Heteroatom-Doped Carbon Spheres from FCC Slurry Oil as Anode Material for Lithium-Ion Battery
2022-04-14YangGuangWangDengkeChenSongZhangYueFuZijianLiuWei
Yang Guang; Wang Dengke; Chen Song; Zhang Yue; Fu Zijian; Liu Wei
(1. Energy & Environmental Research Institute of Heilongjiang Province, Harbin 150090;2. State Key Laboratory of Chemical Resource Engineering, Beijing Key Laboratory of Electrochemical Process and Technology for Materials, Beijing University of Chemical Technology, Beijing 100029;3. Center for Composite Materials and Structures, Harbin Institute of Technology, Harbin 150000)
Abstract: A facile injected pyrolysis strategy to synthesize heteroatom-doped carbon spheres (CSs) with good conductivity is proposed by using the fluid catalytic cracking slurry oil (FCCSO) as the carbon source through a pyrolysis reaction process at 700-1000°C. The structures of CSs are characterized by scanning electron microscopy (SEM), transmission electron microscopy (TEM), X-ray diffraction (XRD), Raman spectroscopy, Fourier transform infrared spectroscopy(FT-IR) and X-ray photoelectron spectroscopy (XPS). The effect of preparation conditions on the morphology and its electrochemical properties of CSs acting as the anode material for lithium-ion battery (LIBs) are investigated. The XPS measurement results show that the CSs mainly contain C, N, O, and S elements. With the increase of pyrolysis temperature,the particle size of CSs decreases but the graphitization degree of CSs increases. As the anode material for LIBs, CSs show excellent electrochemical performance with a maximum reversible capacity of 365 mAh/g and an initial coulombic efficiency of 73.8% at a low current density of 50 mA/g. The CSs exhibit excellent cycling stability in a current range of 50 mA/g to 2 A/g, and still can maintain a stable reversible capacity of 347 mAh/g when the current is cycled back to 50 mA/g. This is mainly ascribed to the existence of suitable heteroatom content and unique spherical structure of CSs. The heteroatom-doped CSs can provide a new choice for the preparation of high efficiency anode materials for LIBs.
Key words: slurry oil; carbon spheres; lithium-ion battery; electrochemical performance
1 Introduction
Nowadays, production from the fluid catalytic cracking(FCC) process will inevitably continue to increase as crude oil is increasingly inferior and heavier. FCCSO, as a kind of carbon-rich by-product of petroleum processing,exhibits the characteristics of high aromatic content, high density, high viscosity, low hydrogen-carbon atom ratio and high carbon residue value. However, the majority of FCCSO is mainly used as a fuel oil, with low added-value and economic benefits[1-2]. On the other hand, since the discovery of carbon nanotubes by Japanese scientist Iijima in 1991, the research of carbon materials was brought to a new stage[3]. Among various forms of carbon, carbon spheres (CSs) are considered to be a promising energystorage material in many applications due to their high power density, excellent rate capability and long cycle life[4]. Many types of carbon sources are used to prepare CSs[5], including pyridine[6]and carbohydrates[7], which generally suffer from high cost and inconvenient storage.Therefore, carbon sources that are easily available,along with low-cost and high-carbon content have been attracting more attention in recent years. Hence, it is quite desirable to develop a simple and economic route to convert FCCSO into high-value carbon materials.
Lithium-ion batteries (LIBs) have attracted great attention in the past two decades due to their high energy density,ultralong cycle life and mature industrial production methods[8]. Carbon-based materials are recognized as the most promising anode materials for commercial applications due to their unique properties such as low price, good conductivity, and stable physical and chemical properties[9]. CSs have proved to be qualified LIBs anode materials for their high bulk density, excellent chemical stability and good electrical conductivity[10-11].However, the LIBs based on traditional graphite anodes cannot satisfy the growing requirements for high energy density. Therefore, a great number of researchers focus on novel anode materials to improve the electrochemical performance. Numerous attempts have been made to design or modify the carbon-based electrode materials to satisfy the requirement for high energy density[6,12-13]. It is recognized that doping heteroatoms (such as N, O and S)can significantly promote the lithium storage capacity of carbon materials by changing the electronic density and increasing the active sites[14]. Heteroatoms can introduce abundant external defects and electroactive sites for lithium storage. The presence of heteroatoms in the carbon skeleton can change the electron distribution and enhance the wettability of carbon materials[15]. In order to enhance the storage capacity of anode materials, heteroatomdoping could be used to improve the electrochemical performance of carbon materials[16]. Nitrogen doping is an effective method to improve the electrochemical performance of carbon-based electrodes, which can improve the wettability and electronic conductivity of the electrode/electrolyte[17-18]. Oxygen and sulfur are crucial factors in the electrochemical performance of electrode materials[19]. The FCCSO contains a trace amount of N/S/O, which is a kind of carbon source with natural advantages for preparing carbon materials[20]. However,the preparation methods have significant influences on the structure and electrochemical properties of CSs[21-22].The traditional chemical vapor deposition suffers from a long preparation period and incomplete carbonization of the precursor, which makes the large-scale production difficult[22-23]. Therefore, the injection pyrolysis method was used to prepare CSs using FCCSO as the carbon source. Compared with the traditional chemical vapor deposition method, the injection pyrolysis strategy is characteristic of shorter preparation cycle, higher production efficiency, and simpler operation.
In this work, the heteroatom-doped carbon spheres were successfully synthesized through the injection pyrolysis strategy using FCCSO as the carbon source.CSs were studied by different characterization methods.The electrochemical performance of CSs used as the anode materials for LIBs was investigated. This study is expected to provide a feasible method for FCCSO to prepare CSs, and can provide new ideas for expanding the deep processing of FCCSO.
2 Experimental
2.1 Synthesis of carbon spheres
Typically, FCCSO (8 g, provided by the Daqing Zhonglan Petrochemical Co., Ltd.) was uniformly dissolved in heptane (200 mL, provided by the Shanghai McLean Biochemical Technology Co., Ltd., with a purity of 99.5%). The solution was then heated to 50°C and refluxed under magnetic stirring for 50 min. The mixture was filtered through a microporous vacuum filter device(0.45 μm, FHLPO 4700, Millipore Fluoropore). The filtrate was evaporated by rotary steaming at 90°C and under a reduced pressure of 100 mm of Hg for 3 h to obtain the treated FCCSO. A vertical tubular resistance furnace (VTL1700, Nanjing Boyuntong Instrument Technology Co., Ltd.) was preheated to 700°C-1000°C.The treated FCCSO was injected into the tubular furnace at a proper flow rate (2 mL/h) through the injection pump in an argon atmosphere[5]. Then the furnace was naturally cooled to room temperature, and the soot-like pyrolysis products (FCSs700, FCSs800, FCSs900 and FCSs1000)in the furnace were collected.
2.2 Electrochemical measurements
Electrodes used for testing electrochemical properties were prepared as follows. The active material, conductive agent (acetylene black) and binding agent (PVDF) were mixed and dispersed into a specified volume of N-methyl-2-pyrrolidone (NMP) in a mortar according to a certain mass ratio of 8:1:1. Then the mixture was coated onto a copper foil collector by drying in a blast oven at 80oC.After rolling and slicing (10 mm) by an electric roller and electrode slicer, the electrode was dried in a vacuum oven at 120oC for 12 h. Then the CR2032 coin cells were assembled in a glove-box filled under high purity argon flow using the lithium tablets as the counter electrodes and reference electrodes, the glass fiber membrane(Whatman GF-D) as the separators, and an active material as the working electrode.
2.3 Structural characterization
The Fourier transform infrared spectra (FT-IR) were recorded using a Bio-Rad FTS-165 spectrometer. Samples were analyzed using pellets prepared by compressing a dispersed mixture of the sample and KBr powder. The Raman spectra were collected on a Renishaw inVia Reflex Rm2000 spectroscope with a wave length of 514.5 nm. The crystal structure of the samples was measured by X-ray diffraction (XRD) recorded on a Rigaku D/max-2500B2 +/PCX system using Cu Kα radiation (λ= 1.5406 Å)over the (2θ) range of 5°-90°. The X-ray photoelectron spectroscopy (XPS) measurements were carried out by using a Thermo Electron Corporation ESCALAB 250 apparatus with an Al Kα X-ray source operating under a base pressure of 1×10-9mbar.
3 Results and Discussion
3.1 Morphology analyses
The morphology and microstructure of CSs were examined by SEM and TEM. As shown in Figure 1, the CSs exhibit a uniform spherical shape with relatively smooth surfaces and severe aggregation. The observation of the broken sphere indicated that these CSs with concentric structures were solid and onion-like (Figure 1(b)). The cross-section of the incomplete carbon microspheres clearly showed that the carbon layer was a microstructure arranged in concentric circles along the surface[6].

Figure 1 TEM images of CSs (a), SEM images of the CSs (b), SEM images of: FCSs700 (c), FCSs800 (d), FCSs900 (e), and FCSs1000 (f)
The SEM image and the size distribution of the products collected at different pyrolysis temperatures are shown in Figure 1(c - f) and Figure 2. It has been found that the pyrolysis temperature plays a crucial role in the synthesis process. According to Figure 1(c), the FCSs700 with many spheres had a good shape and a wide particle size distribution. Most of the CSs had diameters ranging from 3 μm to 4 μm, with an average diameter of about 3.64 μm. When the pyrolysis temperature rose to 800°C, the aggregation phenomenon occurred between CSs, but the size generally decreased. Compared with FCSs700,FCSs800 had a narrower particle size distribution with an average diameter of 2.65 μm. Moreover, some of the CSs were deformed at 900 °C. At 1000 °C, the CSs had the smallest size (480 nm) and better shape, illustrating that the diameter of CSs decreased obviously at high pyrolysis temperature. The decrease in carbon sphere diameter was due to the tight arrangement of carbon atoms. The polymerization of the CSs might be attributed to the phenomenon of mutual fusion between carbon spheres[19,25].

Figure 2 The statistical distribution of the diameter of FCSs700 (a), FCSs800 (b), FCSs900 (c), and FCSs1000 (d)
As shown in Figure 3, the pyrolysis temperature was 900 °C, while the carrier gas flow rate was 10 mL/min,and the heating rate was 10 °C/min. The SEM images of CSs obtained under different reaction time conditions(60 min, 90 min, 120 min, and 150 min) showed agglomeration between CSs. With the increase of pyrolysis time, the diameter of the sample increased relatively (Figure 3(b)). However, there was a phenomenon of aggregation between CSs and a few carbon spheres were slightly deformed. When the pyrolysis time was extended to 120 min, the particle size of CSs ranged from around 1.3 μm to 4.1 μm and the caking phenomenon was serious (Figure 3(c)). In Figure 3(d), the CSs were bigger and still maintained a regularly spherical shape. With the extension of reaction time, the diameter of CSs tended to increase, which was mainly due to the continuous accumulation of gaseous carbon source decomposed by the FCC reaction on the surface to promote the growth of the CSs. The agglomeration between CSs and the fusion of the carbon nucleus growth process could provide conditions for the formation of larger carbon spheres. Therefore, the overall size of CSs also tended to increase as the reaction time increased[26].
3.2 Structural characteristics
X-ray diffractometry (XRD) and Raman spectroscopy were used to characterize the further graphitization degree of CSs. As depicted by Figure 4(a), the typical XRD patterns of CSs displayed two broadened diffraction peaks at the 2θvalues of 25.6° and 43°, which were analogous to the (002) and (100) planes of carbon materials, revealing the presence of amorphous carbon structure and low crystallinity. Moreover, the interlayer spacing d002of the FCSs1000 was calculated by the Bragg equation to be 0.3478 nm, which was higher than that of graphite (0.3354 nm), indicating that the carbon atoms in carbon spheres accumulated in a lower order[6].With an increasing temperature, the (002) peak become narrower and the intensity increased, indicating that the graphitization degree of CSs was enhanced with the increase of pyrolysis temperature.
Raman spectroscopy was an effective tool for characterizing the disordered structure of carbonaceous materials. Figure 4(b) shows the Raman spectra of CSs.The characteristic carbon peaks appeared at 1340 cm-1and 1591 cm-1, corresponding to the D band and the G band,respectively[24]. The D band corresponds to the disordered and imperfect structure of the carbon material, whereas the G band represents the ordered carbon structure with sp2electronic configuration. As the polymerization temperature was reduced from 1000°C to 700°C, further analysis of the Raman spectra revealed that the ratio of D band to G band(ID/IG) became greater (from 0.959 to 1.050), indicating the enhancement of graphitization degree of CSs, which agreed well with the results of XRD.
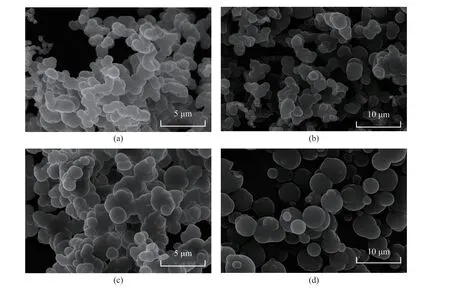
Figure 3 SEM images of the FCSs formed at different reaction time: (a) 60 min, (b) 90 min, (c) 120 min, and (d) 150 min
To study the functional group composition of carbon spheres, CSs were characterized by FT-IR. As shown in Figure 4(c), it can be clearly seen that all the CSs exhibit spectral bands at 3 340 cm-1, 1 630 cm-1and 1 080 cm-1,which are assigned to the -OH stretching vibration peak of the water in the sample, stretching vibration peak of-C=O or C=N, and the C-O or C-N stretching vibration,respectively[25]. It was found that the intensity of -OH band gradually become weaker with the increase of pyrolysis temperature, mainly due to the fact that more-OH functional groups reacted on the surface of CSs during heat treatment[19].
The XPS (X-ray photoelectron spectroscopy) analysis was performed in order to further analyze the chemical state of the elements existing in CSs. All data are summarized in Table 1. The proportion of heteroatoms relatively decreased and the carbon content rapidly increased from 85.18% to 94.13% with the increase of polymerization temperature. This outcome indicated that the polymerization temperature greatly affected the composition and the chemical structure of CSs.

Figure 4 The XRD pattern of CSs (a), Raman spectra of CSs (b), FTIR spectra of CSs (c)

Table 1 Elemental composition of CSs measured by XPS
The XPS spectra of CSs can provide details of the elemental composition, chemical state and electronic state of elements that are present on CSs surfaces. As shown in Figure 5(a), C1s spectrum can be fitted to four individual component peaks corresponding to the graphite peak C=C (284.7 eV), C-N/C-O/C-S (285.9 eV), C=O (287.7 eV), and π-π* (290.0 eV), respectively. From FCSs700 to FCSs1000, the graphite peak intensity gradually increased at 284.7 eV[26]. Analysis of the O1s spectra in Figure 5(b) showed that they consisted of two peaks centered at 531.5 eV and 533.0 eV, which were assigned to oxygen atoms with double bonds to carbon atoms(C=O) and C-OH/C-O groups, respectively. This result was in agreement with the previous IR test. In addition,it was clear that the total content of O decreased with the increase of polymerization temperature, which could be ascribed to the release of heteroatoms during hightemperature treatment[27-28]. The S2p spectrum (Figure 5c) of CSs could be fitted to two peaks at 164.0 eV and 165.3 eV. These two peaks were related to S2p3/2 and S2p1/2 of the -C-S-C- covalent bond, indicating that the sulfur atoms mainly existed in the form of sulfur bridges(-C-S-C-) in CSs[20,28]. In addition, the introduction of high content heteroatom would change the microstructure and hence affect the electrochemical performance[29-30].The XPS analysis was employed to identify the chemical state of nitrogen (Figure 5(d)). The N1s spectrum could be fitted to four peaks containing nitrogen: pyridinic-N(N-6, 398.5 eV), pyrrolic-N (N-5, 401 eV), quaternary-N(N-Q, 402 eV) and pyridine-N-oxide (N-oxide, 403.2 eV). N-6 and N-5 were formed predominately through substituting carbon atom by N at the edges of the plane or the defect sites, since such carbon atoms were much more chemically active than those within the plane of perfect graphene[31]. Nitrogen atom replaced the carbon atom in the graphene plane and could be bonded to three carbon atoms. It should be noticed that low nitrogen content was observed in all samples, as evidenced by the XPS survey spectra. As shown in Table 1, with the pyrolysis temperature increasing to 1000°C, the nitrogen content of CSs gradually decreased to 0.13 at%. This might be attributed to the seriously breaking of the C-N bonds at high polymerization temperature. N-5 might be transformed to N-6 and N-Q when the temperature was higher than 700oC,and the stability of N-Q was superior to that of N-6 at higher temperature[5,27]. Hence, the content of N-6 and N-Q increased and the amount of N-5 decreased. This result was consistent with other reports[32-33].

Figure 5 C1s, O1s, S2p and N1s spectra of CSs
3.3 FCSs-derived anode performance
Figure 6(a) shows the cycling performance of CSs at a current density of 50 mA/g. All of them showed excellent cycling performance. FCSs800 delivered the highest discharge specific capacity of 365 mAh/g, and its reversible capacity was higher than that of the pure carbon spheres[34], pure black graphite[35], and mesocarbon microbeads[35]. This outcome was mainly due to the appropriate heteroatoms doping content. The coulombic efficiency of the first cycle for FCSs800, FCSs900, and FCSs1000 electrodes was 71.86%, 72.7%, and 73.8%,respectively. This tendency suggested that higher heating temperatures reduced the surface defects and functional groups. The stable reversible capacities after 10 cycles were 339.5 mAh/g, 324.1 mAh/g, and 302.7 mAh/g, respectively. It was obvious that the capacity fading that occurred during the initial 10 cycles was largely dependent on the electrochemical stability during the initial cycles with the formation of solid electrolyte interphase (SEI) and other side reactions[6]. It can be observed that the coulombic efficiency for all samples rose rapidly to 94% in the second cycle and maintained a value of 99% for the rest of cycles. These results indicated that CSs had good cycling performance in LIBs. Figure 6(c, d, e) shows the galvanostatic charge/discharge voltage profiles at a current density of 50 mA/g. The voltage plateau at 0.8 V-1.0 V in the first cycle was ascribed to the formation of the SEI[37]. In addition,the formation of SEI on the surface of carbon materials could lead to a part of irreversible capacity and low coulomb efficiency for the first time. The plateau region of the sample appeared at about 1.0 V during the first discharge. The absence of the platform in subsequent cycle demonstrated that Li+ions can form a stable SEI after embedding the carbon spheres for the first time[6].
As shown in Figure 5(b), the specific capacity of FCBs900 electrode at 50 mA/g was 365 mAh/g, and the capacity decreased to 333.8 mAh/g after 60 cycles.When the current density was recovered to 50 mA/g again, the reversible capacity of FCBs1000 was 347 mAh/g. The observed excellent performance of CSs should be attributed to the microstructure of concentric incompletely closed graphitic shells[38]and the heteroatom doping structure. Compared to C atoms in the amorphous carbon, O atom has a lone pair of electrons to serve as an electron-rich donor to interact with Li-ions[39-40]. The existence of O atoms and functional groups in the carbon skeleton could change the electron distribution and enhance the wettability of carbon materials[41]. The radius of N is small enough to pair with Li, thus facilitating an electrostatic dipole-dipole interaction over the Li doping atom bond with appropriate length. According to the Li bond theory, such intermolecular interaction is in favor of the strong binding strength between Li and high-electronegativity atoms[42-43]. Hence, ion diffusion in the interface between the electrode and electrolyte and the interior of bulk electrode is promoted, which can improve the electrochemical performance. Sulfur is an electrochemically active element that can react reversibly with lithium, and sulfur doping has been proved to be an efficient method to improve the electrical conductivity and provide additional reaction sites for the accommodation of lithium. S-doping is beneficial to improving the area of electrode/electrolyte interface, increasing the reaction sites and shortening the transport length of Liions. However, the fact that only one form of the sulfur incorporation, i.e. sulfide -C-S-C-, most probably can contribute to an increase in charge capacity of special importance[44]. Thus, benefiting from the O/N/S-co-doping and the corresponding enhanced interfacial interaction,the actual performance of carbon cathode hosts for the lithium-ion battery is significantly better than that of the undoped carbon materials.

Figure 6 Anode performance of CBs. (a) Cycling performance of FCSs800, FCSs900 and FCSs1000 at a current density of 50 mA/g, (b) Rate performance of FCSs800, FCSs900 and FCSs1000, (c) Charge - discharge curves of FCSs800, (d) Charge -discharge curves of FCSs900, (e) Charge - discharge curves of FCSs1000
4 Conclusions
In this study, the heteroatom-doped CSs were synthesized via pyrolysis process by using the FCCSO as a carbon source, which was a promising energy material. The CSs had a smooth surface, uniform size distribution, and good shape. The morphology, structure,and performance of CSs could be easily controlled by adjusting the pyrolysis temperature and reaction time.The anode performance of CSs in LIBs showed that an appropriate heteroatom content and an unique spherical structure contributed to a high reversible capacity of 365 mAh/g at 50 mA/g and an excellent rate capability(347 mAh/g after 60 cycles). The study demonstrates the great potential of the heteroatom-doped CSs prepared from FCCSO for a variety of uses in high performance electrode material.
Acknowledgements: This work was supported by the Foundation of Heilongjiang Academy of Sciences (KYJJ2019HY01) and the Foundation of Heilongjiang Provincial Institute of Basic Applied Technology Research Project (ZNJZ2018NY01).
杂志排行
中国炼油与石油化工的其它文章
- Study on Viscosity Reducing and Oil Displacement Agent for Water-Flooding Heavy Oil Reservoir
- Electrospinning Nanofiber Membrane Reinforced PVA Composite Hydrogel with Preferable Mechanical Performance for Oil-Water Separation
- Preparation of Solid Waste-Based Activated Carbon and Its Adsorption Mechanism for Toluene
- Antibacterial and Corrosion Inhibition Properties of SA-ZnO@ODA-GO@PU Super-Hydrophobic Coating in Circulating Cooling Water System
- Investigation of Nitrite Production Pathway in Integrated Partial Denitrification/Anammox Process via Isotope Labelling Technique and the Relevant Microbial Communities
- Study on Low-Temperature Properties of the Asphalt Modified by Carbon Nanotubes (CNTs) and Crumb Rubber (CR)
