Novel closed-cycle reaction mode for totally green production of Cu1.8Se nanoparticles based on laser-generated Se colloidal solution
2022-08-01ZhangyuGu顾张彧YisongFan范一松YixingYe叶一星YunyuCai蔡云雨JunLiu刘俊ShouliangWu吴守良PengfeiLi李鹏飞JunhuaHu胡俊华ChanghaoLiang梁长浩andYaoMa马垚
Zhangyu Gu(顾张彧), Yisong Fan(范一松), Yixing Ye(叶一星), Yunyu Cai(蔡云雨), Jun Liu(刘俊),Shouliang Wu(吴守良), Pengfei Li(李鹏飞), Junhua Hu(胡俊华), Changhao Liang(梁长浩),,5,§, and Yao Ma(马垚),6
1Henan Institute of Advanced Technology,Zhengzhou University,Zhengzhou 450001,China
2Key Laboratory of Materials Physics and Anhui Key Laboratory of Nanomaterials and Nanotechnology,Institute of Solid State Physics,Hefei Institutes of Physical Science,Chinese Academy of Sciences,Hefei 230031,China
3Anhui Institute of Optics and Fine Mechanics,Hefei Institutes of Physical Science,Chinese Academy of Sciences,Hefei 230031,China
4School of Materials Science and Engineering,Zhengzhou University,Zhengzhou 450001,China
5University of Science and Technology of China,Hefei 230026,China
6Technology Center,Benecke Changshun Auto Trim(Zhangjiagang)Co.,Ltd.,No.8,Changyang Rd.,Nansha,Jingang Town,Zhangjiagang 215632,China
Keywords: non-stoichiometric copper selenide, green production, selenium colloidal solution, laser irradiation in liquids
1. Introduction
As an important member of copper chalcogenide nanomaterials, Cu2-xSe (x=0.18~0.25) is widely demanded in many fields such as solar cells,[1,2]photothermal therapy,[3–7]photoacoustic imaging,[8]thermoelectric converters[9]and so on, because it has tunable stoichiometric composition, numerous phases and morphologies. In existed reports, various synthetic methods have been explored to generate diversified Cu2-xSe nanomaterials with different shapes and properties.[10]For example, Cu2-xSe nano-cubes,[11]ultrasmall plasmonic Cu2-xSe particles,[5]Cu2-xSe nanowires,[12]and Cu2-xSe nanosheets with controllable thickness[13]can be obtained by the one-pot solution strategy,the ambient aqueous method, the modified composite hydroxide mediated method and the cation exchange reactions, respectively. For decades,these synthesis approaches still cannot avoid the problems from relatively toxic raw materials,air sensitive process,high temperature, and so on.[14,15]The commonly used raw selenium materials include selenium powder, selenite, and selenium dioxide.[1,12,16]Selenite and selenium dioxide both have strong toxicity of human physiology and environment. Elemental selenium powder is relatively low toxic,but it must undergo a reduction process to be an acute toxic selenide. In the above-mentioned methods,to keep the stability of the cuprous ions, some protective measures are necessary, including inert gas,reductants,organic stabilizers,and so on. Meanwhile,the temperature at which Cu2-xSe is formed has been reported to be between 160°C and 250°C,[5,10–13,17]and it is not conductive to effective toxic control of heavily used selenium source.There is still a lack of truly green and convenient synthesis strategy to obtain Cu2-xSe NPs, especially for mass production.
On the other hand, there is a powerful preparation of green and safe Se NPs from the low toxic Se powder by pulsed laser irradiation in liquid (PLIL) technique which has always been deemed as a special methodology compatible with the 12 principles of green chemistry for achievement of nanoparticles.[22,23]In addition,the possibility of remote control and PC control for PLIL make this technology more suitable for continuous gram-scale nanoparticle production.[24,25]Different from other synthesis methods,such asin vivosynthesis, direct solution-phase synthesis, and acrylonitrile-induced synthesis.[26–28]PLIL generated Se colloidal nanoparticles in deionized water possess advantages in highly purity and tunable size according to our reported articles.[29–31]To our surprise,in our experiments,PLIL generated Se NPs in colloidal solution coexist with a little of SeO2due to the mild oxidation so as to the formation of minor H2SeO3in solution. This phenomenon has never been reported in previous reports. It indicates that PLIL generated Se colloidal solution potentially realize oxidation of Cu metal to supply cuprous ions which can catalyze Se NPs for the formation of Cu2-xSe NPs, and promote the recycle of the by-product.
2. Experimental section
2.1. Materials
Herein,the raw materials for obtaining Cu1.8Se NPs just comprised selenium powder (200 mesh, purity 99.99%) and copper sheets (AR, 99.5% metals basis, 0.1-mm thickness)which were both purchased from Aladdin Industrial Corporation. The deionized water used in the whole experiment was produced by a laboratory water purifier(Millipore direct-Q 5 UV).
2.2. Characterization
The images about morphology,size,crystal facets of the products were carefully observed and collected by a fieldemission scanning electron microscope (FESEM, model Hitachi SU8020)and a transmission electron microscope(TEM,FEI Tecnai TF20 operated at 200 kV). X-ray diffraction(XRD) data were tested on a Philips X’ Pert system with Cu Kαradiation (λ=1.5418 ˚A) to investigate the crystal structure of the products synthesized. The absorption properties of the samples were recorded by UV–Vis–NIR spectrophotometer(UV3600-MPC3100 spectrophotometer). X-ray photoelectron spectroscopy (XPS, Thermo ESCALAB 250) was employed to analyze the chemical state of samples. Inductively coupled plasma mass spectrometer (ICP-MS, icap Qc)was used to determine the content of target elements in samples.
2.3. Experiments
2.3.1. PLIL synthesis of selenium colloidal solution
In this work,the pulsed laser irradiation in liquid(PLIL)technique was utilized to prepare selenium colloidal solution as the active precursor for generation of Cu2-xSe NPs. In detail,first,selenium powder was mixed with deionized water to form a suspension at the concentration of 100 ppm after the ultrasonic treatment. Second, such suspension (50 mL) in a glass bottle was irradiated by an Nd:YAG nanosecond pulsed laser with the assistance of the magnetic stirrer. The chosen parameters of the laser included pulse duration 7 ns, wavelength 532 nm, pulse repetition rate 50 Hz, energy per pulse 200 mJ,and output power 10 W.After irradiation for 10 min,the Se suspension transformed to Se colloidal solution, and then displayed obvious Tyndall effect indicating the existence of a large number of Se NPs.This product was labeled as Se-1.For comparation,the laser irradiation duration was changed as 5 min,20 min,and 40 min,and such formed Se colloidal solutions were recorded as Se-2,Se-3,and Se-4,respectively. The schematic diagram of preparation process is shown in Fig.1.
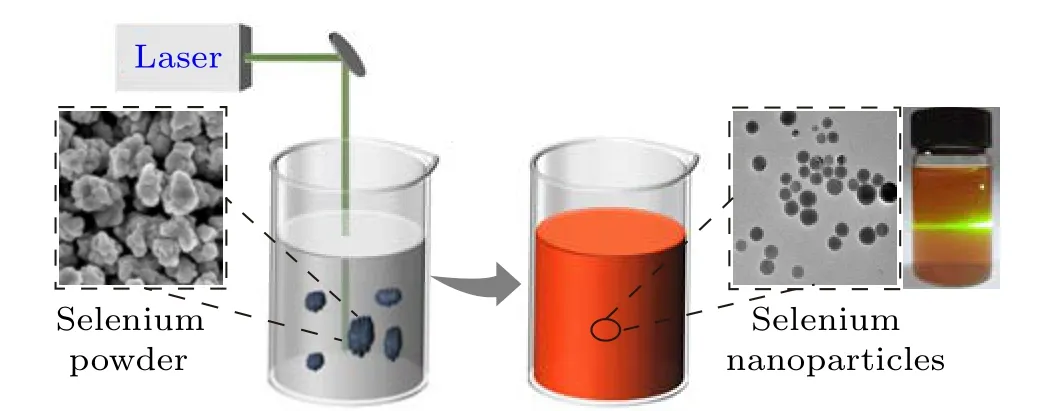
Fig.1. Schematic diagram for preparation of Se colloidal solution by PLIL technique.
2.3.2. Closed-cycle synthesis of monodisperse Cu2-xSe NPs
The copper sheet (1 cm2) was dipped in the abovementioned Se colloidal solution at 70°C for 48 h until the color of the solution changed from brick red to dark green indicating the exhaustion of original Se source and formation of Cu2-xSe NPs. After standing for 12 h, Cu2-xSe NPs precipitated and made the solution clean. The residual copper sheet continued to react with the newly supplied Se colloidal solution until the copper was completely used. As formed Cu2-xSe NPs were successfully synthesized by a closed-cycle process through a series of reactions so as to the reuse of the by-product of H2SeO3and the high conversion rate of Se element. The detail of the synthesis was discussed in the following part.
3. Results and discussion
Figure 2 shows basic characterization of Se NPs in colloidal solutions generated from Se powder by pulsed laser irradiation in deionized water. In Figs. 2(a)–2(d), TEM images of Se NPs in four groups revealed the size change under different laser irradiation durations. The average sizes of Se NPs in Se-1, Se-2, Se-3, and Se-4 groups were counted as 51.51±8.38 nm, 101.11±46.40 nm, 15.87±6.71 nm, and 1.53±0.38 nm, respectively. XRD patterns in Fig. 2(e) and selected area electron diffraction (SAED) pattern inserted in Fig.2(a)both confirmed that laser-generated Se NPs has amorphous state under different laser irradiation durations. Raman spectra for the four samples in Fig. 2(f) display a same peak at around 257 cm-1which corresponds to the A1symmetric stretching mode of amorphous Se.[29]According to our previous reports, pulsed laser irradiation can melt bulk Se so as to generate amorphous Se NPs in liquid with variable sizes and shapes.[30,31]Herein,the chosen selenium powder has the average size of~2 μm (see Fig. S1 in Supporting information),and looks like small Se bulks. When intensively heated by the absorbed laser, the powder will suffer from melting and/or evaporation in water and decomposition into smaller fragments.
In Fig. 2(g) for the sample Se-1, a doublet between 57 eV~54 eV with a spin orbit splitting energy of 0.86 eV was well attributed to the element Se.[32]Interestingly,a weak peak at 59 eV in Fig. 2(g) was also observed. It is well attributed to Se 3d5/2from H2SeO3indicating the existence of Se element with high valency state in solution.[33]This phenomenon was also observed for other samples (see Fig. S2).Meanwhile, in Fig. 2(h), pH values of the four Se colloidal solutions changed in a narrow range from 4.91 to 5.75 indicating a stable acidity feature after laser irradiation. ICP-MS test also confirmed the formation of the ionic Se species with low concentration in solution(see Fig.S3). Especially for Se-1 group,the proportion of oxidized Se element was calculated as about 1.6%. Therefore,it is inferred that a small amount of selenium element undergoes the oxidation change during laser irradiation. Laser irradiation can greatly reduce the size of Se particles,but has no obvious effect on pH value of Se colloidal solution.
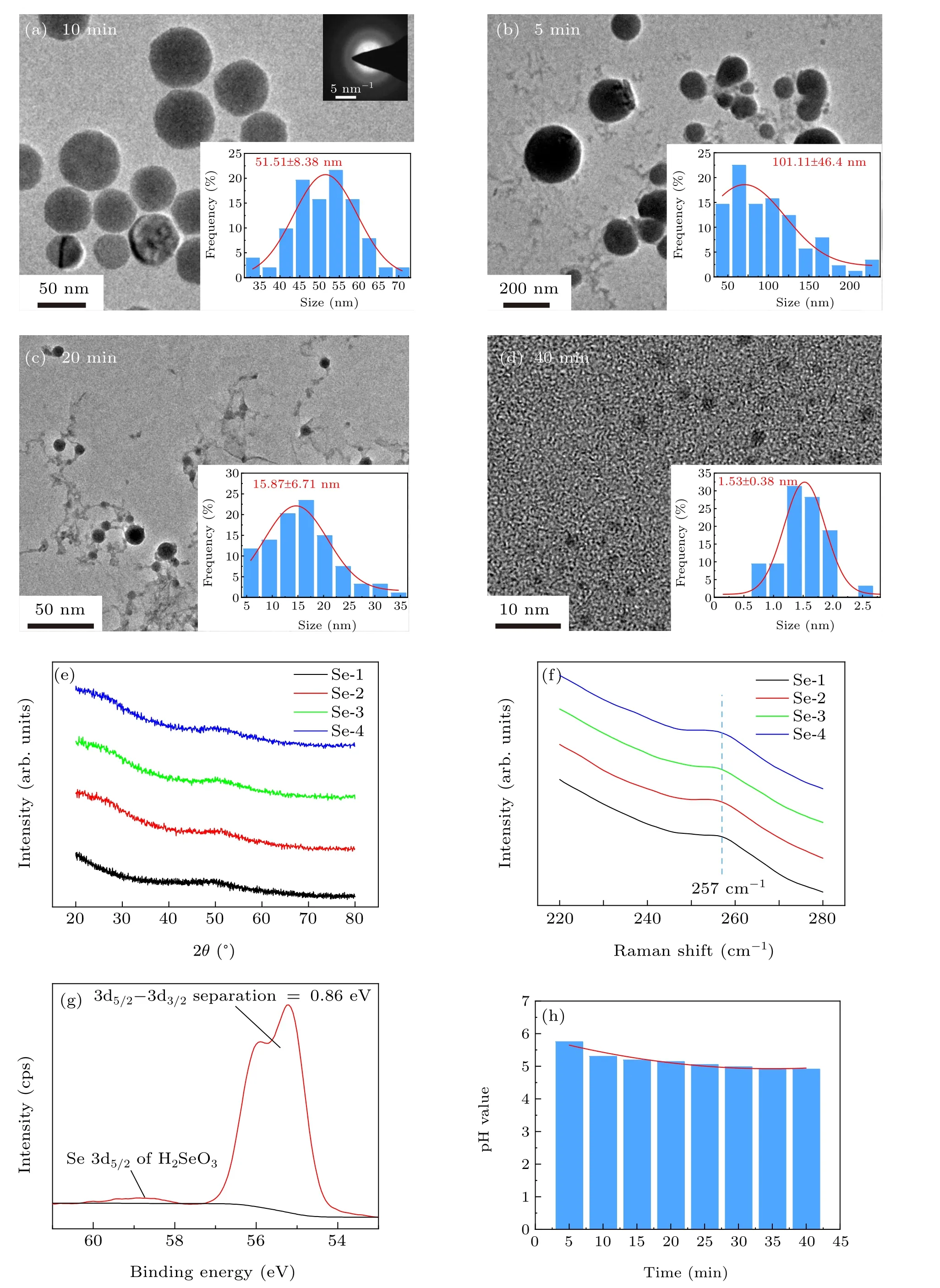
Fig. 2. The characterization of selenium colloidal solutions: (a)–(d) TEM images of Se NPs in the corresponding groups with inserted grain diameter distribution maps;(e)XRD patterns of selenium NPs;(f)Raman pattern;(g)XPS spectrum of sample;(h)relationship between pH value of Se colloidal solution and irradiation time.
Figure 3 outlines the synthesis process from dipping of copper sheet in Se colloidal solution to generation of Cu2-xSe NPs. In Fig. 4, the product generated by Se-1 sample and Cu sheet was analyzed. The obtained NPs possess good dispersibility in water. TEM image in Fig.4(b)shows that these NPs are ovoid with mean size around 70 nm. The highresolution TEM image inserted in Fig. 4(b) displays distinguishable lattice spacing of 0.33 nm corresponding to the plane (111) of berzelianite Cu2-xSe.[3]The XRD pattern in Fig.4(c)of the as-prepared NPs shows three diffraction peaks located at 27.75°, 46.10°, and 54.67°, assigned to the (111),(220),(311)planes of cubic berzelianite Cu1.8Se,respectively(JCPDS No.88-2045).[3]Excellent absorption performance in near-infrared region in Fig. 4(d) was also in line with traditional Cu2-xSe nanomaterials.[34]XPS in Figs. 4(e) and 4(f)identified the valence states of Cu and Se elements in the product. Cu 2p1/2and Cu 2p3/2peaks both can be separated into two peaks, respectively. In detail, the two strong peaks at 952 eV and 932.1 eV both were consistent with the univalent Cu+ion, while the two weak peaks at 953.9 eV and 933.5 eV were indexed well with the divalent Cu2+ion.[35]It indicates that minor Cu+ions are oxidized to Cu2+ions.The two weak satellite peaks at 965 eV–960 eV and 945 eV–940 eV respectively are recognized as shake-up satellite peaks of Cu 2p.[35,36]The peak at 916.9 eV in the Cu LMM spectrum as shown in Fig.S4 further proved the univalent state of Cu species in the product.[13,37]The double peaks at 55.3 eV and 54.6 eV were the typical Se 3d binding energy for lattice Se2-.[38]
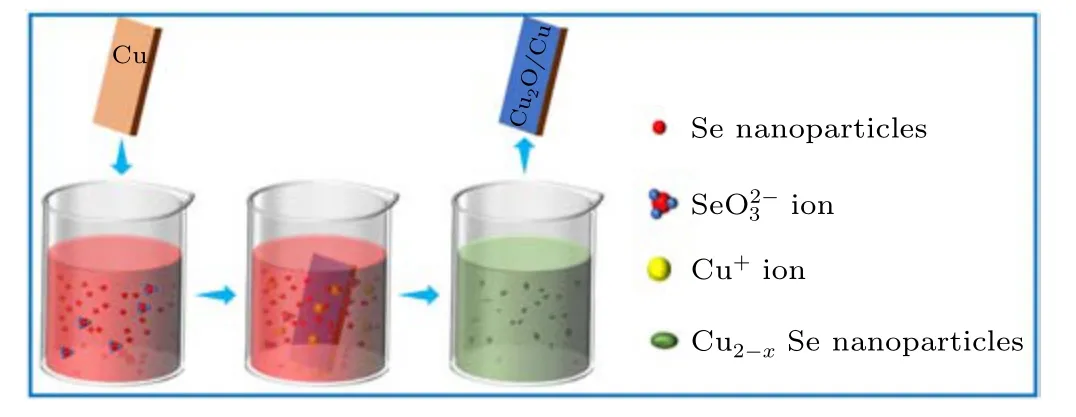
Fig.3. Schematic diagram of synthesis of Cu2-xSe NPs.
To investigate the conversion of Se element, Se content before and after the reaction was compared. For Se-1 reacting with Cu sheet at 70°C, quality ratio of Se element in final Cu1.8Se product to original Se powder was calculated as 75.86% by ICP-MS test in Fig. 4(b). In experiments, laser irradiation can modulate the size of Se NPs. Different Se colloidal solutions were used to react with the same Cu sheets at 70°C, and the conversion rate of Se element was counted listed in Fig.4(a). When the size of Se NPs was smaller than or equal to 50 nm,the conversion rate was about 75%. For Se NPs larger than 50 nm,the conversion rate decreased to about 10%. On the other hand, the effect from the reaction temperature was also analyzed. When temperature increased to 90°C,the conversion rates for Se-1,Se-3,and Se-4 groups all rose slightly,while the conversion rate for Se-2 group dropped slightly. However, when temperature decreased to 50°C,the conversion rate for the four groups all significantly reduced.Herein,it is reasonable to choose the optimal strategy in which Se-1 is the precursor to react with Cu sheet at 70°C to obtain Cu1.8Se NPs.
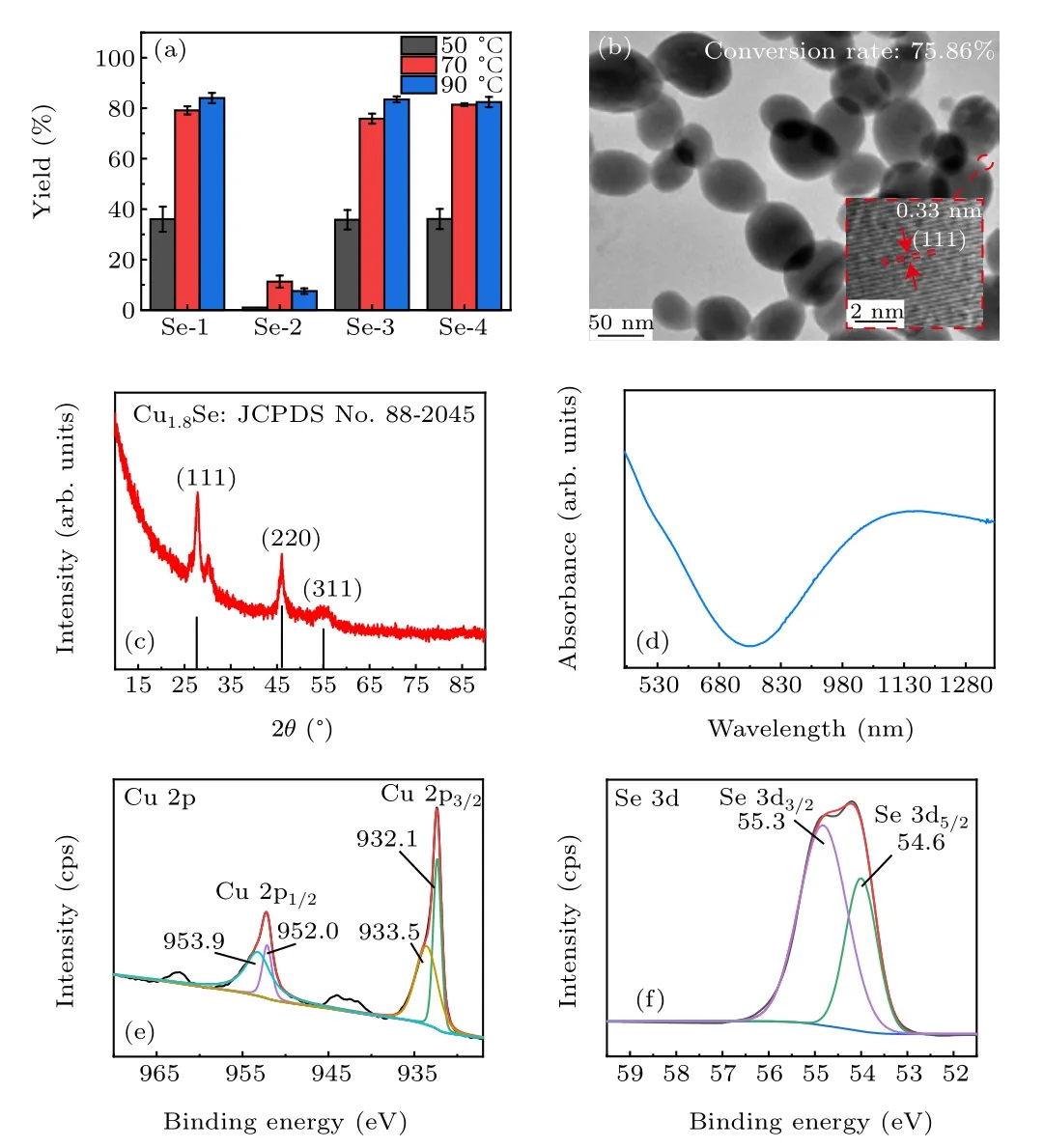
Fig.4.(a)Comparison of production yield of Cu2-xSe NPs with different reaction temperatures and Se colloidal solution; (b)TEM image and HRTEM(inset image)of the as-prepared Cu2-xSe NPs;(c)XRD pattern;(d)UV–Vis–NIR absorbance spectra of the as-synthesized Cu2-xSe colloidal solution;XPS spectra of Cu2-xSe NPs: (e)Cu 2p;(f)Se 3d.
Figure 5 further reveals the synthesis mechanism for Cu1.8Se NPs. In this work, copper sheets and selenium colloidal solutions by PLIL technique are starting reactants for preparation of Cu1.8Se NPs.However,Se NPs in element state cannot directly react with Cu sheet because of unmatched redox potential. It is worth noting that,during the laser irradiation on Se powder,a small amount of selenium can be oxidized by oxygen in air or water to form selenium dioxide (SeO2),which dissolves in water to form selenite(H2SeO3)and makes the colloidal solution acidic.As commonly reported,Cu metal can be oxidized to form Cu2O by H2SeO3in solution.It means that a thin layer of Cu2O film can be generated on the surface of original Cu sheet in our experiments(see Eq.(1)). Due to the acidic environment, Cu+ions quickly dissolve from Cu sheet into the solution(see Eq.(2)). Thereafter,Cu+ions can make elemental Se to undergo the disproportionation catalytic reaction and convert into Cu2Se and H2SeO3species shown in Eq.(3).[19]Finally,in Eq.(4),the as-formed Cu2Se NPs were oxidated into Cu2-xSe NPs through a phase transformation promoted by air exposure and the unreacted oxidants.[20]ICPMS results of colloids at different stages of the reaction well support this theory(Fig.S5(c)and Fig.6(d)). In most reports,Cu+ions work as the initial source for Cu2-xSe and must be protected by reductive conditions.[5,11–13]Interestingly,because of Cu metal as the raw material in all experiments,laser induced H2SeO3works as reaction initiator and reappear as the by-product which can further dissolve Cu element to introduce more and more Cu+ions until the Se NPs are used up. Such processes bring out a special closed-cycle reaction mechanism in our experiments.
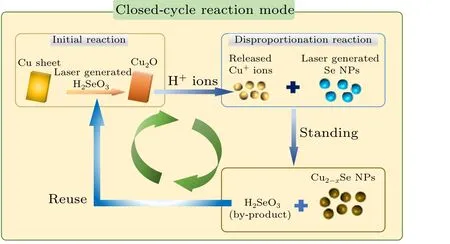
Fig.5. Schematic diagram of the closed-cycle reaction mode.
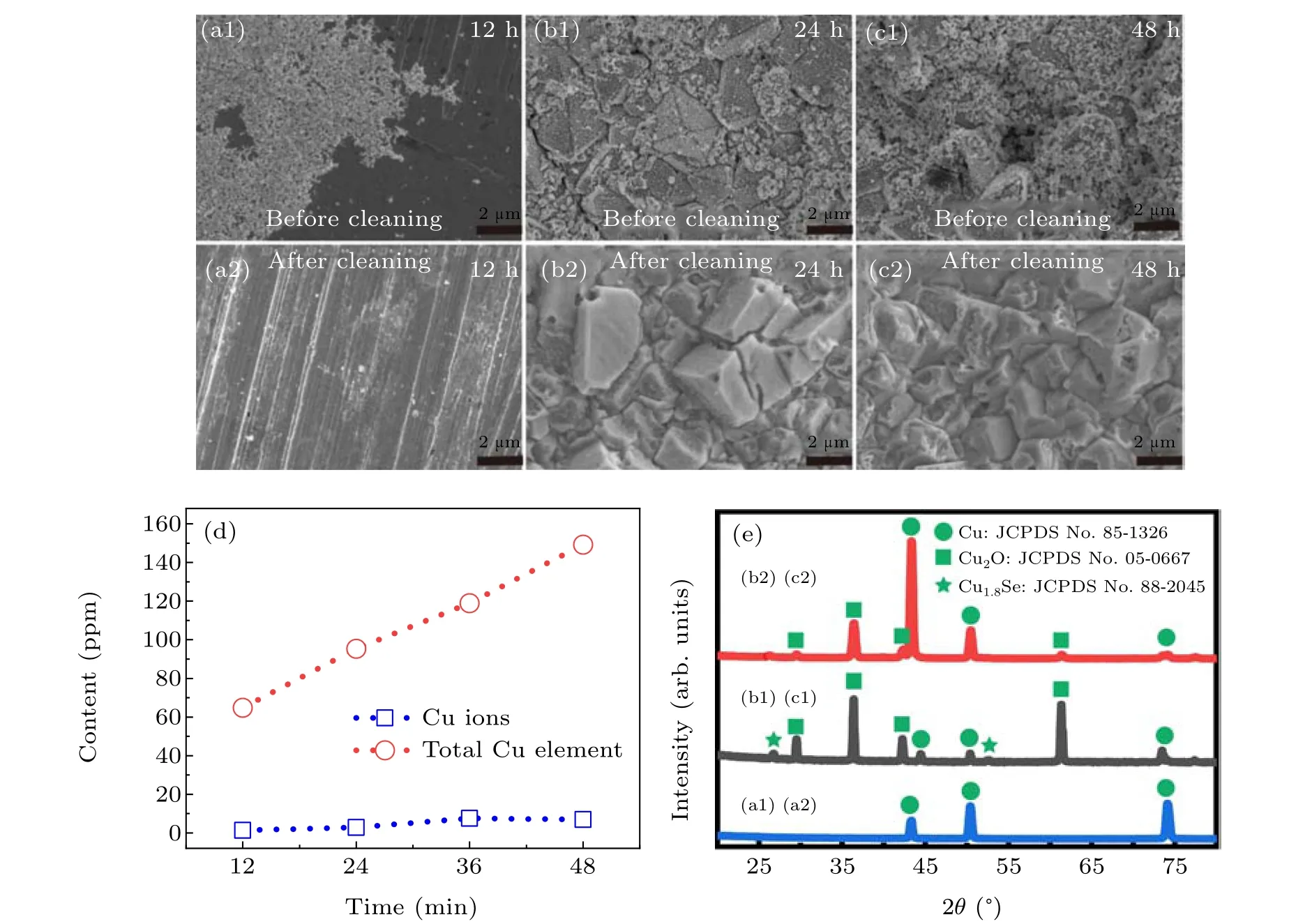
Fig.6. Analysis of reaction intermediate processes: (a1),(b1),and(c1): SEM images of copper sheet surfaces taken from Se colloidal solution at different time (12, 24, 48 hours, respectively); (a2), (b2), and (c2): the images of the corresponding copper sheets after cleaning respectively; (d)ICP-MS results;(e)XRD patterns of the corresponding copper sheets.
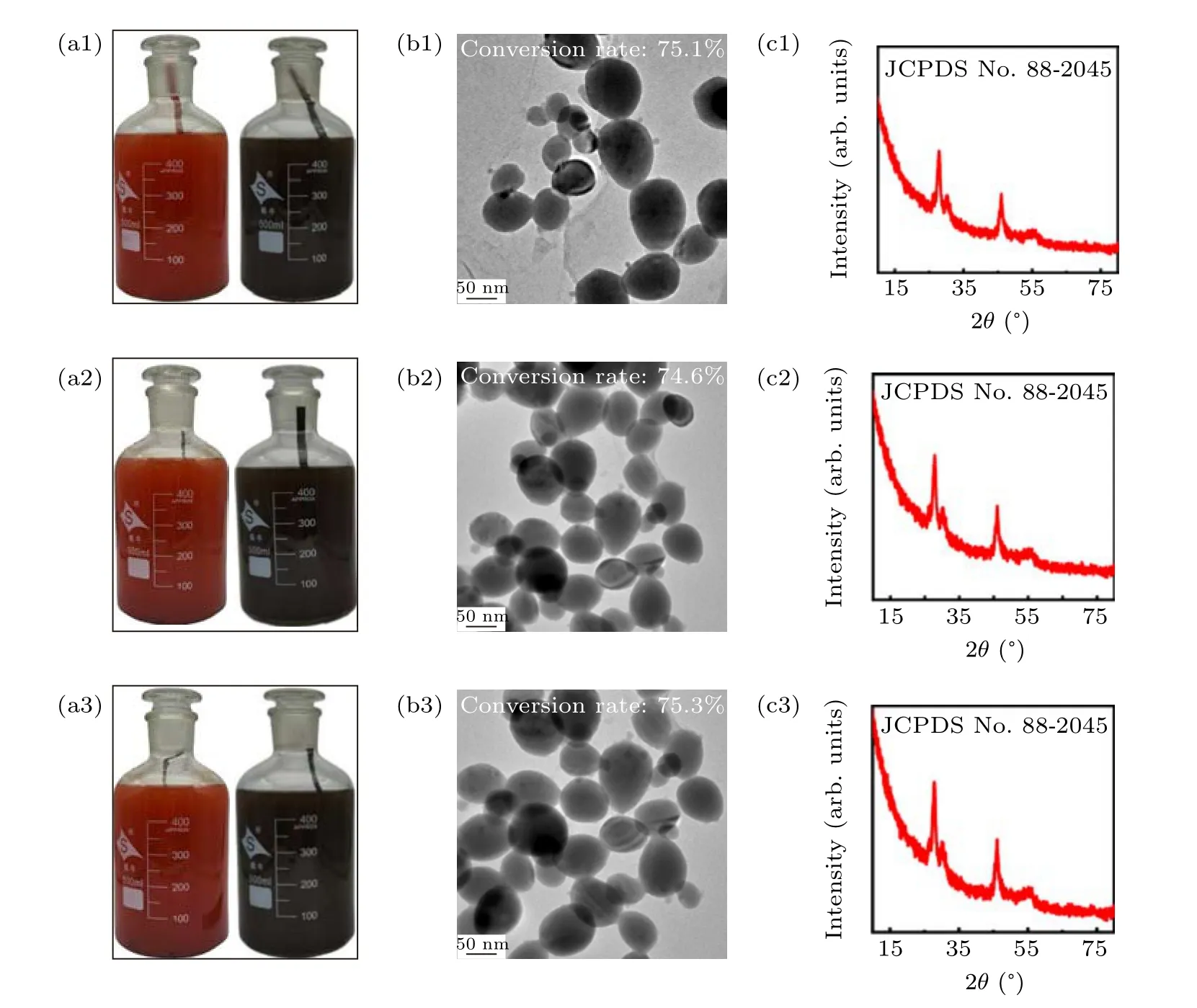
Fig.7. (a1)–(a3)Photographs of the 500-mL large-scale synthesis in glass reaction containers corresponding to the first,second,and third batches of reactions respectively;TEM images(b1)–(b3)and XRD patterns(c1)–(c3)of products obtained in different batches.
To enhance production of Cu1.8Se NPs,the dose of Se-1 solution was increased from 50 mL to 500 mL.In the reaction,copper element is always excessive to Se colloid,so in theory,it is unnecessary to further increase the quality and area of the copper sheet. However,to ensure the sufficient mass and heat transfer in big vessels,we cut the copper sheets in the reaction into long strips with a width of 0.5 cm and length of 12 cm.In addition, the other reaction conditions remain unchanged.After the exhaustion of Se NPs in initial solution,the residual Cu reacted with the following supplied Se colloidal solutions for many rounds. In Fig.7,the products generated in the first three rounds of experiments were compared. In the left column, Figs. 7(a1)–7(a3) were the photographs of Se colloidal solutions before and after reaction with Cu sheets in the first three rounds. The color changes for the three groups followed the same pattern. For the middle column from Figs. 7(b1) to Fig.7(b3),the average size for the products in the 3 groups located between 60 nm~70 nm,which was also similar to the result in Fig.4(b). On the basis of ICP-MS tests, the conversion rates still reached around 75%in several times of enlarged synthesis. XRD patterns in the right column from Fig. 7(c1)to Fig. 7(c3) also confirmed the same Cu1.8Se phase of the three products. All data indicated that copper sheets can be used continuously without subsequent treatment to participate in the next round of synthesis and it would not make any difference to the features of the products. A reasonable explanation is that the excessive copper in the reaction makes the availability of multiple closed-cycle reaction which can be deemed as a quasi-continuous mode of production in which accumulation of toxic by-product H2SeO3can be avoided and no reduction conditions is required.
4. Conclusion
In summary,we first put forward a green and convenient synthesis of Cu2-xSe NPs in the way of closed-cycle reaction which can be scaled up as a quasi-continuous mode of production. The copper sheets worked as the raw source to avoid the requirements for reductive conditions. Se colloidal solution generated by pulsed laser irradiation in water contains a large amount of Se NPs and a little of H2SeO3as the initiator for the closed-cycle reaction. The final product was confirmed as Cu1.8Se NPs with average size of 70 nm. The conversion rate of Se element from Se powder to Cu1.8Se NPs was calculated as about 75%. In the extended experiment, the volume of the Se colloidal solution was increased to 500 mL. After several time of continues reaction with the same Cu sheet,the products were still Cu1.8Se NPs with unchanged size feature and Se conversion rate. Interestingly,compared with common preparation methods for Cu2-xSe,the dose of H2SeO3in this cycle-closed reaction is at a much lower level,while H2SeO3as the subsequent by-product is also reused. This indicates a brand-new way to improve yield of Cu2-xSe products and avoid non-environmentally friendly operations on the synthesis.
Acknowledgments
Project supported by the Fund from Hefei National Laboratory for Physical Sciences at the Microscale (Grant No. KF2020110), the Natural Science Foundation of Anhui Province, China (Grant No. 1908085ME146), the Key Research and Development Plan of Anhui Province, China(Grant No.201904a05020049),the Director Fund of Institute of Solid State Physics, Chinese Academy of Sciences (Grant No.2019DFY01),the National Natural Science Foundation of China(Grant Nos.52071313 and 51971211),and the Hefei Institutes of Physical Science,Chinese Academy of Sciences Director’s Fund(Grant Nos.YZJJZX202018 and YZJJ202102).
猜你喜欢
杂志排行
Chinese Physics B的其它文章
- Real non-Hermitian energy spectra without any symmetry
- Propagation and modulational instability of Rossby waves in stratified fluids
- Effect of observation time on source identification of diffusion in complex networks
- Topological phase transition in cavity optomechanical system with periodical modulation
- Practical security analysis of continuous-variable quantum key distribution with an unbalanced heterodyne detector
- Photon blockade in a cavity–atom optomechanical system
