Sensitivity of Fusarium pseudograminearum isolates to fludioxonil in Henan Province, China
2022-04-09CHENYaweiXUJianqiangWANGShuoXUDaochaoMAShichuangHUANGYulongHOUYing
CHEN Yawei, XU Jianqiang*,, WANG Shuo, XU Daochao,MA Shichuang, HUANG Yulong, HOU Ying
(1. College of Horticulture and Plant Protection, Henan University of Science and Technology, Luoyang 471003, Henan Province, China;2. College of Food and Bioengineering, Henan University of Science and Technology, Luoyang 471003, Henan Province, China)
Abstract: Wheat crown rot caused by Fusarium pseudograminearum has become an important soilborne disease and affects the quality and yield of wheat. In order to detect the sensitivity of F. pseudograminearum to fludioxonil in Henan Province, China, 105 isolates of F. pseudograminearum were collected from six cities in 2019. Sensitivity was determined by the mycelial growth rate method, then the methods of least-significant difference (LSD) and SPSS cluster were used for result analysis. The sensitivity of F. pseudograminearum to carbendazim and tebuconazole was determined and the correlation coefficient which existed between fludioxonil and the two fungicides, carbendazim and tebuconazole was analyzed. The results showed that the minimum inhibitory concentration (MIC) of fludioxonil was 0.240 0 μg/mL. The sensitivity frequency distribution was a continuous single peak curve, and the EC50 value ranged from 0.002 7 to 0.047 0 μg/mL. The average EC50 value of (0.026 3 ± 0.010 1) μg/mL,could be used as the sensitivity baseline of the pathogen to fludioxonil. The variance analysis results showed that the sensitivity of the different cities to fludioxonil was different, and the EC50 value ranged from 0.015 0 to 0.033 5 μg/mL. The maximum EC50 value of the isolate from Zhongmu County Zhengzhou City was 16.78 times bigger than the minimum value. Cluster analysis showed that there was no significant correlation between the sensitivity of isolates to fludioxonil and their geographical origin.The mean EC50 values of carbendazimand tebuconazole against the pathogens were (0.788 1 ± 0.315 3)μg/mL and (0.088 6 ± 0.145 3) μg/mL, respectively. There was no significant correlation between the sensitivity of the isolates to fludioxonil, carbendazim and tebuconazole. The results of greenhouse trials showed that the control efficacy of fludioxonil suspension seed coat agent could reach 58.00% (75.0 μg a.i/g) in 2020 and 63.69% (50.0 μg a.i/g) in 2021 when used to treat wheat as a seed dressing agent. The results of this study provide the basis for the rational use of fludioxonil in the control of wheat crown rot and provide information for monitoring the sensitivity of pathogenic fungi to fungicides.
Keywords: Fusarium pseudograminearum; fludioxonil; mycelial growth rate method; sensitivity;greenhouse control efficacy
0 Introduction
Wheat crown rot caused by a variety ofFusariumspecies is a soil-borne disease, and one of the main diseases on wheat[1-2]. The main pathogens areFusarium graminearumandF. pseudograminearum[3]andF. graminearumis the most pathogenic one[4].F.pseudograminearumwas first reported to cause wheat crown rot in Australia in 1951, causing economic losses of more than 500 million Australian dollars per year[5-6]. The occurrence and harm of the disease have been reported in over 10 countries, including the United States, Australia, Italy, Turkey , Canada etc[7-9].Li et al. reported that wheat crown rot caused byF.pseudograminearumwas first found in Henan Province in China, and it occurred in many provinces across the country in 2016[3-4,10]. In recent years, due to the returning of straws to the field, cross-regional mechanized operation, and the poor resistance of existing cultivated varieties, the disease has spread rapidly over the years and has caused a greater loss. The disease rate in highly affected fields has reached about 70%and has become one of the most important wheat diseases in the Huang-Huai wheat region[3,6,11]. Wheat crown rot can occur from the tillering stage to the maturity stage, which can make the leaf sheath and base of the crown turn brown, and the crown node necrotic. In severe cases, it can cause plant death, white head and affects wheat yield. In the process ofFusariuminfecting wheat, the toxin is transmitted upward through the crown to the wheatear, leading to an increase in the toxin content of wheat, which adversely affects the quality and safety of agricultural products[12].Wheat crown rot is a soil-borne disease. Mixed kinds of seed coating agents does not generally alter the shape and size of seeds and the seed color is often modified to make treated seed less attractive to birds[13]
Fludioxonil is a new type of non-sytemic phenylpyrrole chemical, developed by Swiss Syngenta Crop Co., Ltd. It can prevent and control soil-borne diseases caused by pathogenic fungi and has a high inhibitory effect on the growth of pathogenic fungi[14-15]. It has the characteristics of high efficiency, low risk, and safety, and is currently one of the world's largest seed treatment varieties[16]. Fludioxonil is one of the most effective fungicides for the control ofBotrytis cinereaand is also effective for the control ofSclerotinia[17-18].It is now known that fludioxonil can be used for disease prevention and control in potato, tomato, cucumber,corn, rape, and other crops. Fludioxonil seed coating agent can temporarily increase the vigor of pepper seeds and can be applied to pepper seeds having lower vigor that are sown soon after treatment[19]. Li[20]showed thatBotrytis cinereafrom Beijing tomato has a low to moderate risk of resistance to fludioxonil. At present, there is no report about the sensitivity ofF.pseudograminearumto fludioxonil in Henan Province, but the wheat disease caused byF. pseudograminearumhas reduced crop yields and caused huge economic losses. Therefore, its prevention and control cannot be delayed. In this paper, the mycelial growth rate method was used to determine the sensitivity of 105 strains ofF. pseudograminearumfrom six cities in Henan Province, and the sensitivity baseline was established. The differences in the sensitivity of the strains to fludioxonil in different regions were analyzed. The control efficacy of fludioxonil on wheat crown rot, and the prospect of applying fludioxonil in the control of wheat crown rot were evaluated, thereby laying a foundation for the prevention and control of wheat crown rot in production.
1 Materials and methods
1.1 Materials
Tested strains: From April to May 2019, wheat crown rot disease strains with typical symptoms were collected from six cities in Henan Province: 1-3 crown nodes of wheat turned brown near the ground while the junction of the crown nodes was pink. When separating, the junction (crown section) of the crown node with red or white mildew layer near the ground of the wheat plant was cut off, cultured in PSA with lactic acid, and incubated at 28 ℃. After 2 days of cultivation, if there is no rhizopus contamination, the hyphae on the edge of the colony grown from each crown segment, the mycelial disk is transferred to the fresh PSA to continue the cultivation. During strain preservation, a total of 439 strains ofFusariumwere isolated. About 150 strains were selected for monospore isolation, and the obtained monospore strains wereF.pseudograminearum(Fpg-F, 5′-CGGGGTAGTTT CACATTTCCG-3′/Fpg-R, 5′-GAGAATGTGATGAC GACAATA-3′; amplification length 520 bp) andF.graminearum(Fg-F, 5′-TTCTTTGACATCTG TTCAACCCA-3′/Fg-R, 5′-ACAGATGACAAGATTCA GGCACA-3′, 280 bp) confirmed by PCR amplification, respectively[21]. The above specific primers were used for molecular identification, and 105 strains ofF. pseudograminearumwere obtained.
Tested wheat variety: Zheng Mai 366 was provided by the researcher Yang Lirong, a graduate student majored in plant protection at Henan Academy of Agricultural Sciences.
Tested medium: Potato sucrose agar (PSA): 200 g potato (peeled), 20 g sucrose, 17-20 g agar powder,and 1 000 mL distilled water.
Test agent: The 97.90% fludioxonil was provided by Syngenta (China) Investment Co., Ltd.;98.00% tebuconazole was provided by Guangxi Tianyuan Biochemical Co., Ltd.; 98.00% carbendazim was provided by Shandong Shuangxing Pesticide Factory. Both fludioxonil and tebuconazole were predissolved in acetone, and carbendazim was pre-dissolved in 0.10 μg/mL dilute hydrochloric acid, and both were prepared into 1.0 × 104μg/mL mother liquor and placed in a refrigerator at 4 ℃. Fludioxonil suspension seed coating agent (25 g/L) was provided by Syngenta Nantong Crop Protection Co., Ltd.
1.2 Methods
1.2.1 Determination of the minimum inhibitory concentration (MIC) of fludioxonil onF. pseudograminearumFour strains of crown rot fungi were randomly selected: Wugang County Luohe City JFLW-8 and JFLW-10, Yanjin District Xinxiang City JFXY-17 and JFXY-19. The MIC of fludioxonil on the growth ofF. pseudograminearumwas determined. Using the mycelial growth rate method[22], selected strains of crown rot fungi were cultured on PSA plates for 3 days, then a 5 mm diameter mycelial plug was punched on the edge of the colony using a punch.The mycelial plugs were inserted using an inoculating needle face down onto PSA plates containing the final concentration of 0.03, 0.06, 0.12 and 0.24 μg/mL fludioxonil, one plug per plate, while the control plate was without any agent. Each treatment was done in triplicate, and after inverting the culture for 3 days in an incubator at a constant temperature of 28 ℃, the colony diameter at each concentration was observed.The minimum concentration that inhibits mycelial growth was the MIC againstF. pseudograminearum.The diameter of the colony was measured by the cross method, and the mycelial growth inhibitory rate (I) at each concentration was calculated. The equation used is shown below.
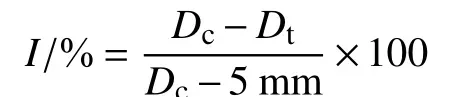
Dc: average of the diameter of the control strain,mm;Dt: average of the diameter of the treated strain,mm.
1.2.2 Determination of the sensitivity ofF. pseudograminearumto fludioxonil The sensitivity of wheat crown rot fungi to fludioxonil was determined using the mycelial growth rate method[22]. According to the determined MIC of fludioxonil which inhibits the growth ofF. pseudograminearumhyphae, the concentrations were set to 0.005, 0.01, 0.02, 0.03, 0.04 and 0.05 μg/mL, and each treatment was performed in triplicates. After culturing 105 strains ofF. pseudograminearumon PSA plates for 3 days, a punch was used to take a 5 mm diameter mycelial plugs on the edge of the colony and an inoculation needle was used to insert the hyphae of the fungus in the abovementioned medicated plates with different concentration gradients of fludioxonil. After inverting culture in a constant temperature incubator at 28 ℃ for 4 days,the diameter of the colony was measured using the cross method, and the mycelial growth inhibition rate at each concentration was calculated.
1.2.3 Sensitivity distribution map drawing and sensitivity baseline determination According to the method of Russell[23], the sensitivity ofF. pseudograminearumto fludioxonil can be divided into several suitable intervals from low to high. The classification is based on the EC50value, and starting from the minimum value. The statistics of each interval number and frequency of strains were determined. Based on the median EC50value of each interval on the abscissa and the strain frequency on the ordinate, the frequency distribution diagram of the sensitivity ofF.pseudograminearumto fludioxonil was drawn.
1.2.4 Correlation between the sensitivity ofF.pseudograminearumto fludioxonil, carbendazim and tebuconazole A total of 71 isolates were randomly selected from 105 isolates ofF. pseudograminearum, and their sensitivity to carbendazim was determined using the hypha growth rate method. The concentration gradients were set to 0.2, 0.3, 0.4, 0.5,0.6 and 0.8 μg/mL. Also, 75 isolates ofF. pseudograminearumwere randomly selected to determine their sensitivity to tebuconazole, and concentration gradients were set to 0.031 25, 0.062 5, 0.125, 0.250,0.50 and 1.0 μg/mL.Three replicates were set for each treatment, and the control (without fungicides) was included. Using Excel 2007 and DPS6.55, the virulence regression equation, the correlation coefficientR, and effective intermediate concentration (EC50) of the agent for inhibiting mycelial growth were calculated. Analysis was performed to determine the correlation regardingF. pseudograminearumvirulence in the presence of fludioxonil, carbendazim and tebuconazole:P<0.05,bvalue is positive,R2>0.800,there is a positive correlation between the two fungicides;bvalue is negative,R2>0.800, there is a negative correlation between the two fungicides; andP>0.05, the correlation between the two fungicides is not relevant.
1.2.5 Control efficacy of fludioxonil on wheat crown rot in greenhouse Preparation of conidia suspension: The fresh mycelial disk of strain LYXA-8 was inoculated into 50 mL mung beans, the spore suspension was prepared by water re-suspension, and the concentration was adjusted to 1 × 106/mL.
Greenhouse efficacy trials were conducted in 2020 and 2021. A total of two greenhouse efficacy trials were carried out and each greenhouse contained 18 cups. The treatment was repeated two times. Six portions of 2 g Zhengmai 366 seeds were collected and labeled as CK0, CK, T1, T2, T3and T4. The seeds were disinfected with 70% alcohol for 1 minute,rinsed with sterile water and placed on sterilized paper to dry. The concentration gradients of fludioxonil suspension seed coat (25 g/L) were 12.5, 25.0, 50.0 and 75.0 μg a.i/g for seed dressing (formulated in accordance with the recommended concentration of fludioxonil suspension agent). The wheat was placed with tweezers into 200 g sterilized and spore suspension (LYXA-8) cups with seven seeds per cup, and then covered with 30 g of soil. The disease incidence was observed after incubation in a greenhouse at 25 ℃(12 hours of light, 12 hours of darkness). CK0was seeds without medicament soaking and spore suspension treatments. CK used as control was seeds without medicament soaking were inoculated spore suspension. For each treatment, three replicates were set up and water was applied for 2-3 days. The roots were washed 21 days later for observation. The disease classification was based on the criteria of Wang et al,and the disease index and control effect were calculated[24]. The experiment was repeated twice.
1.3 Data analysis
Excel 2007 and DPS6.55 were used to calculate the virulence regression equation, correlation coefficientRand EC50value of the agent which inhibited mycelial growth. Shapiro-Wilk normality test was performed using DPS6.55. Significance test was performed using LSD and cluster analysis was performed using the SPSS 20.0 software.
2 Results and analysis
2.1 The minimum inhibitory concentration of fludioxonil on the mycelial growth of F. pseudograminearum
When the concentration of fludioxonil was 0.030 0 μg/mL, the inhibition rate of the mycelial growth of the four strains was more than 50.00% with a increase of fludioxonil concentration to render an elevated inhibition rate. When the concentration of fludioxonil was 0.240 0 μg/mL, the inhibition rate for all the four strains reached 100.0%, indicating that the MIC of fludioxonil required to inhibit the growth ofF. pseudograminearumhyphae was 0.240 0 μg/mL(Fig. 1). It can also be seen from Fig. 1 that the sensitivity of the in the low concentrations.

Fig. 1 Inhibition rate of fludioxonil to mycelial growth of F. pseudograminearum

Fig. 2 Determination (A) and frequency (B) of sensitivity of F. pseudograminearum to fludioxonil
2.2 Sensitivity and baseline of F. pseudograminearumto fludioxonil
As shown in Fig. 2 (A, B), the EC50value range of fludioxonil to 105 isolates ofF. pseudograminearumwas from 0.002 7-0.047 0 μg/mL. The maximum value was 17.41 times higher than the minimum value and the average EC50value was (0.026 3 ± 0.010 1)μg/mL. The results of the Shapiro-Wilk normality test showed that the sensitivity frequency of the tested strains to fludioxonil did not conform to the normal distribution (W=0.973 0,P=0.030 5 < 0.05). However,the sensitivity frequency distribution generated a continuous single-peak curve. Therefore, (0.026 3±0.010 1) μg/mL was used as the sensitivity baseline of wheat crown rot to fludioxonil.
2.3 The sensitivity of F. pseudograminearum to fludioxonil in different regions
Table 1 shows that the susceptibility ofF. pseudograminearumin the same county or city to fludioxonil was quite different. Among them, the largest difference was recorded in the Zhongmu County Zhengzhou City isolates. The EC50value of the lowest and highest sensitivity strains differed by 16.78 times, while the different multiples of the other 6 cities ranged from 2.43 to 4.43.
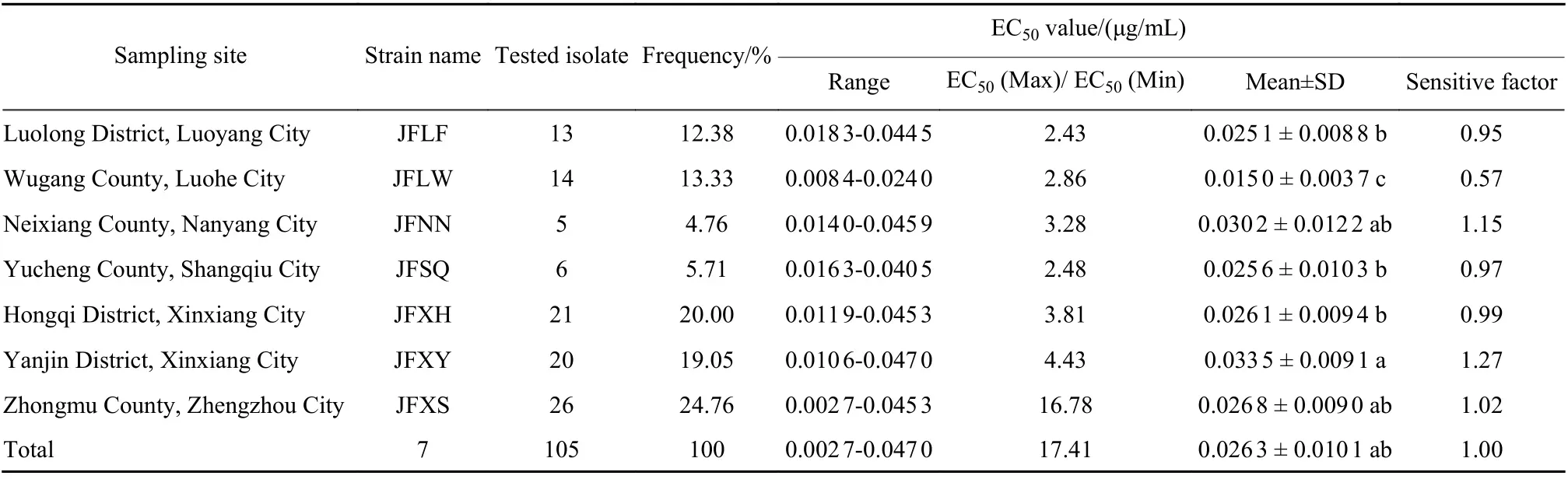
Table 1 Sensitivity of F. pseudograminearum from different areas in Henan Province to fludioxonil
Except for isolates from Wugang County Luohe City and Yanjin District Xinxiang City strains, the sensitivity of wheat crown rot fungi to fludioxonil from the other five cities was relatively insignificant.Fludioxonil had an average EC50of 0.015 0-0.033 5 μg/mL for strains in various cities. The isolates from Wugang County Luohe City strains were the most sensitive while Yanjin District Xinxiang City isolates were the least sensitive, with a difference of 2.23 times in sensitivity. The average EC50value of 105 tested strains was 0.026 3 μg/mL. Compared to the average EC50value of the strains in various regions, the sensitivity of Wugang County Luohe City was higher than the average value of Henan Province, while the sensitivities of Neixiang County Nanyang City and Yanjin District Xinxiang City were lower than the average value of Henan Province. However, isolates from Luolong District Luoyang City, Yucheng County Shangqiu City, Hongqi District Xinxiang City, and Zhongmu County Zhengzhou City strains had values close to the average level of Henan Province.
2.4 Hierarchical cluster analysis of the sensitivity of strains from different geographical sources to fludioxonil
It can be seen from Fig. 3 that according to the EC50value of fludioxonil, 28 strains ofF. pseudograminearumin seven regions can be divided into four categories,and the number of strains included was 8, 5, 7, and 8,respectively. Except for Wugang County Luohe City strains that only appeared in the first cluster analysis group, strains from other regions appeared in different cluster analysis groups. It showed that the susceptibility of wheat crown rot fungi to fludioxonil differed greatly in the same area. However, there was no obvious difference in sensitivity to fludioxonil among strains from different regions. This was consistent with the results using LSD method, indicating that the difference in sensitivity ofF. pseudograminearumto fludioxonil,was not significantly related to the geographic origin of the strain in Henan Province.
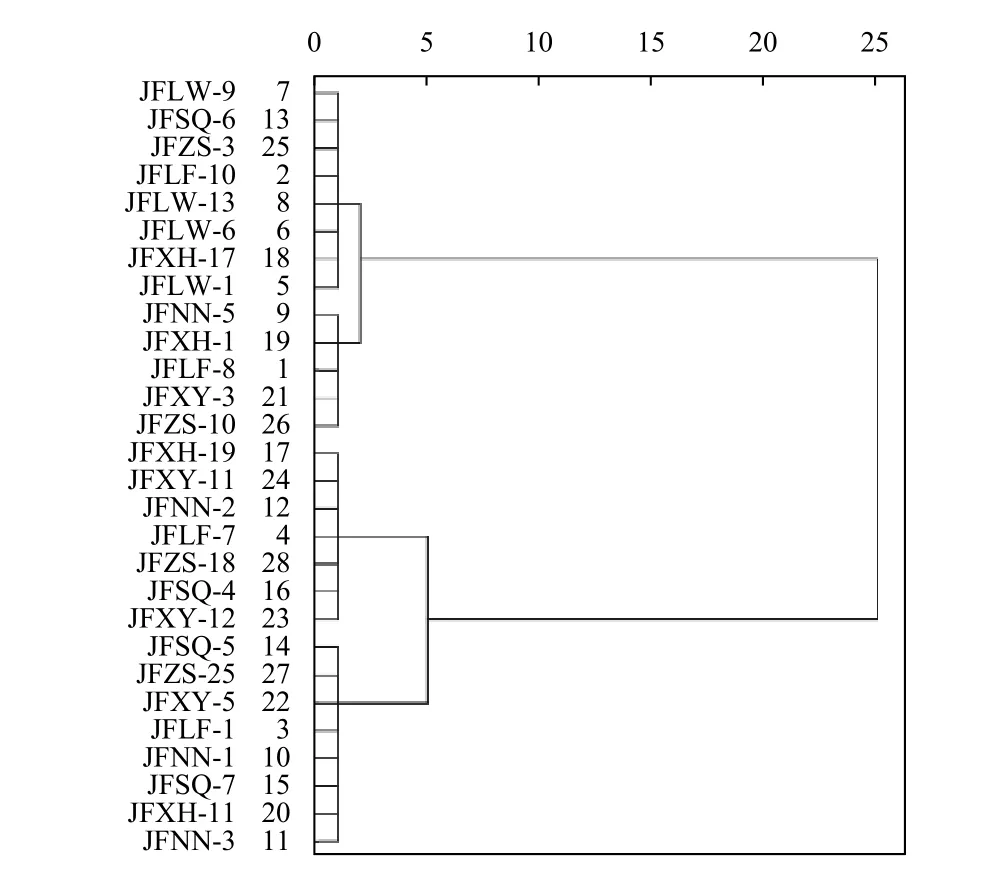
Fig. 3 Hierarchical cluster analysis on EC50 values of fludioxonil to F. pseudograminearum from different areas in Henan Province
2.5 Correlation between the susceptibility of F.pseudograminearum to fludioxonil, carbendazim and tebuconazole
A total of 71 isolates of pathogens were investigated and obtained as follows: 10 from Luolong District,Luoyang City, 4 from Wugang County of Luohe City,4 from Neixiang County of Nanyang City, 6 from Yucheng County of Shangqiu City, 20 from Hongqi District of Xinxiang City, 11 from Yanjin District of Xinxiang City, and 16 from Zhongmu County of Zhengzhou City. As shown in Fig. 4 (A), the average EC50value of fludioxonil to the 71 strains ofF.pseudograminearumwas (0.026 3±0.009 0) μg/mL,and that of carbendazim was (0.788 1 ± 0.315 3) μg/mL.The difference was significant atP=0.05 level. The linear regression equation wasy=0.733 2x+ 0.768 8,wherebwas a positive value,R2=0.000 4, which was less than 0.800 (P=0.000 1 < 0.05). It shows that there was no correlation between the pathogen's sensitivity to fludioxonil and carbendazim.

Fig. 4 Correlation of the sensitivity of F.pseudograminearum to fludioxonil and carbendazim (A) or tebuconazole(B)
Seventy-five (75) isolates of pathogens were investigated and obtained as follows: 11 from Luolong District of Luoyang City, 8 from Wugang County of Luohe City, 5 from Neixiang County of Nanyang City, 5 from Yucheng County of Shangqiu City, 14 from Hongqi District of Xinxiang City, 9 from Yanjin District of Xinxiang City, and 23 from Zhongmu County of Zhengzhou City. As shown in Fig. 4 (B),the mean value of fludioxonil for the 75 strains of pathogens was (0.025 8 ± 0.010 0) μg/mL, and the mean value for tebuconazole was (0.088 6 ± 0.145 3)μg/mL, and the difference was significant atP=0.05 level. The linear regression equation wasy=-2.723 0x+ 0.158 8, wherebwas a positive value,R2=0.034 9, which was less than 0.800 (P=0.000 3 <0.05). It showed that there was no correlation between the pathogen's sensitivity to fludioxonil and tebuconazole.
2.6 Control efficacy of fludioxonil on wheat crown rot in greenhouse
Table 2 shows the control of wheat crown rot by fludioxonil in a greenhouse. It can be seen that the increase in fludioxonil dosage resulted in a decrease in disease index and enhanced the control efficacy.When the dosage of fludioxonil was 75.0 μg a.i/g in 2020, the best effect was obtained, with disease index of (20.00 ± 4.04), and control efficacy of 58.00%.When the dosage of fludioxonil was 50.0 μg a.i/g in 2021, the best effect was obtained, with disease index of (9.52 ± 8.73), and control efficacy of 63.69%. Fludioxonil has an effect on wheat crown rot and can be used to prevent and control the disease.

Table 2 Control efficacy of fludioxonil 25 g/L FSC on wheat crown rot in greenhouse
3 Conclusion and discussion
In this study, the mycelial growth rate method was used to determine the sensitivity of 105F. pseudograminearumin Henan Province. The difference in sensitivity of wheat crown rot fungi strains to fludioxonil in Henan Province was not significantly related to the geographical origin of the strains.F. pseudograminearumin Henan Province was highly sensitive to fludioxonil, and fludioxonil had a good inhibitory effect on the proliferation ofF. pseudograminearumon wheat seedlings. Therefore, fludioxonil has a good effect on wheat crown rot disease. It has good application prospects in the prevention and treatment of the disease.
Xu[25]determined the sensitivity ofF. graminearumto fludioxonil in Henan Province and demonstrated the strong inhibitory effect of fludioxonil on wheat head blight in Henan Province and wheat head blight was sensitive to fludioxonil. The sensitivity baseline was only 0.007 0 μg/mL and fludioxonil can be used as an alternative agent for the chemical control of wheat head blight. In this study, the sensitivity baseline of wheat crown rot in Henan Province to fludioxonil was (0.026 3 ± 0.010 1) μg/mL, which was 3.76 times higher than that of wheat head blight. The reason for this difference may be related to the difference in the target protein sequence of fludioxonil in different pathogens. The target of fludioxonil is the two-component histidine kinase SHK1 in pathogens[26].After the treatment of fludioxonil, the two-component histidine kinase pathway in plant pathogenic fungi was activated, which resulted in the increase and accumulation of intracellular glycerol synthesis, increased intracellular turgor pressure, electrolyte concentration and extravasation, and increased cell membrane permeability. This causes cell rupture and eventually death[18]. Currently, the genome sequence ofF.graminearumhas been published[27], but the genome sequence ofF. pseudograminearumis yet to be announced. Once the genome sequence ofF. pseudograminearumwas released, a comparison of the two SHK1 sequences will reveal the differences in the binding sites of fludioxonil in the amino acid side chains in the two pathogens. The strength of the binding force on the target protein will reveal the difference in the sensitivity of the two species to fludioxonil. On the other hand, the difference in sensitivity of the two pathogens to fludioxonil may also be related to their life history:F. pseudograminearummainly exists on crop disease residues in the form of conidia and mycelium and is heterogeneous. It hardly produces sexual stages in nature and has low sensitivity to chemicals.F. graminearumis a homologous combination, and it easily produces a sexual stage in nature. Therefore, it is more sensitive to fludioxonil,but in the later stage, it can adapt to the selection pressure of the agent by producing mutant offsprings through sexual crossing[28].
The results of this study showed that there was no correlation in susceptibility to fludioxonil,carbendazim and tebuconazole. During production,fludioxonil can be compounded with these two agents to delay the development of pathogen resistance.Zhao[29]reported that 27% bendiocarb-thiamethoxam suspension seed coating agent had the highest control efficacy on wheat stalk basal rot, the effect of chemical treatment was also obvious, and the increased rate was close to or more than 20%. Yang[30]showed that 10% fludioxonil+azoxystrobin suspension seed coating agent had a good control efficacy on potato mole disease, and its effective ingredients consisted of 15,20 and 25 g/100 kg seed treatments, with a control efficacy of more than 75%. These results provide a reference basis for the compound control of wheat crown rot by fludioxonil and other agents.
In greenhouse conditions, the seeds were inoculated with pathogens after the medicament dressing treatment. Fludioxonil suspension seed coating agent had a good preventive effect on wheat crown rot infection and prevented the occurrence of wheat rot.Moreover, the roots, crowns and leaves of wheat seedlings grew normally, indicating that fludioxonil is safe for wheat at the concentration used.F. pseudograminearumis the dominant pathogen of wheat crown rot in Henan Province. However, the field control effect of fludioxonil on wheat crown crown rot still needs further research. Under natural conditions,the main pathogens of crown rot in different regions are different, and even the same wheat field often has multiple pathogens coexisting. In agreement with the results of Xu[25], fludioxonil can be used to control wheat crown rot in areas whereF. pseudograminearumandF. graminearumare the main pathogens.However, in areas where other types ofFusariumor even other types of pathogens are the main pathogens,the inhibitory effect of fludioxonil on the main local pathogens needs to be further clarified.
The farming method in Henan Province is primarily conservation farming, which involves the returning of entire amount of straw from winter wheat to summer corn to the field, result in a large accumulation of fungi in the farmland and providing favorable conditions for the growth of pathogens. In agricultural production, comprehensive control of crown rot should not only use fungicides to inhibit the growth of pathogens but should also focus on improving the composition of the soil microbial community to reduce the number of pathogens; or promote the healthy growth of wheat and increase the resistance of wheat to pathogens. Straw-degrading fungi can compete for nutrients with pathogens on field straws,thereby preventing the spread of pathogens and achieving the goal of preventing soil-borne diseases[31].Biochar has an obvious growth-promoting effect, resisting the invasion of pathogens by making plants grow healthy[32]. Trichoderma is not only capable of competing with pathogens for nutrition, but also has a very good growth-promoting effect, and a dual inhibitory effect on soil-borne pathogens[33]. These soil improvement measures, when combined with chemical seed dressing, are expected to achieve continuous control of wheat crown rot.
