Stem cell-based 3D brain organoids for mimicking,investigating,and challenging Alzheimer’s diseases
2022-08-08FedericaCordellaCarloBrighiAlessandroSolopertoSilviaDiAngelantonio
Federica Cordella,Carlo Brighi,Alessandro Soloperto,Silvia Di Angelantonio
Alzheimer’s disease (AD) is a progressive and irreversible neurodegenerative disorder that causes a decline of cognitive functions and a deterioration of behavioral and social performances. According to current estimations,AD is considered the prevalent cause of dementia,accounting for 60% and 80% of cases every year. The etiology of the disease is related to both genetic and environmental factors,making AD a cultifactorial disorder,with a major classification as late-onset AD and earlyonset AD.
AD’s clinical manifestations,including memory impairment and altered cognitive functions,are caused by synaptic and neuronal loss together with a prominent inflammatory state in the entorhinal cortex,hippocampus,and neocortex (Livingston et al.,2020).
Despite the large number of theories that attempt to explain AD-related pathomorphological changes in the brain,evidences coming fromin vitroandin vivoAD models have highlighted a cytotoxic effect of amyloid β (Aβ) fragments and hyperphosphorylated TAU on neurotransmission,axonal transport,signaling cascades and immune response,leading to synaptic loss and dysfunction (Rajmohan and Reddy,2017).
Mutations in the amyloid precursor protein (APP) or in its processing enzyme,γ-secretase (subunit-PSEN1 (Presenilin 1) and subunit-PSEN2 (Presenilin 2)) correlates with the familial form of AD,and lead to increased β-site cleavage of APP and production of longer,aggregationprone variants of Aβ peptides. The major constituents of amyloid plaques are two abnormally folded products of APP metabolism: Aβ40and Aβ42,which show a higher rate of oligomerization and insolubility.
Another distinctive AD hallmark is represented by the presence of neurofibrillary tangles. These structures are primarily composed of paired helical filaments of hyperphosphorylated TAU proteins,sometimes mixed with straight filaments (Pizzarelli et al.,2020). TAU protein,encoded by theMAPTgene,represents the major microtubule associated protein (MAP) of a normal adult brain. This protein interacts with tubulin enhancing its polymerization into microtubules and helping their stability. The alternative splicing of TAU pre-mRNA originates 6 different isoforms: these proteins differ in containing three (3R) or four (4R) microtubule binding repeats in the carboxyl terminal and one (1N),two (2N) or zero (0N) amino terminal inserts. Thus,the alternative splicing of TAU is developmentally regulated,resulting in the production of two major groups: the 3R TAUs and 4R TAUs. The 2N4R TAU represents the most abundant TAU isoform inside the adult brain,while 0N3R is only expressed in the fetal human brain.Like the other MAP proteins,TAU is recognized as a phosphoprotein and its biological activity is regulated by its phosphorylation level. The presence of several missense mutations on the MAPT gene,such as G272V,P301L,V337M,and R406W,leads to TAU hyperphosphorylation and subsequent fibrillization. Moreover,phospho-TAUper seinhibits the assembly of tubulin into microtubules instead of favoring its stability and promotes the sequestration of both healthy TAU and the other MAP proteins (Chang et al.,2008). Neuropathological study showed that in the AD brains,Tau spreads among several brain areas,possibly transferred by the neuronal network (Braak and Braak,1991). This phenomenon,known as Tau propagation,can be considered a relevant starting point to understand the molecular mechanisms of AD progression,helping to identify new targets and new therapeutic strategies. Indeed,Tau misfolding is associated not only with AD but also with other neurodegenerative pathologies known as Tauopathies,such as fronttemporal dementia or chronic traumatic encephalopathy.
Stem cell-based strategy for investigating neurodegenerationin vitro:Given the late phenotypic onset of AD and its poor prognosis,there is an urgent need to develop novelin vivoandin vitromodels that better recapitulate human AD,to dissect molecular pathological mechanisms,identify novel therapeutic targets,and test drug candidates. Over the past few years,a combination of transgenic animal models has been employed to elucidate the causative link between structural and functional brain changes and genetic mutations characterizing AD. Although several mouse models are able to recapitulate some AD key hallmarks,such as protein aggregation and the timing by which the pathology takes place,most of them recapitulate only the Aβ plaques phenotype by overexpressing human genes commonly associated with familial forms of AD,without taking in account Tau pathology. However,Alzheimer’s condition is defined as the concomitant manifestation of Aβ plaques deposition and neurofibrillary tau tangles which together exacerbates a progressive neuroinflammatory state and leads to a massive neuronal loss.
In this scenario,induced pluripotent stem cells (iPSCs) represent an important bridge to overcome these gaps,fostering the establishment of anin vitrohuman model that might display both Aβ and Tau phenotype with a relevant timeframe as that observed in humans. The discovery that terminally differentiated cells,like fibroblast,can be reverted into a pluripotent condition with an exogenous cocktail of transcriptional factors such as OCT4,SOX2,c-MYC and KLF4 (Takahashi and Yamanaka,2006),has been a groundbreaking leap for the development of human-basedin vitrodisease models. Indeed,the improvement in human somatic cells reprogramming into iPSCs,and in protocols for iPSCs differentiation into brain cells is paving the road for a deeper analysis of development and functions of the human brain in physiological and pathological conditions. Moreover,the availability of patientderived iPSCs,displaying the genome and the molecular phenotype of the affected individuals,allows to go beyond the limitation of transgenic mouse models,and to develop a more faithful disease model to investigate the pathology at a cellular level.
Patient-specific iPSC-derived neurons recapitulate some pathological features of the Alzheimer pathology,such as the presence of soluble Aβ accumulation or insoluble Aβ aggregation,key hallmarks of the AD early stages. However,in 2D cultures,even harboring the most aggressive mutations of familiar AD,only low levels of Aβ species have been detected,without remarkable extracellular β-amyloid aggregation,probably because of the lack of interstitial compartment. Despite the major improvements made over the past years in 2D cell culture techniques,they still remain oversimplified platforms presenting many limitations including the absence of thein vivo-like cytoarchitectural organization and the brain’s spatial synaptic connections,which instead are necessary to faithfully replicate Alzheimer pathophysiology (Liu et al.,2018).
Three-dimensional revolution in cell biology:The number of innovative,clinically relevant constructs for disease modeling is rapidly growing with a variety of 3Din vitroplatforms,including organoid cultures,3D bioprinting,and 3D cellular scaffolding approaches. These advanced technologies have been successfully applied to replicatein vitrothe pathogenesis of several human neurodegenerative diseases such as AD,Parkinson’s disease,and amyotrophic lateral sclerosis,contributing to dissecting the molecular pathways involved in the disease.
Application of iPSC-derived neuronal and glial cells in the generation of brain organoids represent a step forward in order to fill the gap between 2D cultures and animal models (Brighi et al.,2020). Using these constructs,defined by selforganized and self-patterning features,it is possible to generate a complex 3D environment,suitable for the investigation of pathologies characterized by the presence of protein aggregates,such as AD. In current protocols,inhibitors of the dual SMAD signaling are used to enhance neural fate choice,mimicking the complex signaling conditions which characterize the embryonic state. Specifically,after initial neural commitment,the anterior/dorsalization of the neuroepithelium is promoted by high levels of WNT signaling. This process recapitulates thein vivocorticogenesis through which glia and neurons of the 6 cortical layers are generated (Sloan et al.,2018). The heterogeneous but developmentally regulated differentiation of iPSCs into brain cells within 3D structures allow their direct interaction with the extracellular matrix,the formation of cell-to-cell connections and a more consistent gradients of neurogenic growth factors,leading eventually to complex and functional networks (Yakoub,2019). Notably,despite in 2D cultures,the proteins secreted by the cells,like Aβ peptides,are removed every time the medium is changed,thus preventing protein aggregation,in organoids,the presence of a 3D extracellular matrix promotes the interstitial accumulation in microdomains of extracellularly released proteins. It has been speculated that iPSC-derived brain organoids with an AD genetic background may recapitulate the extracellular protein aggregation and the neuronal degeneration evidenced in human post-mortem brain tissues. In this line,patient-derived cortical organoids harboring presenilin-1 M146V mutation showed Aβ42 overexpression and altered matrix remodeling protein expression compared to healthy control derived organoids (Yan et al.,2018). Furthermore,cortical organoids generated from familial AD patient-derived iPSCs spontaneously displayed pathological AD characteristics,including accumulation of structures highly comparable with amyloid plaques and neurofibrillary tangles (Gonzalez et al.,2018).
Even though brain organoids generation represent an outstanding technology,attention is required when applied in neurodegenerative diseases research. As a matter of fact,despite cortical organoids revealed the presence of astrocytes and oligodendrocytes together with an patterned neuronal population,the absence of microglia and a vascular network,both derived from the mesodermal lineage,still represents a major drawback that needs to be rectified. Microglia,the brain’s resident macrophages,express both neuroprotective and immunomodulatory roles and play important functions during neurodevelopment by modulating neuronal connection through synaptic pruning,as well as during adulthood,patrolling brain parenchyma,triggering prompt pro- or anti-inflammatory responses. Given that,the establishment of an organoid model that includes the presence of this cell subtype is paramount for interfacing correctly with the disease. However,most of the currentin vitro3D brain models lack microglia,thus being unable to provide an accurate framework for elucidating the neurodegenerative mechanisms implicated in the disease manifestation and in the pathologyspreading from cell to cell throughout the entire brain. Thus,modelling AD in a dish still represents a big challenge,due to the complex etiology of the disease. A promising step to increase our knowledge,to identify druggable targets,and to foster drug development will be the integration in cortical organoids of iPSC derived immune and vascular cells harboring AD related mutations. In this scenario,the opportunity to exploit genome editing technologies such as CRISPR-CAS9,zinc finger nucleases,and transcription activator-like effector nucleases for genetically engineer healthy iPSCs with a panel of AD-relevant mutations might reveal new biochemical pathways involved in the pathogenesis of the disease and offer novel therapeutic strategies in tackling the pathology (Figure 1).
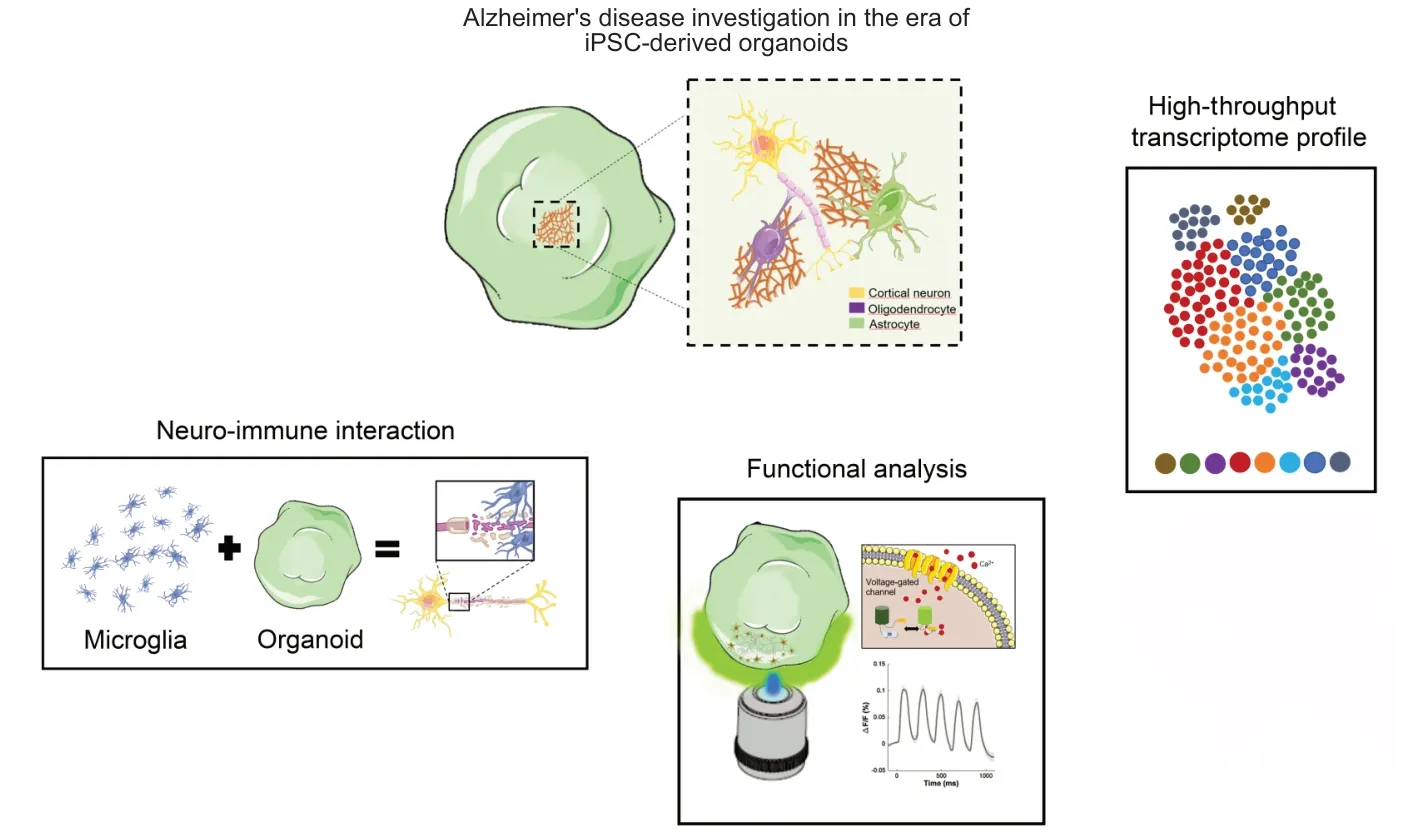
Figure 1|Potential applications of cortical organoids for fighting Alzheimer’s disease.
For example,human iPSC engineeredad hocto express TAU-P301L mutation could be used as bricks to build up specific brain organoids that better characterize the development of the TAU-associated neurodegenerative processes,looking at the cause-effect temporal relationship between Aβ plaques,neurofibrillary tangles and synaptic degeneration. Indeed,TAU-P301L mutation is frequently used in animal models to recapitulate pathological features of human AD,frontotemporal dementia,and parkinsonism. This mutation represents the most common and more aggressive MAPT mutation leading to the formation of twisted ribbon-like filaments of 4R-TAU isoforms in the temporal cortex,hippocampus,and substantia nigra,and to a functional TAU impairment in promoting microtubules assembly. In this manner,a more compliant framework could be generated by dissecting all cellular and molecular events occurring in different cell types in response to specific genetic alteration,posing,from individual evidence,valuable resources to integrate key molecular elements of AD in a stagedependent manner. Furthermore,the use of iPSC derived neurons expressing genetically encoded calcium,chloride,or voltage sensors (as GCaMP,Clop-Hensor or Archon1) will enable a functional high throughput analysis of synaptic and neuronal degeneration induced by TAU misfolding.
参考译文:The Chinese have the custom/habit of eating yuanxiao(sweet dumplings made of glutinous rice flour)and watching festivelanternson thefifteenth eveningof thefirst lunar month.
In a more holistic approach,to better model AD using anin vitro3D system,efforts should be made to implement the organoid model considering the interplay between neuron,astrocytes,microglia,and the neurovascular system,the so called neurovascular unit,thatin vivoensures the correct formation and function and the proper immune response of the brain. This can be achieved combining brain organoid with iPSC derived vascular structures and microglial cells though the hematopoietic differentiation,in microfluidic devices (multi-organ-on chip) or in bio-printed constructs. The combination of cells,with the same genetic background with or without TAU mutation will provide a powerful tool to identify the causative cascade of AD pathology.
In conclusion,human-derived brain organoids might represent an innovativein vitromodel which considers for the very first time the whole complexity of the human brain otherwise not fully replicated within 2D cultures or animal models. iPSC-based organoids provide a unique technology for deepening our understanding of the neuron-gliamicroglia interaction and crosstalk in both physiological and pathological conditions. In this regard,3D brain organoids might help to determine how different brain cells play key roles in the establishment of neurodegenerative mechanisms that underlie AD and other dementia-like phenotypes,expanding our knowledge in their pathogenesis and progression. Lastly,the use of genome editing approaches to introduce AD mutations and/or patientderived iPSC could reveal new molecular targets paving the way to establish more compliant therapeutic treatments likely reducing the use of animal models in drug screening.
Alessandro Soloperto and Silvia DiAngelantonio contributed equally to this article.
Federica Cordella,Carlo Brighi,Alessandro Soloperto*,Silvia Di Angelantonio*
Center for Life Nanoscience,Istituto Italiano di Tecnologia,Rome,Italy (Cordella F,Brighi C,Soloperto A,Di Angelantonio S)
Department of Physiology and Pharmacology,Sapienza University,Rome,Italy (Cordella F,Brighi C,Di Angelantonio S)
*Correspondence to:Alessandro Soloperto,PhD,alessandro.soloperto@iit.it; Silvia Di Angelantonio,PhD,silvia.diangelantonio@uniroma1.it.https://orcid.org/0000-0002-8142-6059 (Alessandro Soloperto)
Date of submission:December 2,2020
Date of decision:January 15,2021
Date of acceptance:March 4,2021
Date of web publication:July 8,2021
https://doi.org/10.4103/1673-5374.317976
How to cite this article:Cordella F,Brighi C,Soloperto A,Di Angelantonio S (2022) Stem cell-based 3D brain organoids for mimicking,investigating,and challenging Alzheimer’s diseases. Neural Regen Res 17(2):330-332.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Liang Yuan,Tufts University,USA.
Additional file:Open peer review report 1.
杂志排行
中国神经再生研究(英文版)的其它文章
- Deciphering the role of PGC-1α in neurological disorders: from mitochondrial dysfunction to synaptic failure
- Dying by fire: noncanonical functions of autophagy proteins in neuroinflammation and neurodegeneration
- Transcranial magnetic stimulation in animal models of neurodegeneration
- SYNGR4 and PLEKHB1 deregulation in motor neurons of amyotrophic lateral sclerosis models: potential contributions to pathobiology
- Cholesterol synthesis inhibition or depletion in axon regeneration
- Challenges in developing therapeutic strategies for mild neonatal encephalopathy
