Diradical Character and Controllable Magnetic Properties in Chitin Through Radical Modification
2022-03-08KONGLingqian孔令乾YANGHongfang杨洪芳QIANZhaohong钱兆红ZHOULianwen周连文ZHANGMeiXUJing
KONG Lingqian(孔令乾), YANG Hongfang(杨洪芳) , QIAN Zhaohong(钱兆红), ZHOU Lianwen(周连文), ZHANG Mei(张 梅)*, XU Jing(徐 静)*
1 College of Textile and Clothing, Dezhou University, Dezhou 253023, China2 College of Chemistry and Chemical Engineering, Dezhou University, Dezhou 253023, China
Abstract: The parent and radical-modified chitin units are computationally studied and the parent chitin unit possesses closed-shell singlet(CS) state as its ground state. Two radicals(CH3· and CH3CH2·) are chosen to modify the parent chitin unit. Calculations predict that CH3CH2·-modified chitin unit possesses the open-shell broken-symmetry(BS) singlet diradical ground state, different from that CH3·-modified chitin unit is a triplet diradical. That is to say, the magnetic conversion can occur from nonmagnetic exchange coupling to antiferromagnetic(AFM) or ferromagnetic(FM) exchange coupling by means of the radical modification method. The present work helps to get a basic understanding of structures and electronic properties of chitin and its radical-modified derivatives at the atomistic level. Moreover, it also provides a feasible way to design new chitin-based magnetic materials and organic magnetic switches.
Key words: radical-modified chitin; diradical; magnetic conversion; density functional theory (DFT)
Introduction
In recent years, along with the economic development and improvement of living standards, people are paying more attention on how to improve their own health and quality of life. As a result, it makes green textile materials and health care textiles more popular. Among them, magnetic or electromagnetic functional textiles had penetrated into people’s daily life, as they might play a crucial part in the protection of magnetic therapy. However, magnetic properties of these above-mentioned textiles almost all originate from the addition of stationary magnetic particles during the finishing process of printing and dyeing, such as coating, dyeing, and pasting. Although these methods can give textiles a certain degree of magnetism, they also restrict textiles’ washing endurance greatly. Using magnetic fibers to weave textiles will avoid the above problems thoroughly. Studies reveal that the structural changes might bring magnetic properties[1-5]. Thus, we can give the building blocks of fibers some degree of magnetism through structural modification, and naturally, fibers made of such kind of magnetic building blocks will possess the function of magnetic health care.
Among various kinds of fibers, we herein choose chitin unit for radical modification as shown in Fig. 1. The choice is founded upon the following considerations.

Fig. 1 Topological representation of (a) CH3·-modified chitin unit and(b) CH3CH2·-modified chitin unit
(1) Chitin exists in the shells of crustaceans like shrimp and crab, and it is the second most common polymer after cellulose in earth. Chitin fiber is a kind of very abundant organic regenerated fiber resource from chitin, and its reserves are second only to cellulose fiber. Especially, chitin fiber has bioactivity of reducing inflammation, relieving pain, and promoting wound healing. Moreover, it exhibits biodegradability which is very friendly to the environment. If chitin fiber can maintain its original excellent biocompatibility while possess flexible and controllable magnetic properties, it will surely become a breakthrough in medical magnetic health products.
(2) The chitin unit contains one secondary hydroxyl group at 3 positions and one primary hydroxyl group at 6 positions. The two free hydroxyl groups make it possible for the hydrogen atoms to be replaced by other groups. In other words, they make chitin units easy to be chemical modified. Because it is generally considered that the reaction ability of the primary hydroxyl group is higher than that of the secondary hydroxyl group, we choose the 6-point primary hydroxyl group for radical modification.
(3) Previous studies have proven that cellulose alkylation can be easily realized by means of some appropriate methods[6-8]. Provided that the similar structures between chitin and cellulose, it is reasonable to suppose that the radical-modified chitin can be synthesized in experiment. In summary, we will modify chitin units by replacing the hydrogen atom of its 6-point primary hydroxyl group with one CH3· and one CH3CH2· respectively, and further explore the resulting magnetic properties.
Although lots of efforts have been made to study chitin’s chemical modification, they are mostly on the extraction, the improvement of functionality, or the better control of chitin or chitosa[9-14]. In our study, we have found that different radical-modified chitin units could show different ground states with different diradical character, in comparison with its closed-shell singlet(CS) parent chitin unit. The research shows a feasible strategy to modify chitin and design novel magnetic chitin fiber.
1 Method of Calculations
In our research, we have calculated the chitin unit and its two radical-modified derivatives by Gaussian 09 program in order to gain a comprehensive understanding of their structural and electronic properties. The molecular structure optimization was carried out at the(U)B3LYP/6-31 G(d) level of theory, and energy calculations had been obtained based on the optimized structures, which was considered to be the most successful method for predicting molecular ground states[15-17]. Their vibrational frequency analyses showed no imaginary frequency, which meant that all the optimized geometries could be recognized as the local minima. Figure 2 shows the optimized structures for the parent and radical-modified chitin units. The relevant electronic properties were also determined, including gap between the T state and the broken-symmetry(BS) state(ΔE(T-BS), T means triplet state, and BS means unrestricted open-shell BS singlet state), and the gap between the highest occupied molecular orbital and the lowest unoccupied molecular orbital (HOMO-LUMO gap). In addition, provided that one of the most widely used methods for quantifying the(poly)radical character is to measure the numbers of natural orbital occupation[18-20], the CASSCF(6, 6)/6-31 G(d) method was used to measure the diradical character of CH3CH2·-modified chitin unit(BS ground state) by calculating the orbital occupation numbers, especially the LUNO(occupation numbers of LUNOs), and the CASSCF(4, 6)/6-31 G(d) method was used for CH3·-modified chitin unit(T ground state)[1, 21]. Besides, to explore their magnetic properties, we also calculated theirJ(magnetic exchange coupling constant) values through the BS approach of density functional theory(DFT) proposed by Noodlemanetal.[22-23]. In detail, we used a formalism developed by Yamaguchietal.[24]to estimateJvalues for parent and methyl-modified cellobiose, which is written as
J=(EBS-ET)/(
whereEBSandETdenote energies of the BS and T states,
2 Results and Discussion
We choose CH3·-modified and CH3CH2·-modified chitin units as our main target which could be regarded as the simplest modification mode(shown in Fig. 1), and we expect that replacing the hydrogen atom of its 6-point primary hydroxyl group with one CH3· group or one CH3CH2· group could lead to different magnetic properties. Different spin states of the parent chitin unit and its two radical-modified derivatives are optimized by(U)B3LYP method, and their relevant geometries and energies as well as magnetic couplings will be discussed.
In Fig. 2, we exhibit the most stable optimized geometries for the parent and two radical-modified chitin units, respectively. The radical-modified chitin unit is formed by the H atom in 6-point primary hydroxyl group replaced by one CH3· group or one CH3CH2· group, which can be seen obviously in Fig. 2. Figure 2 shows the most relevant computationally optimized bond distances and angles, from which we can see that the structural parameters of two radical-modified chitin units are very close to those of the parent one. The main changes brought by the radical modification lie in the interflap angle between two parts and the bond lengths between fused rings where radical groups are located. Whereas an angle of 117.7° was found in the parent chitin unit, the angel of the CH3·-modified one indicates 119.5°, and the angel of the CH3CH2·-modified one indicates 119.1°. Although the angle difference is minor, it still reveals that replacing the H atom by radical groups at the 6-point primary hydroxyl group makes the structure more nonplanar. Moreover, the CH3CH2·-modified chitin unit features a slightly lengthened distance(2.446 Å, 1 Å=0.1 nm ) between the two parts compared with the parent one(2.430 Å), which is caused by the more enhanced steric hindrance in the CH3·-modified one, making the two parts further from each other. Similarly, the distance between the two parts in the CH3·-modified one is also lengthened to 2.448 Å. This value is larger than that of the CH3CH2·-modified chitin unit which might lie in their different ground states, T states in CH3·-modified one and open-shell BS singlet state in CH3CH2·-modified one.

Fig. 2 Optimized geometries: (a) parent chitin unit(CS); (b) CH3·-modified chitin unit(triplet); (c) CH3CH2·-modified chitin unit(open-shell BS singlet) (the main bond lengths are indicated in Å and the angles in (° ))
In summary, structural characteristic is only slightly affected, and the bond length differences between the three molecules are no more than 0.018 Å. That is to say, though a radical group replaced the initial H atom at the 6-point primary hydroxyl group, the basic geometric skeletons of chitin units are hardly affected. Therefore, it could be reasonably predicted that chitin units’assembling abilities remain. In other words, the slight structural differences caused by the radical modification do not threaten the possibility of radical-modified chitin units to form relatively stable chitin fibers. And the differences in structures for these molecules inevitably leads to the corresponding differences in electronic properties.
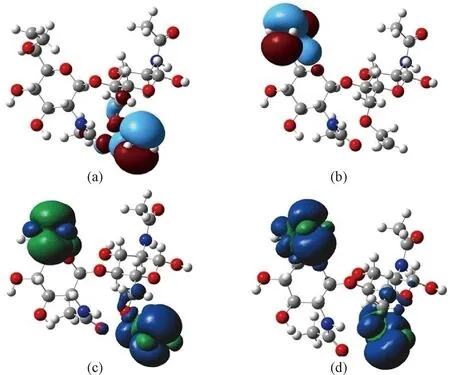
The structures of possible spin states(CS, BS, T) of the parent chitin unit and two radical-modified ones are optimized. Table 1 gives the energy differences between BS and T states and that between BS and CS states which are all calculated by the DFT approach. According to our calculations, the energy of parent chitin unit possesses two spin states, and its CS state is lower than its T state by 78.35 kcal/mol. Obviously, the CS state is chitin unit’s ground state. For radical-modified ones, the energy order is T Table 1 Energies of the singlet-triplet gaps, spin-squared values of BS state, energy differences between BS and CS states of chitin unit and radical-modified ones The parent chitin unit molecule is a typical CS state, but after radical modification, the radical-modified ones possess different and interesting spin states. For CH3·-modified one, it is originally a triplet diradical with two spin-parallel unpaired electrons(triplet ground state), and thus has the possibility to form ferromagnetic(FM) solids as building blocks[24]. Particularly, we calculated CH3·-modified one’s HOMO-LUMO gap in its triplet ground state, and the value is 4.55 eV. From the chemical viewpoint, the HOMO combined with the LUMO plays a dominant role in numerous reactions, and the gap between them(HOMO-LUMO gap) is responsible for the conductivity and the redox reactivity[24, 28-29].In more detail, molecules with large values of HOMO-LUMO gaps possess low chemical reactivity, because these molecules are energetically unfavorable for extracting electrons from the low-lying HOMO and adding electrons to the high-lying LUMO[29-30]. Therefore, this triplet diradical(CH3·-modified chitin unit) is relatively stable according to its HOMO-LUMO gap[29]. For CH3CH2·-modified one, its HOMO-LUMO gap of BS ground state is also calculated and the value is 3.91 eV. The value is sufficiently low so that it provides a possibility for the promotion from the HOMO to LUMO of an electron. Next the two orbitals(HOMO and LUMO) reorganize and then form two lower nonbonding singly occupied molecular orbitals(SOMOs), which leads to an open-shell BS singlet diradical[31]. Clearly, the result also further confirms the above conclusion that the CH3CH2·-modified chitin unit has an open-shell BS singlet diradical ground state and explores the essence of the fact that the BS state is more stable than the CS state. It is worth pointing out that the above-mentioned conclusions agree well with magnetic properties which will be discussed in detail below. The magnetic exchange coupling constant(J) of two radical-modified chitin units has been calculated to estimate the exchange interaction between radical centers. Herein, we use a formalismJ=(EBS-ET)/( Table 2 Energies, For CH3CH2·-modified chitin unit, once the BS state is estimated to be the ground state, its diradical character should be clarified, because it might prove electromagnetic properties. In detail, for the diradical CH3CH2·-modified chitin unit, the preliminary(6, 6) CASSCF method has been used to calculate its orbital occupation numbers(shown in Table 3), and it can further prove the diradical character and describe its diradical character quantitatively. Among the occupation numbers of HOMO and LUMO as well as the numbers of electrons outside closed-shell bonding orbitals, we choose the LUMO occupation number to measure the amount of the diradical character for the CH3CH2·-modified chitin unit[1, 32-33]. In detail, the calculated LUMO occupation number is 1.000( Table 3 Occupation number of every orbital in active space, CASSCF(4, 6)/6-31G(d) for CH3·-modified chitin unit (T) and CASSCF(6, 6)/6-31G(d) for CH3CH2·-modified chitin unit(BS) Besides, in order to exhibit the diradical character intuitively and to further obtain an insight of the nature of the BS singlet diradical ground state, the SOMOs and the spin density distribution of CH3CH2·-modified chitin unit is visualized in Fig. 3. We could clearly see two unpaired electrons mainly reside in two glucose units separately and the spin density distributes on both units but being spin-up and spin-down distinguishable[31]. Fig. 3 Electronic structure diagram of modified chitin unit:(a)-(b) SOMOs;(c) spin density map in BS state of CH3CH2·-modified chitin unit;(d) spin density map in T state of CH3·-modified chitin unit The above results indicate that different radical modifications could make radical-modified chitin units possess different magnetic characters from their parent chitin units. And it is important to note that the different magnetic characters of parent and radical-modified chitin units are caused by their different ground states. Further, we might understand their respective energetically favorable spin state from another viewpoint through the analysis of their spin density maps for each spin state(BS, CS, T). In detail, the energetically favorable spin states of CH3·-modified and CH3CH2·-modified chitin units could be excellently explained by the spin alternation rule[34-35]. For CH3·-modified chitin unit, the high-spin state(FM coupling, T state) is favorable because the spin alternation is retained in T state, while for the CH3CH2·-modified chitin unit, the low-spin state(AFM coupling, BS state) is favorable because the BS state follows the spin alternation pattern ofα-spin andβ-spin densities. Consequently, the ground states of parent and methyl-modified cellobiose are T and BS states, respectively. Naturally, CH3·-modified and CH3CH2·-modified chitin units have potential to assemble FM and AFM materials, respectively. In this work, we have designed two radical-modified chitin units(CH3·-modified and CH3CH2·-modified chitin units) which could be viewed as the simplest radical-modified derivatives of chitin units. DFT and CASSCF calculations prove that radical modification makes the ground state of parent chitin unit changing from CS state to different open-shell states. In detail, CH3CH2·-modified chitin unit possesses the open-shell BS diradical state, and CH3·-modified chitin unit is a triplet diradical. Besides, the values for the magnetic exchange coupling constant from positive ones to negative ones show us a magnetic conversion from FM to AFM, by means of CH3· modification and CH3CH2· modification, respectively. This work offers a theoretical perspective that we could study the magnetic properties brought by the radical modification and modify chitin units for some special purpose, such as the realization of controllable magnetic characters. The research offers us a promising strategy of designing new chitin-based magnetic materials which might possess tunable magnetic properties.


3 Conclusions
杂志排行
Journal of Donghua University(English Edition)的其它文章
- Recombinant Pyriform Spider Silk Expression and Wet-Spinning
- Experimental Study on Grinding Force of Electrostatic Coated Grinding Wheel
- Influence of Three Sizes of Sliding Windows on Principle Component Analysis Fault Detection of Air Conditioning Systems
- Consensus for High-Order Linear Multi-Agent Systems with Unknown but Bounded Measurement Noises
- Preparation of Polyaniline/Cellulose Nanofiber Aerogel for Efficient Removal of Cr(VI) from Aqueous Solution
- Theoretical Calculation and Analysis of Muffler Based on Multilayer Sound Absorbing Material
