Risk factors and outcomes for patients with pancreatic cancer undergoing surgical exploration without resection due to metastatic disease: A national cohort study
2022-06-02EmilShlstrPulinBerezCrlsonJohnNilssonBobbyTingstedtBodilAndersson
Emil Shlström , Pulin Berez-Crlson , b , John Nilsson , d , Bobby Tingstedt , e ,Bodil Andersson , e,*
a Department of Clinical Sciences Lund, Surgery, Lund University, Sweden
b Department of Surgery, Central Hospital Kristianstad, Sweden
c Department of Translational Medicine, Cardiothoracic surgery and bioinformatics, Lund University, Sweden
d Department of Thoracic and vascular surgery, Skåne University hospital, Lund, Sweden
e Department of Surgery, Skåne University Hospital, Lund, Sweden
Keywords:Pancreatic cancer Surgery Metastasized Exploration
ABSTRACT
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is an aggressive cancer with a dismal long-term prognosis. The treatment options when diagnosed are limited since an overwhelming majority of the tumors respond unsatisfactorily to systemic therapy alone [1] .Surgical treatment is the only curable alternative, but many tumors are already unresectable when diagnosed, mainly because symptoms are vague and occur late in the course of the disease.Approximately 20%-25% of all pancreatic tumors are deemed resectable [2] . Patients who have undergone pancreatic resection should receive adjuvant chemotherapy, such as FOLFIRINOX (5-fluorouracil, leucovorin, irinotecan, and oxaliplatin) or gemcitabinecapecitabine, as it increases survival [3] . Neoadjuvant chemotherapy, with the intention of downsizing the tumor and extinguishing micrometastatic cells prior to radical surgery, thus increasing the resection possibility, is used on borderline resectable and locally advanced PDAC [4] .
When pancreatic cancer is found to be unresectable, the underlying cause is most often metastasized disease (usually liver metastases or peritoneal carcinomatosis) [5] . PDAC can also be considered unresectable due to locally advanced disease, which is defined as arterial tumor invasion (i.e., invasion of the superior mesenteric artery, the celiac axis or the hepatic artery) or extensive venous tumor invasion (i.e., extensive invasion of the portal vein or the superior mesenteric vein) [6] . The predicted resectability is mainly based on the findings on a preoperative computed tomography(CT) [7] . The diagnostic accuracy of CT is, however, low, which leads to the risk of underestimated tumor spread. This includes detecting both local tumor ingrowth and metastatic disease. Approximately 10% to 25% of all patients planned for surgical treatment turn out to have unresectable cancer intraoperatively [7] , which not only delays the start of palliative treatment but also increases the costs [8] . The use of other imaging methods, such as magnetic resonance imaging (MRI), is debated but can be valuable in selected cases preoperatively [9] .
The primary aim of this study was to identify preoperative risk factors for metastatic disease diagnosed during surgical exploration for pancreatic cancer. The secondary aim was to investigate survival in both resected and non-resected pancreatic cancer patients.The latter group was further divided into metastasized and locally advanced unresectable disease.
Method
Patients and data collection
This study was a multicenter, nationwide cohort based on information from the Swedish National Pancreatic and Periampullary Cancer Registry, which was established in 2010. All patients with pancreatic cancer are included in the registry and also all patients are planned for pancreatic surgery regardless of diagnosis. Preoperative, perioperative and postoperative data are collected, and a full list of variables is accessible online [10] . The registry data are provided by all Swedish pancreatic surgeons. Survival is continually updated against a central registry (SPAR, the Swedish state personal address registry). National and regional data are analyzed and E-published yearly [11] . Overall registry validity and coverage are at satisfactory levels in a study published recently [12] . Register data were received on August 28, 2018.
Patients diagnosed with PDAC confirmed by histopathology of adenocarcinoma registered between January 2010 and August 2018 were included in the study. Patients that received neoadjuvant chemotherapy and cases with all other diagnoses, including unknown histopathology, were excluded. The study focused on unresectable cancer caused by metastasized disease (M1). This group also included patients with a combination of M1 disease and locally advanced tumor. Patients with solely a locally advanced tumor due to extensive venous tumor invasion and/or arterial tumor invasion that made resection impossible (M0) were not included in the risk factor analysis but in the survival analysis.
The preoperative parameters analyzed included age, sex, body mass index (BMI), involuntary weight loss, diabetes mellitus (DM),smoking, American Society of Anesthesiologists (ASA) score, and routine blood tests [hemoglobin, C-reactive protein (CRP), bilirubin, white blood cell (WBC) count] and tumor marker carbohydrate antigen 19-9 (CA19-9).
The patients were divided into three groups based on age at diagnosis (<65 years, 65-75 years and>75 years). The same age classification was used in a study performed by van der Geest et al. [13] . Overweight was defined according to the World Health Organization (WHO) classification ( ≥25 kg/m2) [14] . Anemia was defined as hemoglobin levels below 120 g/L (according to the WHO classification for women) [15] . A bilirubin cutoff value of 50 μmol/L was chosen, which represents the approximate limit for jaundice [16] . CA19-9 and CRP were divided into three groups,with the lowest values representing the normal values (<35 U/mL and ≤3 mg/L) and the highest close to the upper quartile of the present cohort ( ≥600 U/mL and>15 mg/L). WBC counts were divided into groups based on the normal reference range (4 × 109/L to 10 × 109/L) [17] .
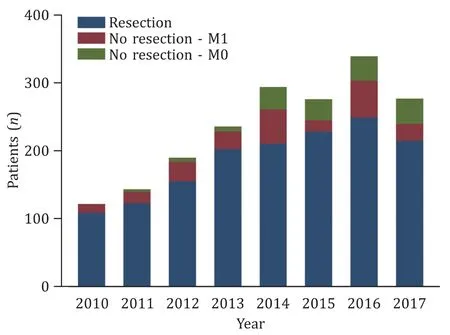
Fig. 1. Clustered bar charts illustrating the number of patients with pancreatic cancer undergoing surgery during the study period except 2018 as the data were only available until August.
A cutoff value at 30 days was chosen when analyzing days between diagnosis and surgery in accordance with a previously conducted Swedish study, which concluded that surgery within 32 days after CT resulted in a 50% risk reduction for intraoperative unresectability in comparison to when a patient had to wait more than 32 days [18] .
Statistical analysis
Descriptive data are presented as number and percentage, mean with standard deviation (SD) and median and interquartile range(IQR), as appropriate. Differences between resectable and unresectable disease were evaluated by Chi-square analysis for categorical variables, and Student’sttest or Mann-WhitneyUtest for continuous variables. Adjustment for possible confounding variables was made by developing a multivariable logistic regression model.Variables that were not normally distributed were log-transformed.Clinically relevant variables withP<0.25 in the univariable analysis were included in the multivariable analysis. In an iterative process of variable selection using backward stepwise selection, covariates were removed from the model if they were nonsignificant and not a confounder, as described by Hosmer-Lemeshow [19] .Missing values were imputed using the chained-equations multiple imputation technique as described by White et al. [20] . The Kaplan-Meier estimate of the survival function was used to estimate long-term survival. The log-rank test was used to compare survival differences between the groups.
A two-sidedPvalue<0.05 was considered significant. Statistical analyses and graphs were performed using Stata MP statistical package version 15.1, 2017 (StataCorp LP, College Station, Texas,USA).
Results
In total, 1938 patients undergoing surgery presented with a histopathological diagnosis of PDAC. The median age was 67.6 years. The proportion of patients undergoing resection, or only explorations due to M0/M1 disease during 2010 to 2017 is presented in Fig. 1 . In total, 1539 patients (79.4%) with pancreatic cancer were considered resectable, 234 patients (12.1%) were found to have unresectable disease due to a metastatic tumor situation,and 165 patients (8.5%) had unresectable tumors due to locally advanced cancer.
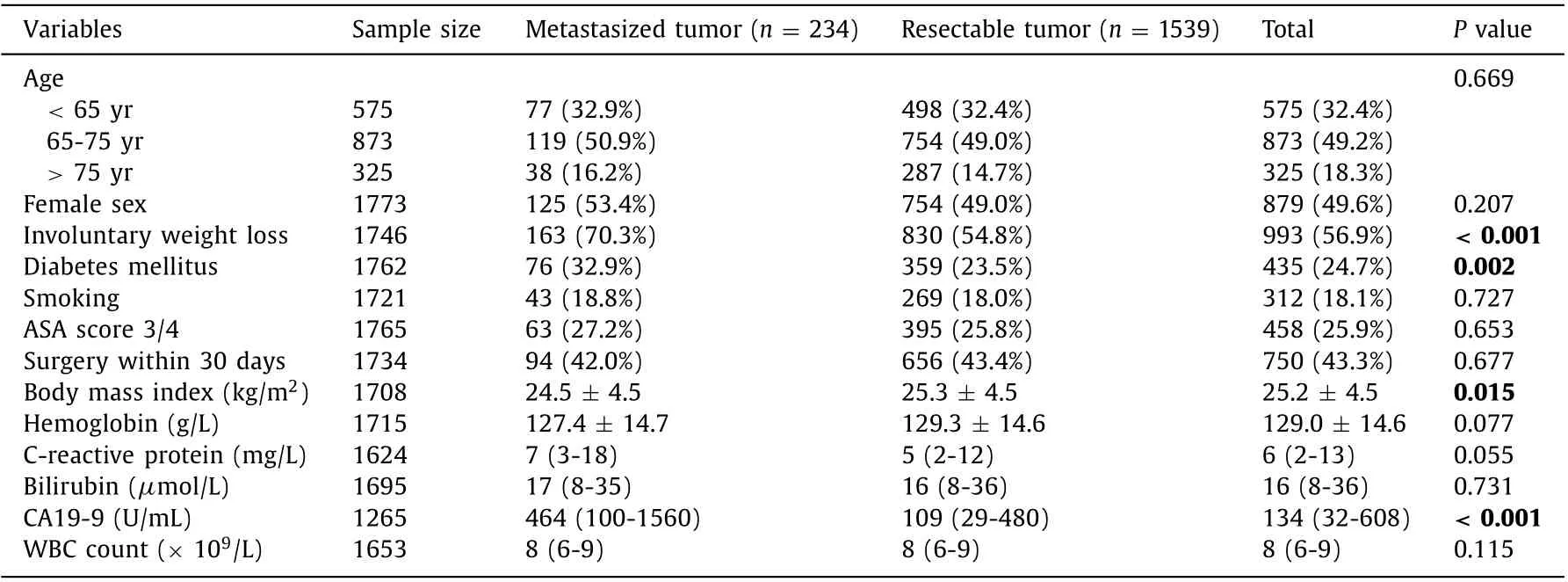
Table 1 Preoperative characteristics of the patients with a metastasized pancreatic cancer at surgical exploration in comparison to the patients with a resectable pancreatic cancer.
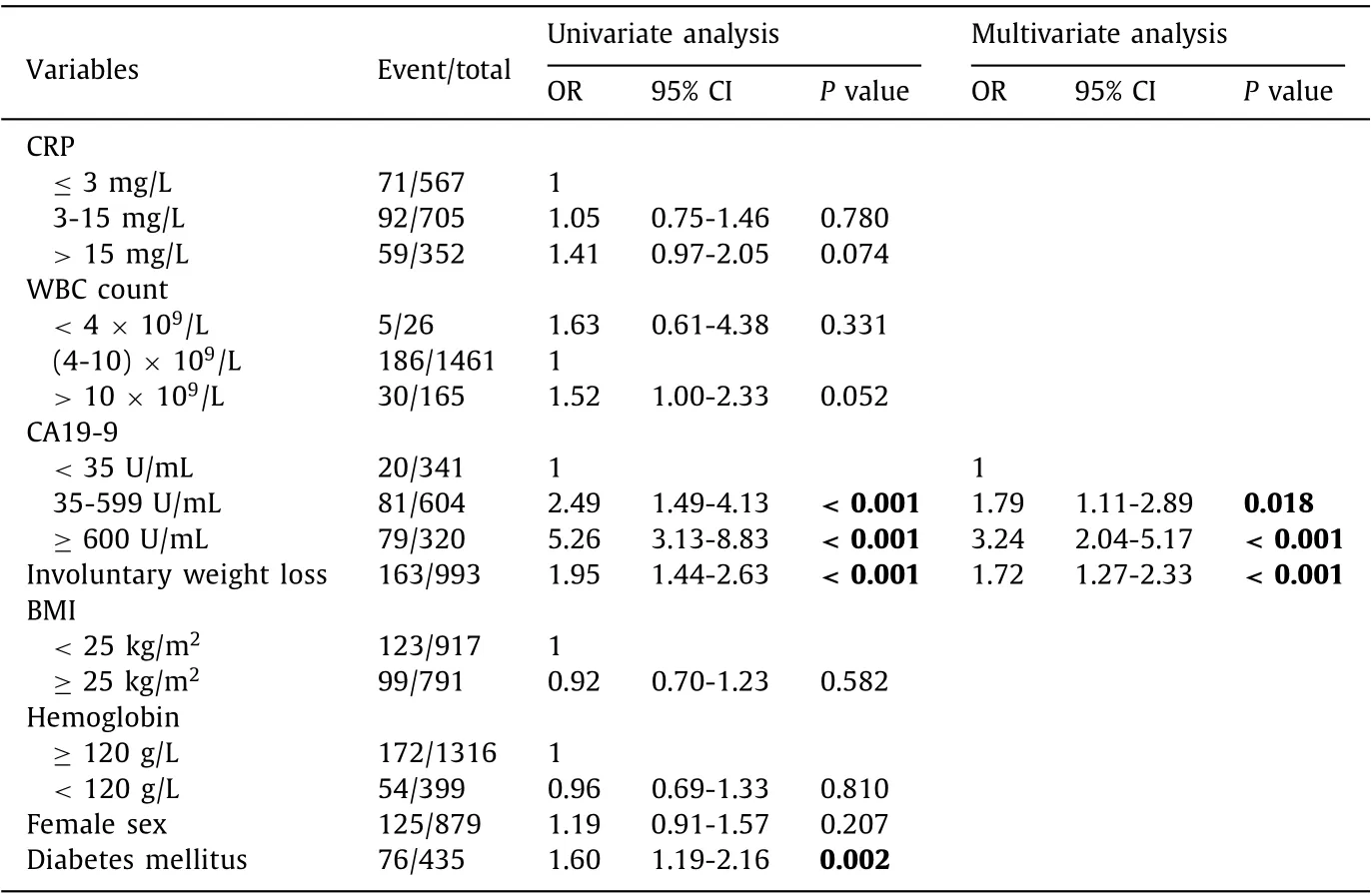
Table 2 Univariate and multivariate analysis identifying risk factors for having a metastasized pancreatic cancer at surgical exploration.
There was no difference between patients who underwent surgical exploration without resection due to metastasized disease and those who underwent pancreatic surgery with resection regarding preoperative parameters such as age, sex, comorbidity and whether the surgery took place earlier or later than 30 days after diagnosis. Additional preoperative comparisons are presented in Table 1 . Four preoperative characteristics (involuntary weight loss,diabetes mellitus, BMI and CA19-9) differed significantly between the groups in the univariate analysis ( Table 2 ). The only independent risk factors were involuntary weight loss (OR = 1.72, 95% CI:1.27-2.33) and elevated CA 19-9 (35-599 U/mL, OR = 1.79, 95% CI:1.11-2.89; ≥600 U/mL, OR = 3.24, 95% CI: 2.04-5.17) ( Table 2 ).
Median survival after surgery was 25.3 months for resected patients compared to 6.8 months for patients with metastasized disease and 11.2 months for patients with locally advanced disease. Overall, 39 patients (2.2%) died within 30 days of surgery,97 patients (5.5%) died within 90 days of surgery, and 532 patients(30.0%) died within one year of surgery. The 30-day mortality was higher among patients in the M1 group than that among resected patients [14 (6.0%) vs. 25 (1.6%),P<0.001]. The same result was seen when analyzing 90-day mortality [51 (21.8%) vs. 46 (3.0%),P<0.001] and 1-year mortality [169 (73.5%) vs. 363 (25.3%),P<0.001], excluding patients alive but followed-up less than one year.The survival rate was lower among patients with exploration with a finding of metastatic disease or locally advanced disease than that among patients with a resected tumor (P<0.001) ( Fig. 2 ).
Discussion
The present study aimed to identify preoperative risk factors for having metastasized PDAC not being diagnosed before the surgery but during the scheduled surgical resection. Independent risk factors were involuntary weight loss and elevated CA19-9. As expected, the survival rate was lower among patients with an unresectable tumor compared to that among patients underwent resection for pancreatic cancer.
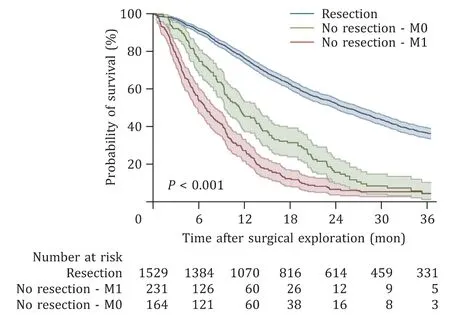
Fig. 2. Kaplan-Meier curve illustrating the difference in survival between patients with unresectable pancreatic cancer and patients with resectable cancer. In total 14 patients were excluded due to missing information regarding date of death. The blue line represents the group that was resected. The red line represents the group that did not undergo resection due to a metastasized tumor (M1). The green line represents the group with a locally advanced tumor and those who were not resected for other reasons (M0). The difference between the groups was statistically significant ( P < 0.001).
In a previous publication from our group, all patients with a suspected malignant pancreatic or periampullary tumor identified from the Swedish National Pancreatic and Periampullary Cancer Registry (12 377 cases) were included, with the intention to describe all patients subjected to pancreatic surgery but found unresectable intraoperatively. During surgery, 14.4% were found to have an unresectable tumor. The proportions of patients who underwent exploration and who underwent exploration without resection were unchanged during the study period [21] .
The current cohort of patients was diagnosed with PDAC at various sites in the pancreas, including patients with and without preoperative intervention of the bile ducts. An elevated CA19-9 correlated with an increased risk of metastasized disease at surgical exploration. This result is in line with previous studies on the subject [ 22 , 23 ]. The international consensus on CA19-9 as the criterion of borderline resectability is 500 U/mL, whereas higher levels indicate disseminated disease [24] . Persistent high levels of CA19-9 after resection also correlate with metastatic or unresected disease [25] .
CA19-9 does have limitations, partially due to its low sensitivity.Approximately 10% of the Caucasian population lack Lewis antigen A and do not express CA19-9. The specificity of CA19-9 is said to be impaired in the presence of obstructive jaundice [26] , but CA19-9 can still predict unresectability of pancreatic adenocarcinoma even in jaundiced patients [22] .
Involuntary weight loss was another independent risk factor for a metastasized disease in the present cohort. BMI was also lower but did not reach significance in the multivariate analysis.The correlation between weight loss and a lower probability of being resected corresponded with a study conducted by Slaar et al.,where this symptom turned out to be a key predictor of metastases at surgical exploration [27] . Weight loss prior to chemoradiotherapy also indicates poor prognosis and impaired long-term survival, even among normal-weight patients [28] . There are several reasons why this symptom might imply an aggressive and rapidly progressing cancer. Weight loss could be a product of malnutrition, sarcopenia and/or cachexia, since all these conditions often occur in patients with pancreatic malignancies. They are known to be risk factors for a more advanced tumor stage [29–31] , which would worsen surgical outcome regardless of the result of a preoperative CT. It is also conceivable that a rapidly growing tumor turns unresectable in the time period between preoperative imaging and surgical treatment. However, the metastasized group did not have more days between diagnosis and surgery than the resectable group. A possible explanation for our result is that patients with a more advanced but still potentially primarily resectable tumor are scheduled earlier for surgery than patients with a less advanced tumor.
Several of the analyzed routine blood tests were included based on the hypothesis that they signal increased inflammatory activity,so that could correlate with tumor burden. However, hemoglobin,CRP and WBC count were not independent predictors of unresectability. There were also no sex differences in the number of patients who had unresectable tumor intraoperatively.
Diabetes mellitus more frequently occurred among patients with metastatic disease, although it did not turn out to be an independent risk factor. A previous study suggested that higher levels of hemoglobin-A1c are associated with a more advanced tumor stage [32] . Diabetes itself can also be an early manifestation of pancreatic cancer. Sharma et al. found that patients with PDAC had noticeable hyperglycemia for a mean period of 30 to 36 months prior to diagnosis [33] .
Higher age at diagnosis was not a risk factor for having metastasized disease. Even though age alone is not considered a reason to exclude someone from potentially curable surgery, the threshold for being considered resectable both before and during surgery might be higher for elderly patients. The complication rate is higher for more advanced pancreatic surgery (including vascular resection) than for standard pancreaticoduodenectomy, and older patients tend to tolerate complications worse, with increasing postoperative mortality as a result [34] .
The preoperative risk factors identified in this study might help surgeons identify which patients are at risk of having an unresectable disease. This could affect whether the patient might become subject to additional examinations, such as diagnostic laparoscopy, endoscopic ultrasound (EUS) or MRI. The use of diagnostic laparoscopy was the subject of a systematic Cochrane review published recently [7] . Performing both diagnostic laparoscopy and CT increased the sensitivity by 18% in comparison to when CT was used alone. False positives are not possible, and the injury rate associated with the procedure is low (0.23%). However, diagnostic laparoscopy also comes with certain disadvantages. Advanced local tumor ingrowth and lymph node metastases are nearly impossible to detect.
The clinical use of preoperative EUS among patients with pancreatic cancer with curative intent has also been subject to a systematic Cochrane review [35] . EUS might facilitate the assessment of resectability in selected cases [ 36 , 37 ]. However, the use of EUS seems to be limited in a standard diagnostic algorithm. The number of false positives was 13%, which must be considered quite high since resection is the only potentially curative treatment for pancreatic cancer. Refraining from performing surgery on these patients would therefore markedly impair their prognosis [35] .
The use of MRI in the diagnosis of pancreatic cancer has been the subject of debate for a long time. MRI tends to be better than CT with regard to detecting liver metastases [38] . However,the overall gain in sensitivity is limited and the procedure is expensive [ 36 , 38 ]. Liver metastases can also be hard to distinguish from hemangiomas or abscesses [38] . The use of MRI in a standard diagnostic algorithm therefore seems quite limited, but it might be useful in situations where the standard CT protocol is insuffi-cient [39] or in patients with risk factors for unresectability.
The strengths of the present study are the large patient cohort and that the registry is validated for registered information. The accuracy and coverage are also E-published yearly [12] . A limitation is that the study is constrained by the variables that construct the registry. Some risk factors identified in previous studies, e.g.tumor size [ 23 , 40 ] and albumin [23] , could therefore not be addressed. Another limitation is missing data.
In conclusion, we identified involuntary weight loss and elevated CA19-9 as independent risk factors for an unresectable pancreatic tumor diagnosed at the time of surgical exploration. As expected, unresectability indicates a dismal prognosis when diagnosed with PDAC. When the risk of having an unresectable tumor is high, additional preoperative examinations instead of upfront surgery might be suggested.
Acknowledgments
We would like to thank all Swedish pancreatic surgeons for providing information and maintaining high coverage of the data in the Swedish National Pancreatic and Periampullary Cancer Registry.
CRediT authorship contribution statement
Emil Sahlström: Data curation, Formal analysis, Writing original draft, Writing - review & editing. Paulina Bereza-Carlson:Writing - review & editing. Johan Nilsson: Conceptualization, Data curation, Formal analysis, Software, Supervision, Validation, Writing - review & editing. Bobby Tingstedt: Writing - review & editing. Bodil Andersson: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing - review &editing.
Funding
This research work was supported by a Government Grant for Clinical Research ( http://www.skane.se/fou/alf ), The Bengt Ihre foundation, and The Swedish Cancer Foundation.
Ethical approval
This study was approved by the Regional Human Ethics Committee at Lund University (Dnr 2018/499 ).
Competing interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Surgical outcomes and quality of life following exercise-based prehabilitation for hepato-pancreatico-biliary surgery: A systematic review and meta-analysis
- Primary biliary cholangitis in pregnancy: A systematic review with meta-analysis
- Navigated liver surgery: State of the art and future perspectives
- Obesity is an important determinant of severity in newly defined metabolic dysfunction-associated fatty liver disease
- The characteristics of liver injury induced by Amanita and clinical value of α-amanitin detection
- Simplified calculation of bile cholesterol saturation index
