Effect of decoction of Fuzheng Jiedu Xiaoji formula (扶正解毒消积方) plus chemoembolization on primary liver cancer in patients
2022-07-20WUTongYANGZhiyunYANGYuyingJIANGYuyongMENGPeipeiLIUHuiminTIANYehongZHANGQiaoli
WU Tong,YANG Zhiyun,YANG Yuying,JIANG Yuyong,MENG Peipei,LIU Huimin,TIAN Yehong,ZHANG Qiaoli
WU Tong,YANG Zhiyun,YANG Yuying,JIANG Yuyong,MENG Peipei,LIU Huimin,Department of Integrated Traditional Chinese and Western Medicine,Beijing Ditan Hospital,Capital Medical University,Beijing 100015,China
TIAN Yehong,Acupuncture and Minimally Invasive Oncology,The Third Affiliated Hospital,Beijing University of Chinese Medicine,Beijing 100029,China;Institute of Acupuncture and Moxibustion,Shaanxi University of Chinese Medicine,Shaanxi 712046,China
ZHANG Qiaoli,Acupuncture and Minimally Invasive Oncology,The Third Affiliated Hospital,Beijing University of Chinese Medicine,Beijing 100029,China
Abstract OBJECTIVE:To investigate the effect of the decoction of Fuzheng Jiedu Xiaoji formula (扶正解毒消积方,FJXF)plus chemoembolization (TACE) on primary liver cancer(PLC) in patients,and study the underlying mechanism.METHODS:Patients with PLC who met the inclusion criteria were randomized into case group and control group.The case group was treated with FJXF combined with TACE.The control group was treated with TACE alone.The short-term clinical effect was evaluated;liver biochemistry,liver function index and multidrug resistance-associated indicators were detected.RESULTS:FJXF combined with TACE in the case group significantly increased the disease control rate than TACE alone in the control group (83.3% vs 61.1%).There was a reduction in the serum alpha-fetoprotein at 8 weeks after treatment in each group,while no difference between the two groups.The same trend can be observed for transaminase and direct bilirubin in both groups.In the case group,it showed a significant increase for albumin at 8 weeks after treatment,while no change in the control group.Multidrug resistanceassociated indicators for multidrug resistance protein 1 and p-glycoprotein were upregulated in the case group but remained stable in the control group.CONCLUSIONS:FJXF combined TACE had a better short-term effect than TACE alone in patients with PLC.The potential mechanism was probably associated with alleviated multidrug resistance induced by FJXF.Additionally,FJXF didn’t increase the risk of liver damage in the combined therapy.
Keywords:liver neoplasms;ATP binding cassette transporter,subfamily B,member 1;chemoembolization,therapeutic;Fuzheng Jiedu Xiaoji formula
1.INTRODUCTION
Primary liver cancer (PLC) is a common malignancy,particularly in China.Global cancer statistics indicated that PLC was the sixth most commonly diagnosed cancer and the fourth leading cause of cancer death worldwide in 2018,with about 841 000 new cases and 782 000 deaths.1The American Cancer Society estimated that in 2020 there was 42 810 new cases and 30 160 deaths of PLC.2China has a high incidence of PLC accounting for 46.6% new cases and 47.1% deaths.1Therefore,active prevention and treatment are of great significance for PLC.However,due to the insidious onset and rapid progression of PLC,only 30% patients benefited in surgical resection and liver transplantation.Most patients have no opportunity of surgery after first diagnosed,they are only treated with palliative care intervention,such as transcatheter arterial chemoembolization (TACE),radiofrequency ablation,cryoablation or molecular targeted drugs.Furthermore,current treatment for PLC did not significantly improve 5-year survival in which multi-drug resistance is an important factor of treatment failure.3Fuzheng Jiedu Xiaoji formula (扶正解毒消积方,FJXF) from Traditional Chinese Medicine (TCM) is a clinically effective empirical prescription of the Center for Integrative Medicine of Beijing Ditan hospital.Our previous clinical study indicated that integrative therapy based on FJXF can control the progression and improve the survival rate for PLC.4Based on the previous studies,this study investigated the clinical effect of FJXF combined with TACE for PLC and elucidated the potential mechanism from the phenotype of multi-drug resistance.
2.METHODS
2.1.Patients
A total of 72 patients with PLC admitted to Beijing Ditan hospital from July 2017 to December 2018 were eligible for the study.The included patients were randomized into case group and control group by random number table in the ratio of 1∶1.The details about baseline demographic and clinical characteristics in the two groups are shown in Table 1.There was no significant difference between the two groups on the baseline data(P >0.05).The study was approved by the Ethics Committee of Beijing Ditan Hospital (Jing Di Lun Ke Zi(2017) No.(011)-01).
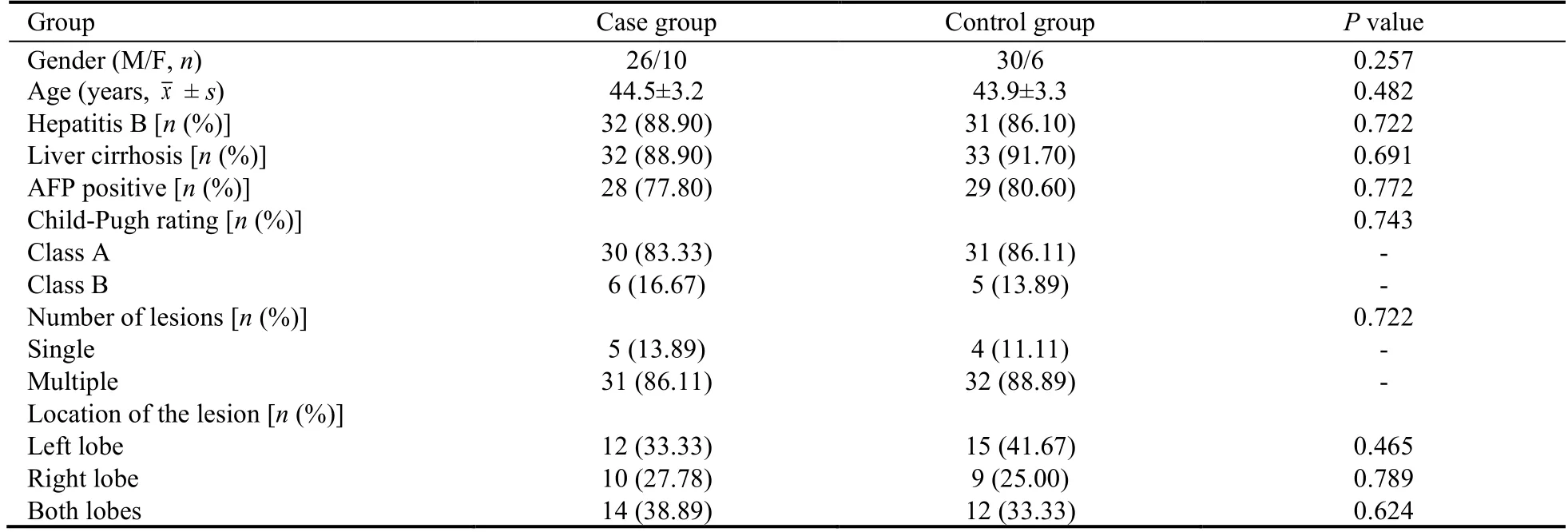
Table 1 Baseline information of the patients between the two groups
2.2.Inclusion criteria
(a) Conformed to the diagnostic criteria of“Chinese Society of Clinical Oncology Guidelines for the Diagnosis and Treatment of Primary Liver Cancer”;5(b)unresectable PLC according to the“2018 practice guidance by the American Association for the Study of Liver Diseases”;6(c) Karnofsky performance score (KPS)≥ 70;(d) aged 35 to 65 years;(e) life expectancy > 3 months;(f) Child-Pugh class A or class B;(g) patients signed the informed consent.
2.3.Exclusion criteria
(a) Extrahepatic metastases or other primary malignancies;(b) received chemoradiotherapy before enrolled;(c) uncontrollable serious infection,long-term chronic infection or non-healed wound;(d) critical diseases in heart,lung,kidney or other organs;(e)patients with mental disorder;(f) pregnant or lactating women;(g) had contraindications of arterial embolism;(h) patients unable to cooperate.
2.4.Treatments
The patients were treated with TACE alone in the control group and FJXF combined TACE in the case group.
The operation of TACE is as follows:Seldinger technique was used to insert the catheter through the right femoral artery into the hepatic artery,then the blood supply artery of the tumor was observed with mesenteric angiography.Next,chemotherapeutic drug perfusion was completed after superselection of the blood supply artery of the lesion.Chemotherapeutic drugs included fluorouracil (Shenyang Pharmaceutical Co.,Ltd.,Shenyang,China,500 mg/m2) and Epirubicin (Pfizer S.r.l.,Wuxi,China,40 mg/ m2).Finally,perfusion tissue was embolized with iodized oil,and the embolization was further strengthened with gelatin sponge debris till blood flow slowed down.The dose of the embolization was determined according to the tumor size and vascular density.7,8
FJXF was taken orally on the first day after TACE treatment.FJXF consisted of Dangshen (Radix Codonopsis) 20 g,Huangqi (Radix Astragali Mongolici) 20 g,Baizhu (Rhizoma Atractylodis Macrocephalae) 15 g,Fuling (Poria) 15 g,Beishashen (Radix Glehniae) 15 g,Maidong (Radix Ophiopogonis Japonici) 15 g,Danggui(Radix Angelicae Sinensis) 15 g,Shudihuang (Radix Reh-manniae Preparata) 15 g,Wugong (Scolopendra)10 g,Qiyeyizhihuagen (Rhizoma Paridis) 15 g and Biejia(Carapax Trionycis) 15 g.The formula was modified with clinical symptoms.Dosages and schedules are as follows:decocted in water for oral administration at a dose of 200 mL twice per day,with 14 d as a cycle for 4 consecutive cycles.It was provided and decocted by the TCM pharmacy of Beijing Ditan Hospital Capital Medical University.
2.5.Reagents
Reverse transcription-polymerase chain reaction (RTPCR) kit was from TaKaRa Biotechnology Co.,Ltd.,(Dalian,China,3587A),and rabbit anti-human Pglycoprotein monoclonal antibody was purchased from Jackson ImmunoResearch Laboratories Inc.,(PA,USA,111-035-003).
2.6.Experimental methods
Real-time PCR:total RNA was extracted and the firststrand cDNA was synthesized by RT-PCR Kit instructions.The PCR was carried out in a 25 μL reaction system.Glyceraldehyde-3-phosphate dehydrogenase was used as an internal reference,and the ratio was used as the multidrug resistance protein 1 (MDR1) mRNA expression.MDR1 upstream primer was 5’-GGCCTAATGCCGAACACATT-3’,and downstream primer was 5’-CTTCTTCACCTCCAGGCTCA -3’.
Western blot:the specimens were added with protein lysate,homogenized,placed on ice for 15 min,and centrifuged at 12 000 rpm for 10 min.Then the supernatant was pipetted,loaded to the lane,and separated by electrophoresis,transferred to PVDF membrane for incubation with primary antibody at 4℃overnight,then reacted with secondary antibody at room temperature for 4 h.After that,the specimens were examined with X-ray.Finally,the images were scanned and analyzed using LabWorks software.
2.7.Observation indicators
(a) The evaluation of short-term clinical effect according to Response Evaluation Criteria in Solid Tumors(RECIST) 1.1.Complete response (CR) defined as complete disappearance of tumor for more than 4 weeks.Partial response (PR) defined as a decrease of ≥ 30% in the sum of the longest diameter for more than 4 weeks.Stable disease (SD) defined as a reduction of < 30% or increase of < 20% in the sum of the longest diameter.Progressive disease (PD) defined as an increase of ≥ 20%in the sum of the longest diameter or occurrence of new lesions.9Disease control rate (DCR) describes the percentage of patients with advanced cancer in which therapeutic intervention has led to a complete response,partial response,or stable disease.The formula of DCR is as follows:
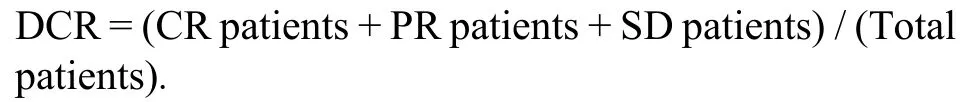
(b) Liver-associated biochemistry and function parameters in peripheral blood included alanine aminotransferase (ALT),aspartate transferase (AST),alpha-fetoprotein (AFP),direct bilirubin (DBIL),prealbumin (PAB),albumin (ALB) and international standardized ratio of coagulation (INR).The parameters were detected before treatment,at 1 week and 8 weeks after treatment.
(c) Indicators associated with multidrug resistance in peripheral venous blood involved in MDR1 and pglycoprotein (P-gp),which were detected before treatment and 8 weeks after treatment,respectively.
2.8.Statistical analysis
SPSS statistics (version 22.0) was used for data analysis.The count data was expressed as percentage (%) with χ2test.The measurement data were expressed by mean ±standard deviation () witht-test.A threshold ofPvalue was set at 0.05.
3.RESULTS
3.1.Comparison of short-term clinical effect between the two groups
The DCR was 83.3% in the case group and 61.1% in the control group.There was a significant difference between the two groups (Table 2).
3.2.Comparison of liver-associated biochemistry and function
The serum ALT,AST and DBIL levels increased at 1 week after the treatment and returned to pre-treatment levels at 8 weeks after the treatment in each group.Meanwhile,they had no difference between the two groups.AFP decreased significantly at 8 weeks after treatment in each group,while it didn’t show a more significant decrease between case and control group.As to the PBA,there was no change after treatment in the case group,while in the control group it was significantly increased at 1 week after TACE,then returned to pretreatment level at 8 weeks after the treatment.Serum ALB level had no difference at 1 week after treatment in each group.Interestingly,there was a significant elevation for ALB at 8 weeks after treatment in the case group but not in the control group.INR remained stable at all times in each group (Table 3).

Table 2 Comparison of the short-term clinical effect between the two groups [n (%)]
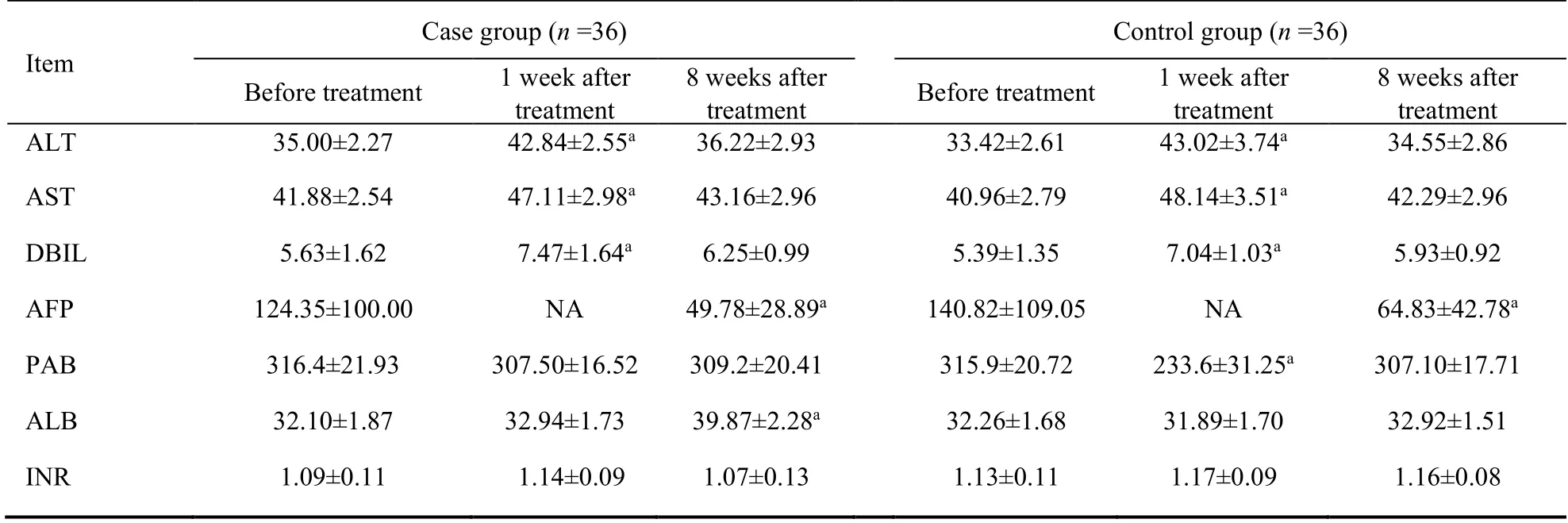
Table 3 Comparison of liver biochemistry/function before and after treatment
3.3.Comparison of MDR1 mRNA expression in both groups
Before treatment,there was no difference for MDR1 expression between case group and control group.In the case group,the mRNA expression of MDR1 slightly decreased at 8 weeks after treatment compared with before treatment (1.10 ± 0.08vs1.03 ± 0.10,P> 0.05).In the control group,the mRNA expression of MDR1 significantly increased at 8 weeks after treatment compared with before treatment (1.08 ± 0.09vs1.30 ±0.07,P< 0.05).
3.4.Comparison of P-gp protein expression between in both groups
Before treatment,there was no difference of P-gp expression between case group and control group.In the case group,P-gp kept unchanged at 8 weeks after treatment compared with before treatment (0.59 ± 0.05vs0.60 ±0.06,P> 0.05).In the control group,P-gp significantly increased at 8 weeks after treatment than before treatment (0.58 ± 0.06vs0.82 ± 0.08,P< 0.05) (Figure 1).
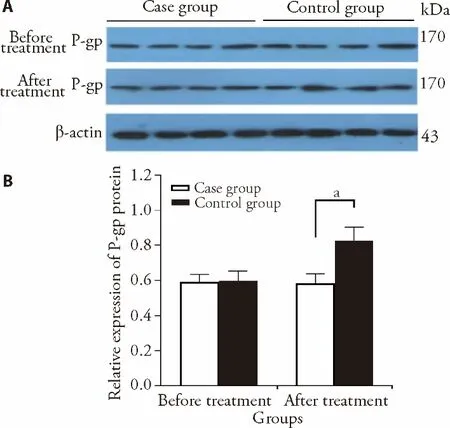
Figure 1 The protein expression of P-gp detected by Western blot in both groups
4.DISCUSSION
There is a high incidence for PLC in China accounting for almost half of worldwide cases.Due to the diversity for risk factors,individual variability and tumor heterogeneity,early diagnosis for PLC is limited.Therefore,most patients are diagnosed in advanced stage with poor therapeutic effect.Although some progress has been made for hepatic arterial chemoembolization,minimally invasive treatment and molecular targeted therapies,the 5-year survival rate for PLC was only 10.1%.10-12
TCM plays an important role in the treatment of refractory disease,such as cancer.In recent years,the combined treatment of TCM and western medicine for malignant tumors has been widely accepted.In Chinese ancient literature,PLC can be classified into the"accumulation","hypochondriac pain",or“liver accumulation".According to the theory of TCM,PLC is induced by long-term combined effects of internal and external factors.The internal factors mainly include liver dysfunction leaded by emotional depression and spleen dysfunction induced by fatigue.The external factors mainly include dampness and heat invading in the liver and gallbladder to form toxicity and stasis.Therefore,the pathogenesis for PLC is characterized with the deficiency complicated with excess so that it is difficult to cure.FJXF,which is the clinically effective empirical prescription of the Center for Integrative Medicine of Beijing Ditan hospital,consists of Dangshen (Radix Codonopsis),Huangqi (Radix Astragali Mongolici),Baizhu (Rhizoma Atractylodis Macrocephalae),etc.The formula has the effects of benefiting qi and blood,promoting blood circulation,removing blood stasis,and softening hardness to dissipate stagnation.A Metaanalysis evaluated the effectiveness and safety of multiple treatments (including TACE combined with TCM,TACE combined with sorafenib,TACE or sorafenib alone) for patients with advanced liver cancer,and the results showed that TACE combined with TCM had the best effect and the lowest incidence of adverse events than other treatments.13In a previous clinical study,we had investigated the effect of the integrative therapy based on FJXF for PLC.The results showed that it can delay disease progression and improve survival rate in patients with PLC.4Consistently,the results in this study showed that the DCR of FJXF combined with TACE in the treatment of patients with PLC was 83.3%and was significantly higher than that of TCEA alone(61.1%).Besides,a previous study found that TACE combined with TCM treatment was beneficial to patients with PLC in improving AST,DBIL,ALB and AFP.14Our study indicated that the levels of ALT,AST and DBIL in both case group and control group were increased transiently at 1 week after treatment,then returned to pre-treatment level at 8 weeks after treatment,which were similar to other studies.15-17Therefore,FJXF didn’t increase risk of liver damage in the combined therapy.As to the hepatic synthetic function,ALB in the case group at 8 weeks after treatment was significantly elevated.We speculated that the treatment of FJXF based on the TACE may be beneficial to the improvement of liver synthetic ability.
TACE is one of the common treatment methods for patients with advanced liver cancer.This method can achieve a median survival of 11-20 months,however more than 40% of patients with PLC have poor therapeutic effect.18One of critical reasons is the multidrug resistance of hepatoma cell to chemotherapeutic drugs.19-21The overexpression of MDR1 and its encoded protein P-gp is the main causes of tumor resistance.P-gp is the most important member of the ABC transporter family,which can actively pump the hydrophobic drug against the concentration gradient from the inside to the outside of the cell in an ATP energy-dependent manner.22,23Drug resistance of hepatoma cell can also be induced by pumping chemotherapeutic drugs out of the cancer cells.The results of our study showed that the expression of both MDR1 and P-gp significantly increased in the control group after treatment,while kept stable in the case group.It suggests that TACE induced multidrug resistance.Importantly,FJXR alleviated TACE-induced multidrug resistance by the stable expression of MDR1 and P-gp,which is also one of the mechanisms for better therapeutic effect in the treatment of FJXF combined with TACE.
The results of this study showed that FJXF combined with TACE has a better effect in the treatment of unresectable patients with PLC.Its potential mechanism is probably associated with the stable expression of MDR1 and P-gp through avoiding acquired multidrug resistance induced by TACE treatment.Meanwhile,this combination therapy is convenient for clinical application and universal availability.
5.REFERENCES
1.Bray F,Ferlay J,Soerjomataram I,et al.Global cancer statistics 2018:GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries.CA Cancer J Clin 2018;68:394-424.
2.Siegel RL,Miller KD,Jemal A.Cancer statistics,2020.CA Cancer J Clin 2020;70:7-30.
3.Zhou X,Ding J,Li H,et al.Hedgehog signalling mediates drug resistance through targeting TAP1 in hepatocellular carcinoma.J Cell Mol Med 2020;24:4298-311.
4.Gu LL,Liu HM,Zhou XB,et al.Efficacy analysis of integrative therapy based on Fuzheng Jiedu Xiaoji formula for primary liver cancer.J Tradit Chin Med 2014;55:576-9.
5.Zhou J,Sun HC,Wang Z,et al.Guidelines for diagnosis and treatment of primary liver cancer in China (2017 Edition).LIVER CANCER 2018;7:235-260
6.Marrero JA,Kulik LM,Sirlin CB,et al.Diagnosis,staging,and management of hepatocellular carcinoma:2018 practice guidance by the American Association for the Study of Liver Diseases.Hepatol 2018;68:723-50.
7.Grandhi MS,Kim AK,Ronnekleiv-Kelly SM,et al.Hepatocellular carcinoma:from diagnosis to treatment.Surg Oncol 2016;25:74-85.
8.Han K,Kim JH.Transarterial chemoembolization in hepatocellular carcinoma treatment:Barcelona clinic liver cancer staging system.World J Gastroenterol 2015;21:10327-35.
9.Schwartz LH,Litiere S,de Vries E,et al.RECIST 1.1-Update and clarification:grom the RECIST committee.Eur J Cancer 2016;62:132-7.
10.Fu J,Wang H.Precision diagnosis and treatment of liver cancer in China.Cancer Lett 2018;412:283-8.
11.Lurje I,Czigany Z,Bednarsch J,et al.Treatment strategies for hepatocellular carcinoma -a multidisciplinary approach.Int J Mol Sci 2019;20.
12.Cyranoski D.China embraces precision medicine on a massive scale.Nat 2016;529:9-10.
13.Chen QF,Wu PH,Huang T,et al.Efficacy of treatment regimens for advanced hepatocellular carcinoma:a network Meta-analysis of randomized controlled trials.Medicine (Baltimore) 2019;98:e17460.
14.Wan YM,Li YH,Xu ZY,et al.The effect of transarterial chemoembolization in combination with Kang’ai injection on patients with intermediate stage hepatocellular carcinoma:a prospective study.Integr Cancer Ther 2017;17:477-85.
15.Chen J,Zhang SL.Study of liver function impairment after TACE in liver cancer.Shi Yong Lin Chuang Yi Yao Za Zhi 2017;21:202-4.
16.Ma MX,Sun XL,Guo YG,et al.Effect of TACE on liver function indexes in patients with primary liver cancer.Hainan Yi Xue Yuan Xue Bao 2017;23:1705-8.
17.Zhang SW,Lan Y,Sun YM.Effect of hepatic artery chemoembolization on hepatic function in the treatment of hepatic malignancy.Lin Chuang Yi Yao Wen Xian Za Zhi 2017;4:1011.
18.Martin S,Fako V,Dang H,et al.PKM2 inhibition may reverse therapeutic resistance to transarterial chemoembolization in hepatocellular carcinoma.J Exp Clin Cancer Res 2020;39.
19.Buschauer S,Koch A,Wiggermann P,et al.Hepatocellular carcinoma cells surviving doxorubicin treatment exhibit increased migratory potential and resistance to doxorubicin re-treatmentin vitro.Oncol Lett 2018;15:4635-40.
20.Perez-Tomas R.Multidrug resistance:retrospect and prospects in anti-cancer drug treatment.Curr Med Chem 2006;13:1859-76.
21.Song JE,Kim DY.Conventionalvsdrug-eluting beads transarterial chemoembolization for hepatocellular carcinoma.World J Hepatol 2017;9:808-14.
22.Roninson IB.The role of the MDR1 (P-glycoprotein) gene in multidrug resistancein vitroandin vivo.Biochem Pharmacol 1992;43:95-102.
23.Tian QE,De LH,Yan M,et al.Effects of Astragalus polysaccharides on P-glycoprotein efflux pump function and protein expression in H22 hepatoma cellsin vitro.BMC Com-plement Altern Med 2012;12:94.
猜你喜欢
杂志排行
Journal of Traditional Chinese Medicine的其它文章
- Efficacy of meridian massage for motor function after a stroke:a systematic review and Meta-analysis
- Antiviral Activity of Medicinal Plants against Human Coronavirus:a systematic scoping review of in vitro and in vivo experimentations
- Fuzheng Kang' ai decoction (扶正抗癌方) inhibits cell proliferation,migration and invasion by modulating mir-21-5p/human phosphatase and tensin homology deleted on chromosome ten in lung cancer cells
- Correlation between slow transit constipation and spleen Qi deficiency,and gut microbiota:a pilot study
- Efficacy of Kushen decoction (苦参汤) on high-fat-diet-induced hyperlipidemia in rats
- Hyperpolarization-activated cyclic nucleotide-gated 2 contributes to electroacupuncture analgesia on lumbar disc herniation-induced radicular pain through activation of microglia in spinal dorsal horn
