table 4 Differences of two-dimensional shear wave elastography results among different types of malignant focal liver lesions
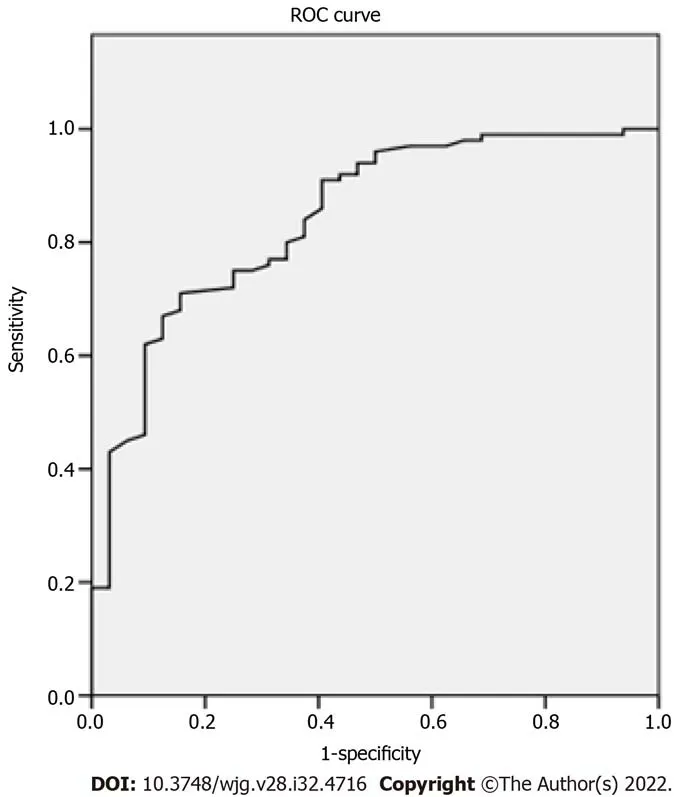
Figure 2 Receiver operating characteristic curve of maximum elasticity for malignant and benign focal liver lesions.
The elastic differences among different types of malignant FLLs are still uncertain. Our results showed that the stiffness of liver metastases was significantly higher than that of primary liver tumors,including CCCs and HCCs, while the stiffness of CCCs and HCCs had no significant differences. This was similar to the study of Parket al[21], which showed that the stiffness of HCCs was significantly lower than that of liver metastases. However, some other studies had the opposite results[10,11]. One study reported that CCCs had the lowest stiffness and HCCs the highest stiffness[10]. Another study found that CCCs had the highest stiffness and liver metastases the lowest[11]. One important reason for the conflicting results is that the sample number in every study was not large enough, and it is necessary to carry out further studies with large samples to confirm our results.
Figure 3 Ultrasound and pathologic images in a 51-year old male patients with hepatocellular carcinomas. A: A tumor shown on conventional ultrasound were diagnosed as undetermined; B: Tumor shown as stiffer, compared with surrounding liver tissue in two-dimensional shear wave elastography velocity mode; C: Maximal elasticity of the tumor was higher than 1.905 m/s and the tumor was diagnosed as malignant; D: Pathological examination confirmed the tumor as hepatocellular carcinomas (HE, × 200).
There were some limitations to our study. First, as mentioned above, only a few cases of CCCs and liver metastases were included. Second, no pathological methods were used to confirm the stiffness of the tumors and explain the differences among different pathological types of malignant FLLs.
CONCLUSlON
In conclusion, malignant FLLs were stiffer than benign FLLs and liver metastases were stiffer than primary liver carcinomas. 2D-SWE with Emax was a useful complement to conventional ultrasound for the differential diagnosis of FLLs.
ARTlCLE HlGHLlGHTS
Research background
Conventional ultrasound is the first imaging choice for liver diseases with the diagnostic efficiency not good enough, which promotes the development of new ultrasound technologies, such as contrast enhanced ultrasound and ultrasound elastography. Two dimensional shear wave elastography (2DSWE) is convenient, less time-consuming and inexpensive. SWE plays an important role in the assessment of liver fibrosis and in the differential diagnosis of benign and malignant focal liver lesions(FLLs). However, its value for the differential diagnosis among different types of malignant FLLs has not been proved.
Research motivation
Though the value of SWE for the differential diagnosis between malignant and benign FLLs was not widely recognized, our previous study showed promising results using maximal elasticity (Emax) as the parameter to differentiate malignant FLLs from benign ones. As the clinical management and prognosis of different pathological types of malignant FLLs are different,it is important to diagnose accurately of the possible pathological types. So, it was necessary to explore the value of 2D-SWE with Emax in differential diagnosis of different pathological types of malignant FLLs.
Research objectives
We aim to explore the value of 2D-SWE using Emax in the differential diagnosis of FLLs, especially among different pathological types of malignant FLLs.
Research methods
In this study, we diagnosed all FLLs as benign, malignant and undetermined using conventional ultrasound. And the stiffness of FLLs and the periphery of FLLs were evaluated using 2D-SWE and the quantitative parameter Emax. Emax of FLLs and the periphery of FLLs were compared between benign and malignant FLLs or among different pathological types of malignant FLLs.
Research results
There were totally 132 FFLs in 127 patients enrolled in the study, including 32 benign FLLs, 16 cholangiocellular carcinomas (CCCs), 72 hepatocellular carcinomas (HCCs) and 12 liver metastases. Thirty-five FLLs were diagnosed as undermined by conventional ultrasound. Emax of malignant FLLs (2.21 ± 0.57 m/s) were significantly higher than those of benign FLLs (1.59 ± 0.37 m/s) (P= 0.000). Emax of the periphery of malignant FLLs (1.52 ± 0.39 m/s) were significantly higher than those of benign FLLs (1.36± 0.44 m/s) (P= 0.040). The cut-off point of Emax of the tumors was 1.905 with AUC 0.843. The sensitivity, specificity and accuracy were 71.00%, 84.38% and 74.24% respectively using Emax > 1.905 m/s for diagnosis as malignant and 23 of 35 (65.74%) FLLs with undetermined diagnosis by conventional ultrasound were diagnosed correctly. Emax of liver metastases (2.73 ± 0.99 m/s) was significantly higher than that of primary liver carcinomas, including CCCs (2.14 ± 0.34 m/s) and HCCs (2.14 ± 0.46 m/s) (P= 0.002).
Research conclusions
Malignant FLLs were stiffer than benign ones and liver metastases were stiffer than primary liver carcinomas. 2D-SEW with Emax was a useful complement to conventional ultrasound for the differential diagnosis of FLLs.
Research perspectives
In this study, we demonstrated the differences of 2D-SWE with Emax between benign and malignant FLLs and among different pathological types of malignant FLLs. Prospective study to explore the value of 2D-SWE with Emax in the evaluatation the different stiffness of liver metastases from different sources or the different stiffness of HCC with different microvascular invasion grade will be necessary.
FOOTNOTES
Author contributions:Guo J and Jiang D contributed equally to this work; Guo J, Jiang D, Yu J and Zhang HP designed the study; Guo J, Jiang D, Qian Y and Gu YJ performed the research and collected data; Zhang HP and Zhou YQ performed statistical analysis; Guo J and Jiang D wrote the manuscript; all authors have read and approved the final manuscript.
Supported byNatural Science Foundation of Shanghai of China, No. 19ZR1441500, No. 22ZR1458200; Science Research Foundation of Shanghai Municipal Health Commission, No. 202140378; and Key Program of Science and Technology Commission Foundation of Changning, Shanghai, China, No. CNKW2020Z04.
lnstitutional review board statement:The study was approved by the Institutional Ethical Review Board of Eastern Hepatobiliary Surgery Hospital Affiliated to Naval Medical University (Approval No. EHBHKY2021-K-017).
Clinical trial registration statement:This study is registered at clinical hospital center ‘Eastern Hepatobiliary Surgery Hospital, Naval Medical University’ trial registry. The registration identification number is ChiCTR2100049831.
lnformed consent statement:All patients provided written informed consent prior to study enrollment before ultrasound examinations.
Conflict-of-interest statement:All the authors report no relevant conflicts of interest for this article.
Data sharing statement:No additional data are available.
CONSORT 2010 statement:The authors have read the CONSORT 2010 statement, and the manuscript was prepared and revised according to the CONSORT 2010 statement.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORClD number:Yu-Qing Zhou 0001-0002-0001-0002; Hui-Ping Zhang 0000-0002-3890-6436.
S-Editor:Gong ZM
L-Editor:A
P-Editor:Gong ZM
杂志排行
World Journal of Gastroenterology的其它文章
- The mechanism of Yinchenhao decoction in treating obstructivejaundice-induced liver injury based on Nrf2 signaling pathway
- Anoctamin 5 regulates the cell cycle and affects prognosis in gastric cancer
- Effects of Granule Dendrobii on chronic atrophic gastritis induced by N-methyl-N'-nitro-N-nitrosoguanidine in rats
- Machine learning predicts portal vein thrombosis after splenectomy in patients with portal hypertension: Comparative analysis of three practical models
- Sirolimus increases the anti-cancer effect of Huai Er by regulating hypoxia inducible factor-1α-mediated glycolysis in hepatocellular carcinoma
- lnternational patterns in incidence and mortality trends of pancreatic cancer in the last three decades: A joinpoint regression analysis
