Synthesis of new non-fluorous 2,2'-bipyridine-4,4'-dicarboxylic acid esters and their applications for metal ions extraction in supercritical carbon dioxide
2022-05-08NIEHengWENQinYUANXunxinYANGHaijian
NIE Heng,WEN Qin,YUAN Xunxin,YANG Haijian
(College of Chemistry and Materials Science,South-Central Minzu University,Wuhan 430074,China)
Abstract Eight new non-fluorous 2,2'-bipyridine-4,4'-dicarboxylic acid esters were designed and synthesized as chelating ligands to transport various metal ions from solid matrix into supercritical carbon dioxide(scCO2).The metal ions extraction efficiencies(Kex)of the series of ligands were studied systematically and the extraction constants(Kex)of the metal ions were calculated.The results indicated that all compounds showed selectivity for Cu2+,Co2+and Ni2+to some extends,and the extraction efficiency could reach over 90%.In addition,the Kexvalues of the eight compounds increased with the increase of extraction efficiency for the same metal ion in the same extraction system.
Keywords non-fuorous bipyridine derivatives;supercritical CO2;metal ions extraction;extraction constant
The health risks of heavy metals in environment are fairly well known because of their hazardous propertiesto human beings,such astoxicity,persistence,bioaccumulation,and carcinogenicity[1].Removal of heavy metals from environment is generally achievedviafollowing manners:conventional solvent extraction,electrochemical methods,adsorption onto activated carbon,and biochemical methods[2].However,each of them has inherent limitations.For instance,in the conventional solvent extraction process,efficiency of extraction and stripping(reverse extraction)is limited by the equilibrium distribution coefficient of the solute between phases and the high ratio of solvent to feed;excess energy is usually necessary for the regeneration of activated carbon;and electroplating is not effective for dilute solutions[3].
During the past decades,supercritical fluid extraction(SFE)has become a promising eco-friendly alternative medium for replacement of traditional toxic organic solvents.Among many supercritical fluids,supercritical carbon dioxide(scCO2)isthe most commonly used due to its abundant,nontoxic,chemical inertness,non-flammable,lower viscosity,higher diffusion coefficient,variable density and reasonable accessible critical constants,i.e.,Tc(critical temperature)=31.1℃,Pc(critical pressure)=7.38 MPa[4].As a non-polar compound,CO2is ineffective to extract metal ions from matrices directly owing to the weak solute-solvent interactions and the charge neutralization requirement[5].However,when metal ions are chelated with organic ligands and form neutral complexes,their solubilities in scCO2can be dramatically increased and the formed complexes can be easily dissolved in scCO2and removed from matrixes[6].Up to now,various chelating ligands have been developed and utilized to extract metal ions in scCO2,e.g.,fluorinated compounds,β-diketones,crown ethers,organophosphates,amines and dithiocarbamates[7].
Based on our previous research results and according to the literatures,bipyridine and its derivatives are strong chelating ligands,which can form stable complexes with almost all heavy metal ions[8].However,bipyridine itself is not very CO2-philic and must be structurally modified to be deployed in scCO2formetalionextraction.Hence,in the present work,a series of new 2,2'-bipyridine-4,4'-dicarboxylic acid esters were synthesized and used as chelating ligands for the extractions of eight different metal ions(Ni2+,Co2+,Cu2+,Mn2+,Cd2+,Zn2+,Pb2+,Fe3+)in scCO2.Finally,the relevant extraction constants(Kex)were calculated.
1 Experimental section
1.1 Chemicals and experimental apparatus
All chemicals were purchased from Acros Chem.Co.and used directly.
NMR spectra were recorded on a Mercury Plus 400 MHz instrument at ambient temperature using TMS as an internal standard.IR spectra were measured on a Nexus 470 FT-IR spectrometer.Melting point was measured by micro melting point apparatus from Beijing TaiKe InstrumentCo..Elementalanalysis was performed by using a PE 2400 series II CHNS/O elemental analyzer.Atomic absorption spectrophotometry(AAS)was recorded by AA-6300 from Shimadzu.A CO2delivery pump(JASCO PU-CO2)was used to cool and deliver CO2fluid and a back pressure regulator(JASCO BP-1580-81)was employed to keep the stable pressure in the range of 0-35.0 MPa.The temperature was controlled using a temperature controller jacket with an accuracy of±0.5 K.
1.2 Synthesis of chelating ligands 1-8
The chelating ligands1-8 were synthesized according to the following modified procedures given by Garelli and Vierling(Tab.1)[9].
Tab.1 Synthesis of chelating ligands 1-8表1 螯合配体1~8的合成
Synthesis of butyl 2,2'-bipyridine-4,4'-dicarboxylate(chelating ligand 1):A mixture of 2,2'-bipyridine-4,4'-dicarboxylic acid(1.0 g,4.09 mmol)and SOCl2(40 mL,0.55 mol)was refluxed for 8 h under N2and then cooled to room temperature,the excess of SOCl2was remove from the reaction system under reduced pressure.Then the residue was added dropwise to a CH2Cl2solution(30 mL)of 1-butanol(0.75 mL,8.3mmol)andtriethylamine(1.2mL,8.6mmol)under a N2atmosphere.After stirring overnight,the reaction was stopped and washed with 1%aq.HCl,saturated aq.NaHCO3,and twice with water.The collected organic phase was dried over anhydrous Na2SO4and concentrated in vacuum.The residue was puri fi ed by silica gelcolumn chromatography(ethylacetate/petroleum ether volume ratio of 1∶1)to obtain a white solid(78%).M.p.99-100℃.FT-IR(KBr,cm-1):1721.6(CO),1595.1,1555.6(bipy),1291.2,1140.6(C—O—C).1H NMR(400 MHz,CDCl3):δ1.01-1.02(d,J=2.4 Hz,6H),1.28(s,4H),1.83(s,4H),4.43(s,4H),7.93-8.05(d,J=48 Hz,2H),8.90-8.97(d,J=28 Hz,2H),9.02-9.15(d,J=52 Hz,2H).13C NMR(100 MHz,CDCl3):δ13.73(s,2C),19.20(s,2C),30.66(s,2C),65.8(s,2C),120.69(s,2C),123.29(s,2C),139.13(s,2C),150.02(s,2C),156.37(s,2C),165.17(s,2C).Anal.calcd for C20H24N2O4:C 67.40,H 6.79,N 7.86;found C 67.42,H 6.77,N 7.90.
Other chelating ligands 2-8 were prepared with the similar procedure as chelating ligand 1.
Amyl 2,2'-bipyridine-4,4'-dicarboxylate(chelating ligand 2).A white solid with 70%yield.M.p.90-91℃.FT-IR(KBr,cm-1):1727.9(CO),1619.7,1556.9(bipy),1287.9,1139.1(C—O—C).1H NMR(400 MHz,CDCl3):δ0.933-0.957(t,J=7.2 Hz,6H),1.39-1.47(m,8H),1.802-1.848(m,4H),4.39-4.41(t,J=7.2 Hz,4H),7.905-7.913(t,J=3 Hz,2H),8.867-8.876(d,J=5.4 Hz,2H),8.95(s,2H).13C NMR(100 MHz,DCCl3):δ13.862(s,2C),22.246(s,2C),27.982(s,2C),28.227(s,2C),65.93(s,2C),120.476(s,2C),123.109(s,2C),138.866(s,2C),149.98(s,2C),156.43(s,2C),165.09(s,2C).Anal.calcd for C22H28N2O4:C 68.73,H 7.34,N 7.29;found C 68.76,H 7.31 N 7.26.
Hexyl-2,2'-bipyridine-4,4'-dicarboxylate(chelating ligand 3).A white solid with 74%yield.M.p.78-79℃.FT-IR(KBr,cm-1):1724(CO),1598.1,1559.9(bipy),1300.6,1134.1(C—O—C).1H NMR(400 MHz,CDCl3):δ0.93(s,6H),1.37(s,8H),1.48(s,4H),1.81-1.85(t,J=6 Hz,4H),4.409-4.424(d,J=6Hz,4H),7.92(s,2H),8.88(s,2H),8.97(s,2H).13C NMR(100 MHz,CDCl3):δ13.97(s,2C),22.51(s,2C),25.61(s,2C),28.58(s,2C),31.42(s,2C),66.05(s,2C),120.57(s,2C),123.19(s,2C),139.01(s,2C),150.06(s,2C),156.55(s,2C),165.21(s,2C).Anal.calcd for C24H32N2O4:C 69.88,H 7.82,N 6.79;found C 69.90,H 7.80,N 6.81.
Heptyl-2,2'-bipyridine-4,4'-dicarboxylate(chelating ligand 4).A white solid with 80%yield.M.p.57-58℃.FT-IR(KBr,cm-1):1727(CO),1619.7,1557.2(bipy),1289.4,1137.7(C—O—C).1H NMR(400 MHz,CDCl3):δ0.884-0.907(t,J=7.2 Hz,6H),1.312-1.401(m,12H),1.429-1.478(m,4H),1.793-1.841(m,4H),4.39-4.41(t,J=7.2 Hz,4H),7.902-7.91(t,J=4.2 Hz,2H),8.865-8.873(d,J=4.8 Hz,2H),8.952(s,2H).13C NMR(100 MHz,CDCl3):δ14.011(s,2C),22.521(s,2C),25.854(s,2C),28.562(s,2C),28.875(s,2C),31.649(s,2C),65.977(s,2C),120.461-120.520(d,2C),123.154(s,2C),138.911(s,2C),150.032(s,2C),156.468(s,2C),165.142(s,2C).Anal.calcd for C26H36N2O4:C 70.88,H 8.24,N 6.36;found C 70.86,H 8.26,N 6.34.
Octyl-2,2'-bipyridine-4,4'-dicarboxylate(chelating ligand 5).A white solid with 78% yield.M.p.67-68℃.FT-IR(KBr,cm-1):1726.4(CO),1595.2,1557.3(bipy),1287.7,1135.7(C—O—C).1H NMR(400MHz,CDCl3):δ0.896(s,6H),1.305(s,16H),1.467(s,4H),1.812-1.827(d,J=6 Hz,4H),4.391-4.421(d,J=6Hz,4H),7.915(s,2H),8.877(s,2H),8.964(s,2H).13C NMR(100 MHz,CDCl3):δ14.06(s,2C),22.62(s,2C),25.94(s,2C),28.61(s,2C),29.16-29.21(d,4C),31.76(s,2C),66.06(s,2C),120.57(s,2C),123.20(s,2C),139.01(s,2C),150.05(s,2C),156.53(s,2C),165.20(s,2C).Anal.calcd for C28H40N2O4:C 71.76,H 8.60,N 5.98;found C 71.78,H 8.62,N 5.96.
Nonyl-2,2'-bipyridine-4,4'-dicarboxylate(chelating ligand 6).A white solid with 73% yield.M.p.65-66℃.FT-IR(KBr,cm-1):1727.9(CO),1591.4,1561.5(bipy),1292.1,1135.1(C—O—C).1H NMR(400 MHz,CDCl3):δ0.866-0.889(t,J=7.2 Hz,6H),1.271-1.309(m,20H),1.428-1.477(m,4H),1.792-1.839(m,4H),4.382-4.41(t,J=7.2 Hz,4H),7.902-7.910(t,J=3.6 Hz,2H),8.865-8.873(d,J=4.8 Hz,2H),8.95(s,2H).13C NMR(100 MHz,CDCl3):δ14.07(s,2C),22.625(s,2C),25.914(s,2C),28.577(s,2C),29.194(s,4C),29.425(s,2C),31.813(s,2C),66.027(s,2C),120.491(s,2C),123.176(s,2C),138.94(s,2C),150.055(s,2C),156.505(s,2C),165.179(s,2C).Anal.calcd for C30H44N2O4:C 72.55,H 8.93,N 5.64;found C 72.57,H 8.95,N 5.62.
Decyl-2,2'-bipyridine-4,4'-dicarboxylate(chelating ligand 7).A white solid with 69% yield.M.p.68-69℃.FT-IR(KBr,cm-1):1726.1(CO),1597.9,1560(bipy),1293.4,1137.9(C—O—C).1H NMR(400 MHz,CDCl3):δ0.889(s,6H),1.285-1.382(d,J=38.2 Hz,24H),1.452-1.467(d,J=6 Hz,4H),1.812-1.844(t,J=6.4 Hz,4H),4.391-4.422(t,J=6 Hz,4H),7.916-7.919(d,J=1.2 Hz,2H),8.88(s,2H),8.965(s,2H).13C NMR(100 MHz,CDCl3):δ14.09(s,2C),22.66(s,2C),25.94(s,2C),28.62(s,2C),29.25(s,2C),29.29(s,2C),29.51(s,2C),31.87(s,2C),66.06(s,2C),120.58(s,2C),123.20(s,2C),139.01(s,2C),150.05(s,2C),156.53(s,2C),165.20(s,2C).Anal.calcd for C32H48N2O4:C 73.25,H 9.22,N 5.34;found C 73.26,H 9.24,N 5.32.
Hendecyl-2,2'-bipyridine-4,4'-dicarboxylate(chelating ligand 8).A white solid with 75%yield.M.p.76-77oC.FT-IR(KBr,cm-1):1729.7(CO),1592.7,1561.6(bipy),1292.1,1135.1(C—O—C).1H NMR(400 MHz,CDCl3):δ0.863-0.887(t,J=7.2 Hz,6H),1.265-1.379(m,28H),1.428-1.477(m,4H),1.791-1.839(m,4H),4.382-4.405(t,J=7.2 Hz,4H),7.901-7.911(m,2H),8.864-8.872(d,J=4.8 Hz,2H),8.951(s,2H).13C NMR(100 MHz,CDCl3):δ14.085(s,2C),22.648(s,2C),25.914(s,2C),28.577(s,2C),29.232-29.299(d,4C),29.477-29.566(d,6C),31.865(s,2C),66.027(s,2C),120.483-120.550(d,2C),123.176(s,2C),138.94(s,2C),150.055(s,2C),156.505(s,2C),165.179(s,2C).Anal.calcd for C34H52N2O4:C 73.87,H 9.48,N 5.07;found C 73.89,H 9.46,N 5.09.
1.3 Preparation of extracted sample and metal ion extraction procedure
Please refer to the supporting information for details[10-11].
2 Results and Discussion
2.1 Screening of optimal conditions for scCO2 extraction of Ni2+
Ni2+extractions with compounds 1-8 as chelating ligands in scCO2were systematically investigated to define the optimal conditions
2.1.1 Effect of pressure on extraction efficiency
The fluid pressure played an important role in SCF extraction because of the direct correlation between fluid density and pressure.By increasing the pressure,the density of the CO2phase increased,which increased its solubilizing ability.The effect of pressure on the Ni2+extraction efficiencies of the eight chelating ligands was shown in Fig.1.As expected,the extraction efficiencies for all eight chelating ligands were improved by increasing the CO2pressure from 10 MPa to 25 MPa,at 323 K,30 min of extraction time.Moreover,the result also showed that chelating ligand 4 was the most efficient ligand in extraction under the same conditions,suggesting highest solubility of the complex of ligand 4 with Ni2+.
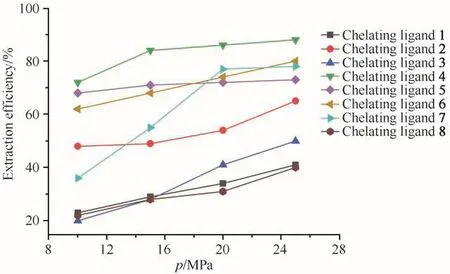
Fig.1 Effect of pressure on extraction efficiency of Ni2+(at 323 K,30 min,15µL ultrapure water and Ni2+/chelating agentmolar ratio of 1∶50)图1 压力对Ni2+萃取效率的影响(在323 K、30 min、15µL超纯水和c(Ni2+)∶c(螯合剂)=1∶50条件下)
2.1.2 Effect of temperature on extraction efficiency
The effect of temperature on the extraction of Ni2+was investigated at four different temperatures(313,323,333,353 K)for all eight chelating ligands.As shown in Fig.2,on increasing the temperature of the system from 313 K to 333 K,the extraction efficiencies were increased.Nevertheless,the efficiency decreased when the temperature was increased further.The extraction temperature affected the density of SCF,the volatility of the analytes and desorption of the analytes from the matrix.On increasing the temperature,the saturated vapor pressure increased and the thermal motion of the complexes at the active sites of the matrix intensified.Meanwhile,the density of the scCO2decreased with the increase in temperature.These three competitive factors might come up with the optimum extraction temperature representing the best compromise among them.For all eight chelating ligands 1-8,the highest extraction efficiency was obtained at 333 K.
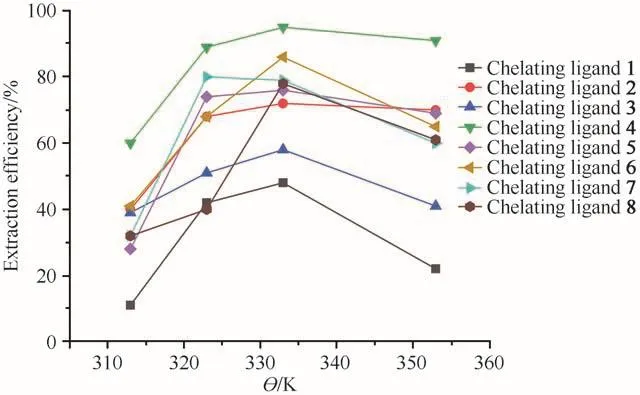
Fig.2 Effect of temperature on extraction efficiency of Ni2+(at 25 MPa,30 min,15µL ultrapure water and Ni2+/chelating agent molar ratio of 1∶50)图2 温度对Ni2+萃取效率的影响(在25 MPa、30 min、15µL超纯水和c(Ni2+)∶c(螯合剂)=1∶50条件下)
2.1.3 Effect of time on extraction efficiency
The effect of time on the extraction efficiency of Ni2+was investigated at four different cases(15,30,60,90 min)for all 1-8 chelating ligands.The pressure and temperature were maintained at constant value of 25 MPa and 333 K,respectively.As shown in Fig.3,the extraction efficiencies initially increased steeply with extraction time and reached their maximum levels at 30 min for all eight ligands.After 30 min,the extraction efficiencies for all eight ligands decreased dramatically,which may possibly have been due to decomposition of the complexes or the ligands during the prolonged exposure to the acidic environment of scCO2containing water.
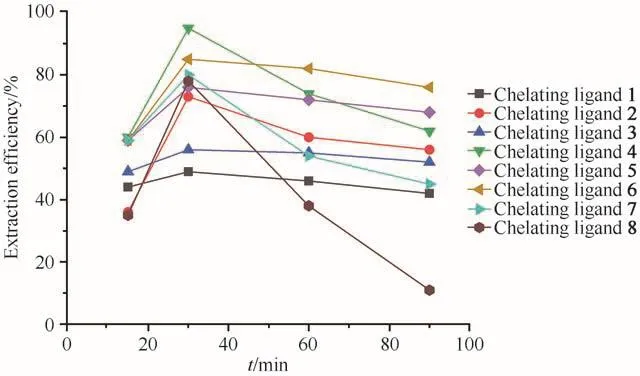
Fig.3 Effect of time on extraction efficiency of Ni2+(at 25 MPa,333 K,15µL ultrapure water and Ni2+/chelating agent molar ratio of 1∶50)图3 时间对Ni2+萃取效率的影响(在25 MPa、333 K、15µL超纯水和c(Ni2+)∶c(螯合剂)=1∶50条件下)
2.1.4 Effect of ligand to metal ratio on extraction efficiency
In the case of chelating ligand 4,the effect of ligand to metal ratio on extraction efficiency was studied at 25 MPa,333 K,and 30 min,respectively.As shown in Tab.2,the extraction efficiency was almost constant after the ligand to metal ratio reached 50.

Tab.2 Effect of the ratio of ligand 4 to Ni2+on extraction efficiency表2 配体4与Ni2+的比例对萃取效率的影响
2.2 Metal ions extraction efficiencies and extraction constants
Based on the above investigation,the optimal extraction conditions were screened as 25 MPa,30 min,333 K,15µL ultrapure water and M/chelating agent/PFOAT molar ratio of 1∶50∶25.[8,12-14]After that,the extraction performances of scCO2for other heavy metal ions(Co2+,Cu2+,Zn2+,Pb2+,Fe3+,Cd2+and Mn2+)were carried out under the conditions of 25 MPa,333 K and ligand to metal molar ratio of 50 without any additives,and the results were shown in Fig.4.The extraction efficiencies were low for most ions except for Ni2+.The possible reason is that the formed complexes have low solubility in scCO2.
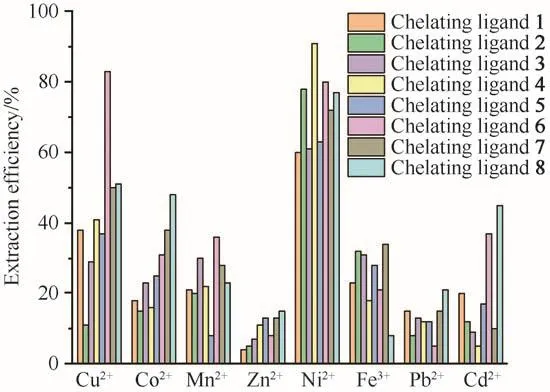
Fig.4 Single metal ion extraction efficiency without PFOAT as additive(in scCO2,25 MPa,333 K,30 min,15µL ultrapure water and M/chelating agent molar ratio of 1∶50)图4 不含PFOAT作为添加剂的单一金属离子的萃取效率(在scCO2、25 MPa、333 K、30 min、15µL超纯水和c(M)∶c(螯合剂)=1∶50条件下)
It is well-known that using PFOAT(tetraethylammonium perfluoro-1-octanesulfonate)as co-extractant to extract ligand-metal complexes as ion pairs into scCO2could enhence the affinity of the complex for scCO2to increase the extraction efficiency[15-16].As shown in Fig.5,the extraction efficiencies for all the metal ions increased notably for all eight ligands when PFOAT was introduced into the extraction system.Moreover,Cu2+was extracted almost completely in the presence of chelating ligands 3-8 and PFOAT.The extraction efficiencies of Co2+and Ni2+were also more than 90%under the same conditions.Unfortunately,the extraction efficiencies for the other five metal ions(Mn2+,Zn2+,Fe3+,Pb2+,Cd2+)were not satisfactory(less than 90%).The results suggested that in the presence of PFOAT,these chelating ligands seemed to show some selectivity for the extraction of Cu2+,Co2+and Ni2+.

Fig.5 Single metal ion extraction efficiency with PFOAT as additive(in scCO2,25 MPa,333 K,30 min,15µL ultrapure water and M/chelating agent/PFOAT molar ratio of 1∶50∶25)图5 以PFOAT为添加剂的单金属离子的萃取效率(在scCO2、25 MPa、333 K、30 min、15µL超纯水和c(M)∶c(螯合剂)∶c(PFOAT)=1∶50∶25条件下)
Mixed metal ion extraction experiments were carried out to confirm the selectivity assumption under the conditions of 25 MPa,333 K,and ligand to metal molar ratio of 50 with and without PFOAT.As shown in Fig.6,without PFOAT,the extraction efficiencies for all eight metal ions were still low.As expected,the metal ion extraction efficiencies increased for all of the chelating ligands in the presence of PFOAT(Fig.7).Cu2+was still extracted completely in the presence of PFOAT and the extraction efficiencies for Co2+and Ni2+were also more than 90%with ligands 2-7.Thus,it is suggested that irrespective of whether or not PFOAT was added,among these metal ions,although it was not so notable,all compounds showed selectivity for Cu2+,Co2+and Ni2+to some extend.
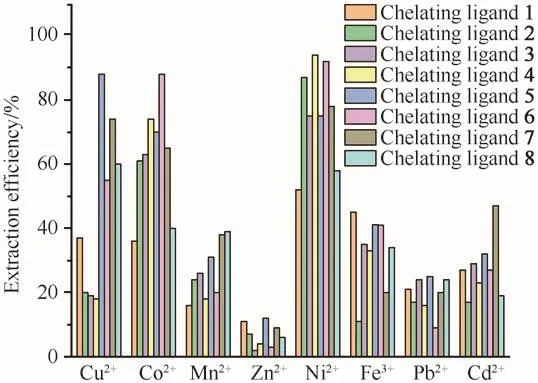
Fig.6 Mixture metal ions extraction efficiency without PFOAT as additive(in scCO2,25 MPa,333 K,30 min,15µL ultrapure water and M/chelating agent molar ratio of 1∶50)图6 不添加PFOAT的混合金属离子的萃取效率(在scCO2、25 MPa、333 K、30 min、15µL超纯水和c(M)∶c(螯合剂)=1∶50条件下)
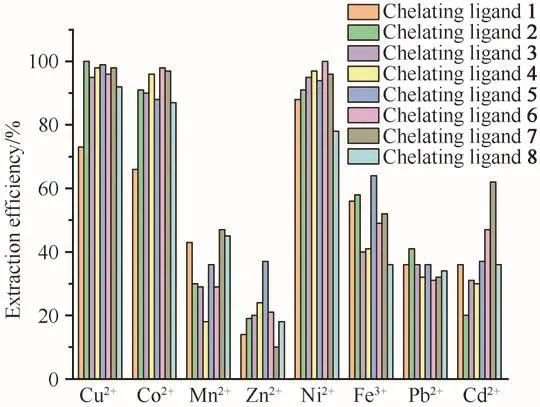
Fig.7 Mixture metal ions extraction efficiency with PFOAT as additive(in scCO2,25 MPa,333 K,30 min,15µL ultrapure water and M/chelating agent/PFOAT molar ratio of 1∶50∶25)图7 以PFOAT为添加剂的混合金属离子的萃取效率(在scCO2、25 MPa、333 K、30 min、15µL超纯水和c(M)∶c(螯合剂)∶c(PFOAT)=1∶50∶25条件下)
2.3 Reduction of the extraction constants
According to literature[7],the kinetics of the solvent extraction of cations is complex since it involves mass transfer coupled with chemical reaction in a heterogeneous system.Based on the measurements and experience with these systems,the reaction of bipyridine ligands with metal ions in the solid phase can be represented as Eq.(2):
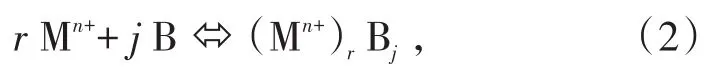
wherejandrdenote the stoichiometric numbers;Mn+,B and Mn+Bjrepresent the metal ions,the extractant and the metallic complex,respectively.This reaction is characterized by an overall extraction constantKex,which is defined by Eq.(3):

where[Mn+],[B]and[(Mn+)rBj]represent the equilibrium concentrations of the metal ions,chelating ligands and chelates,respectively.The measured extraction constants for the extraction of single metal ions without PFOAT are listed in Tab.3.
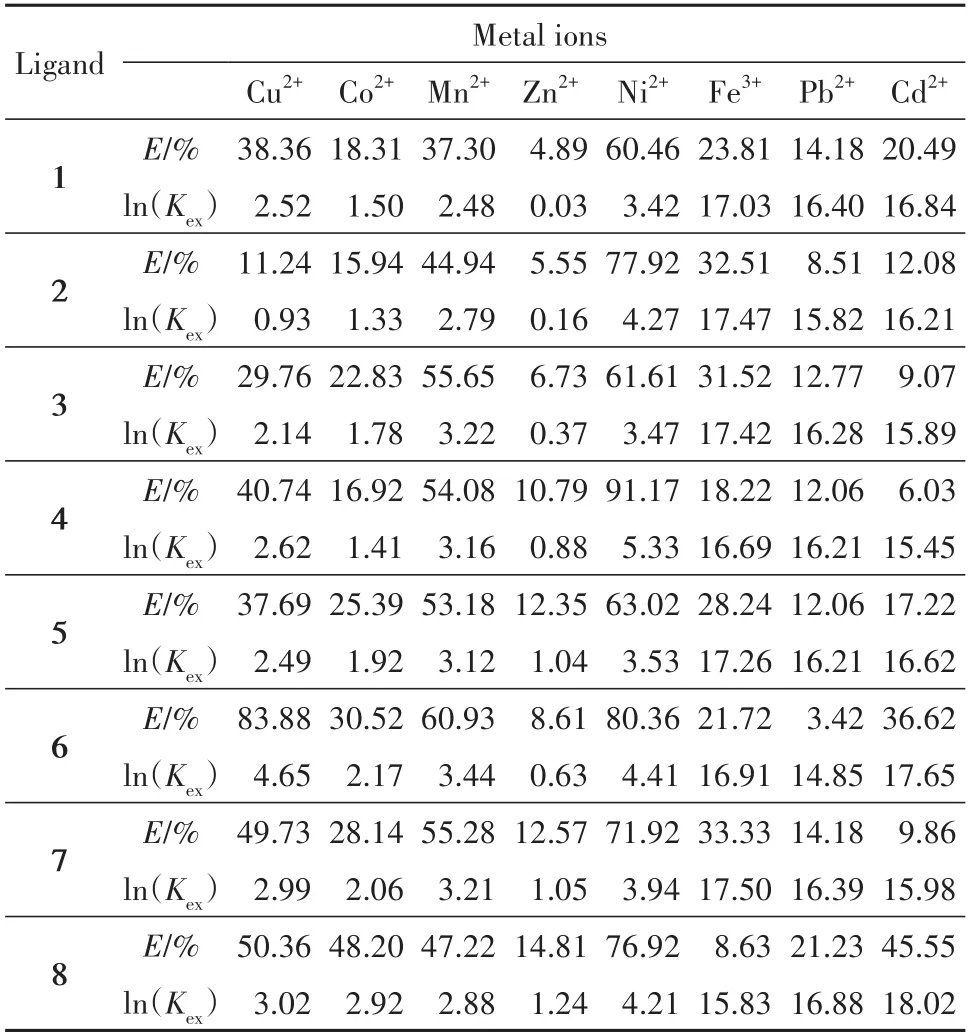
Tab.3 Extraction efficiency and Kexof single metal ion extraction without PFOAT表3 无PFOAT单金属离子萃取的萃取效率和Kex
On the other hand,when PFOAT was used as a co-ligand to extract metal ions from the paper matrix,in the presence of water as an additive,part of the PFOAT dissolved in the scCO2phase became distributed on the surface of the solid phase.PFOAT can dissociate to give a CO2-philic PFOA-anion and this associates with the(Mn+)rBjcomplex to form an ion pair.The reaction between the species can be represented by Eq.(4):
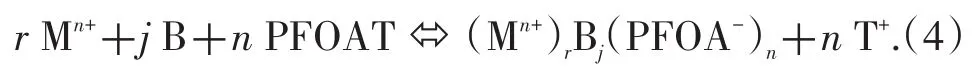
Likewise,the overall extraction constant of this extraction process can be expressed by Eq.(5):

The measured extraction constants for the extraction of single metal ions in the presence of PFOAT are given in Tab.4.
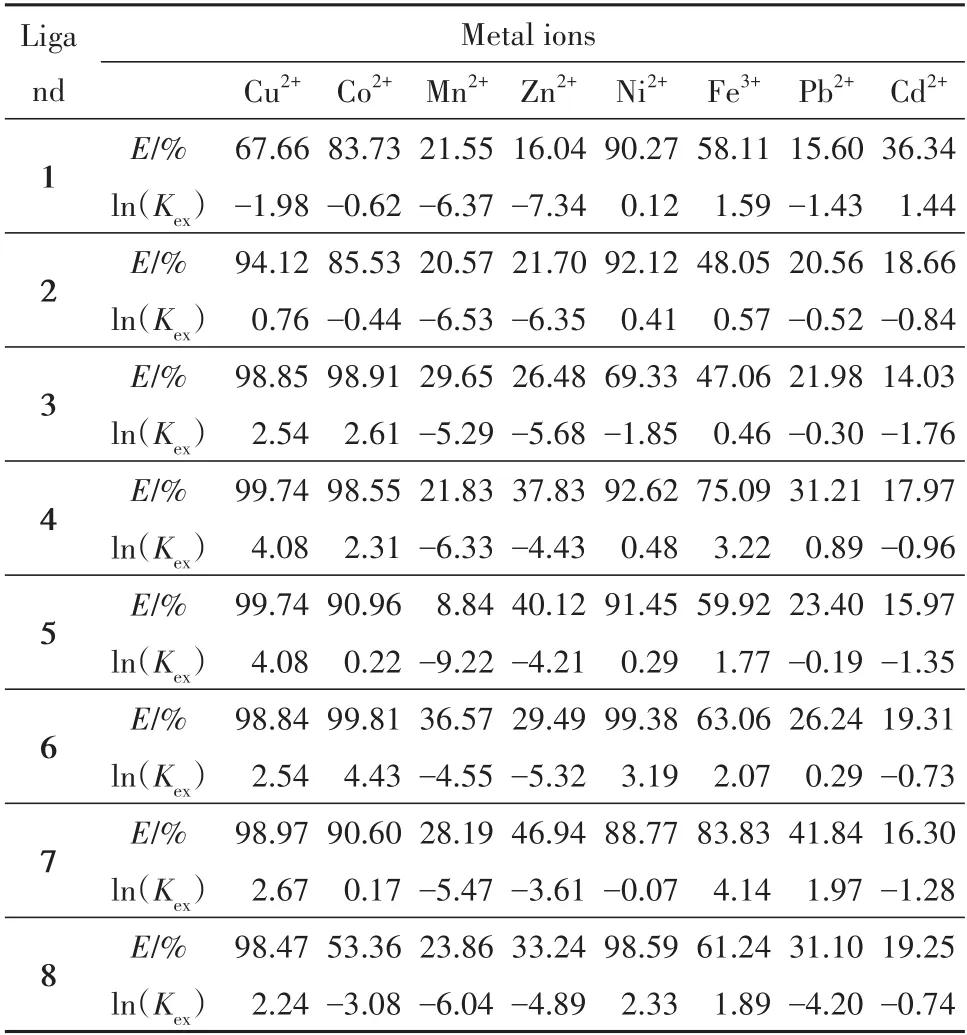
Tab.4 The extraction efficiency and Kexof single metal ion extraction with PFOAT表4 PFOAT萃取单金属离子的萃取效率和Kex
As shown in Tab.3 and Tab.4,all theKexvalues of the eight chelating compounds increased with the increase of the extraction efficiency for the same metal ion in the same extraction system.The ratio between metal ion and chelating compound is different because of the difference of metal property.For the same chelating ligand,the metal ions(Co2+,Cu2+,Ni2+,Zn2+,Mn2+)and the chelating ligand formed complexes in a 1∶1 ratio,and the valueKexof the chelating ligand increased as the extraction efficiency increased.However,the metal ions(Cd2+,Pb2+,Fe3+)formed complexes with the chelating ligand at the ratio of 1∶3[17].Accordingly,the value ofKexwas not only related to the concentration of metal ions,but also to the values of other factors of(r,j,andn).
3 Conclusions
In conclusion,eight new non-fluorous 2,2'-bipyridine-4,4'-dicarboxylic acid esters were synthesized and used as chelating ligands for metal ions extraction in scCO2.Metal ion extractions with the eight compounds as chelating ligands in scCO2were carried out from spiked filterpaper,and almostallthe ligands exhibited good efficiency for Cu2+,Co2+,Ni2+extraction.The extraction constantsKexof the eight chelating ligands increased with increasing extraction efficiency for the same metal ion in the same extraction system.
