Acute myocardial injury in patients with COVID-19: Possible mechanisms and clinical implications
2022-03-07RusuTurlacuMicheuMM
INTRODUCTION
Since December 2019, coronavirus infectious disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus (SARS-CoV)-2 has quickly become a global health issue that is having a major impact on the healthcare system worldwide.High infectivity and rapid transmission of the virus have led to an international public health crisis. A wide range of symptoms had been reported, with most infected patients developing respiratory tract disease with different severity level. Not only the lungs are affected, and other organs are involved, with COVID-19 affecting multiple organs and systems, with different cardiovascular implications. Also, cardiovascular comorbidities have an important impact on the severity of COVID-19 and they seem to be linked with severe clinical outcomes and higher risk of death. Clinical studies have reported that COVID-19 can significantly affect the heart, causing acute myocardial injury (MI)[1-3], in patients with and without pre-existing cardiovascular disease[4].MI is defined as an elevation of at least one cardiac troponin (cTn) concentration above the 99th percentile upper reference limit[5]. COVID-19-related MI can have various clinical manifestations, of which the main ones are myocarditis[6-8], stress cardiomyopathy[9,10], acute coronary syndrome[11-13], and pulmonary embolism[14-16]. In this review, we aim to provide an overview of the potential mechanism involved in MI induced by COVID-19, and the progress in the therapeutic strategies addressing it.
专职辅导员在专业知识和能力方面的缺乏,难以对学生做出专业的引导和相关职业的规划。培养专业辅导员既弥补了此方面的不足,又对专业课教师参与学生思政工作起到了抛砖引玉的作用。
总而言之,对于宫颈腺癌患者行根治性放疗后行筋膜外全子宫切除能减少肿瘤复发转移,而且该手术安全可行,可以在临床上做进一步的推广应用。但由于本研究所选用的样本量较小,因此其得到的结果还不具备充足的说服力,因此下一步有必要进行样本扩大化研究。
PUTATIVE MECHANISMS OF MI
COVID-19 may cause MIvarious mechanisms, either directly, or indirectly. The first mechanism might be a direct injury to myocardial cells due to a viral invasion of endothelial cells and cardiomyocytesangiotensin I converting enzyme (ACE)2.Other possible mechanisms are: downregulation of ACE2, cytokine storm/cytokine releasing syndrome, and hypercoagulation (Figure 1).
信息化背景下的高校思政教育协同管理的过程中,要建立学校、家庭、社会与学生间的相互沟通的渠道,使信息传达与共享能够更加便捷迅速。教师可以通过QQ、MSN、微信等平台与学生进行交流,进而实现思政教育的零距离互动。思政教育工作者要转变角色,加强学习,以自身的人格魅力及学识引导学生。思想政治教育工作者面对网络与学生具有平等的地位,在网络中与学生进行交流讨论,给学生树立正确的榜样,用自己的行动感染学生,把正能量传递给学生。大学生也可以通过博客、QQ 等进行情感倾诉、释放心情。管理者也可以通过电子邮件、短信群发实现对大学生思政教育的提醒和告知。
Direct injury
SARS-CoV-2 is an RNA virus with a high affinity for ACE2. For virus attachment to the receptor, SARS-CoV-2 uses the S protein and the transmembrane protease serine 2(TMPRSS2) to cleave the S protein and facilitate infection[18,19]. The receptors of ACE2 are located in the lung, heart, endothelial cells and immune cells[20]. These locations could explain intracellular viral replication in the myocardium and other tissues, resulting in degeneration, necrosis and dysfunction. Recently, it has been showed that ACE2 and other mediators of SARS-CoV-2 entry (such as cathepsin B and cathepsin L) are preferentially enriched in cardiomyocytes, explaining at least in part the cardiac susceptibility to COVID-19[21].
In acutely ill hospitalized patients with COVID-19, anticoagulant thromboprophylaxis is recommended. The CHEST guidelines are in favor of anticoagulation with LMWH or fondaparinux over unfractionated heparin (UFH) or DOAC[106]. UFH is not preferred, in order to limit staff exposure, and DOAC is not recommended as a primary prevention strategy due to possible risk of interactions between therapies for COVID-19 and oral anticoagulants[106]. The American Society of Hematology guidelines do not recommend any specific anticoagulant to be used as first-choice treatment[107]. There is no recommendation to increase intensity of anticoagulation thromboprophylaxis, and the current standard dose should be used over intermediate or full treatment dosing[106-109]. However, the Italian Society on Thrombosis and Haemostasis suggests that the use of intermediate dose of LMWH should be considered in patients with multiple risk factors for VTE[110]. Also, the Royal College of Physicians suggests that a higher dose of LMWH may be considered in these patients[105].
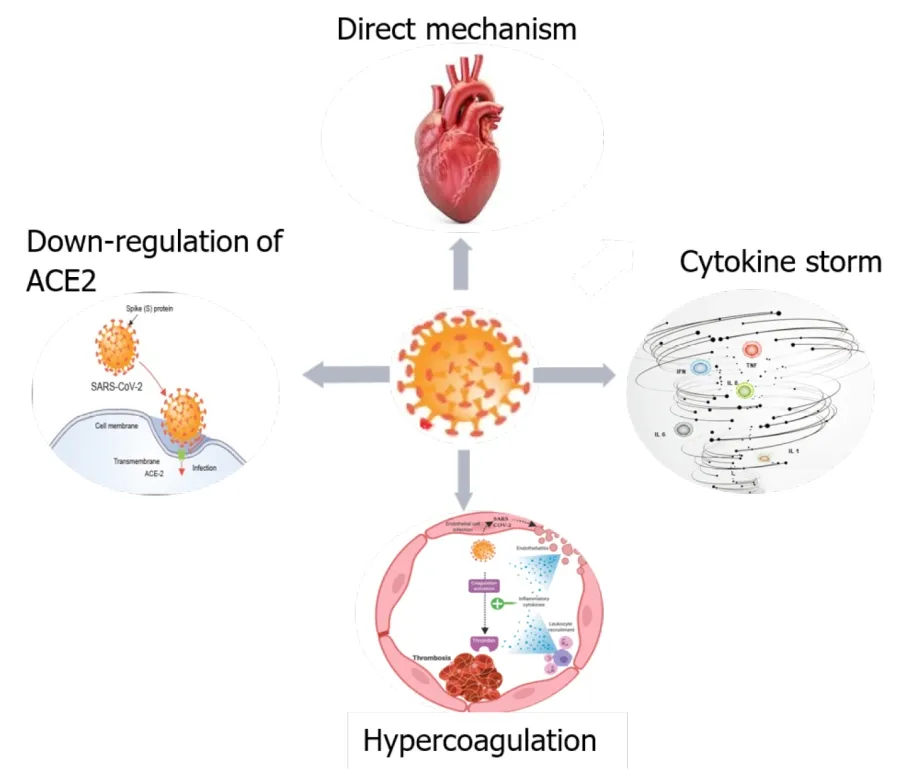
Downregulation of ACE2
Recognition of ACE2 as the primary human receptor for the SARS-CoV-2 was the first step to identify the virus tropism and pathogenicity[29,30]. The literature shows that ACE2 is expressed in type II alveolar epithelial cells, myocardial cells, vascular endothelium, esophageal and bladder epithelium cells, and renal cells[31,32]. The virus uses S protein for binding to the ACE2 receptor of target cells, and the cellular serine protease TMPRSS2 cleaves the S protein into two functional domains, S1 that binds to ACE2 and S2 designed for membrane fusion[30,33-37]. The cleavage can be produced near a fusion peptide located within the S2 domain[33,38]. This mechanism helps the virus priming and entry into the cells and promotes virus infectivity[30,33,39,40]. Lai[39] have demonstrated that SARS-CoV fusion depends on calcium level, so a low level of calcium decreases infectivity. It is known that ACE2 and ACE are linked to the renin–angiotensin–aldosterone system, which promotes angiotensin I maturation, and has a crucial effect on the cardiovascular system[41].
Endothelial dysfunction, hyperinflammation, and hypoxia induced by SARS-CoV-2 contribute to a procoagulant status with majors effects on the cardiovascular system[66]. Inflammation and coagulation play a bidirectional role in vascular disease[67].The inflammation causes endothelial dysfunction, which activates coagulation and together with the coagulation factors, increases cytokine production by the endothelial and mononuclear cells[67]. Endothelial dysfunction by activating the complement system, causes a hypercoagulant state and promotes inflammation[68].
假说3a 企业家信心会强化宽松货币政策对过度投资的增加效应;而在货币政策趋紧时,企业家信心会弱化货币政策对过度投资的削减作用。
Angiotensin I hydrolyzation produced by ACE2 yields angiotensin 1-9 peptide, on which ACE acts to produce angiotensin 1-7 (Ang 1-7)[17]. Ang 1-7 is the ligand for the G-protein Mas receptor that provides cardioprotective effects as vasodilatory, antiproliferative and antioxidative effects[17]. ACE2 has a direct effect on angiotensin II,producing Ang 1-7, but also acts on bradykinin ligand receptor, Des-arg9-bradykinin,thereby inactivating an inflammatory response[17,41,42].
The IL-6 mechanism results in oversecretion of vascular endothelial growth factor,MCP-1, IL-8 and IL-6, and decrease of E-cadherin on endothelial cells, promoting apoptosis of cardiac cells and left ventricular remodeling[49,62,65]. Del Turco[62]have shown that hyperinflammation promotes vascular permeability, leakage,endothelial dysfunction, and hypercoagulation with a significant impact on the cardiovascular system. Also, the production of matrix metalloproteinase by the monocytes/macrophages increases the risk of atherosclerotic plaque rupture and the probability of MI[62].
Ang II activates both mitogen-activated protein kinase and ADAM-17 phosphorylation that generates reactive oxygen species (ROS), which promote endothelial dysfunction and thrombosis[44,45].
Most guidelines recommend the use of current standard dose over intermediate or full treatment dosing due to insufficient data regarding intensified treatment[106-108].Nevertheless, the Anticoagulation Forum suggests, based on expert opinion, that an increased dose of anticoagulant thromboprophylaxis such as enoxaparin 40 mg or 0.5 mg/kg subcutaneous twice daily, UFH 7500 U subcutaneous three times daily or lowintensity heparin infusion, should be considered for these patients[109]. The Royal College of Physicians also suggests intermediate dose of LMWH[105]. In critically ill COVID-19 patients with confirmed VTE, the CHEST guidelines recommend parenteral anticoagulation with LMWH or fondaparinux over UFH[106]. The CHEST and the Royal College of Physicians guidelines recommend a minimum duration of 3 mo of anticoagulation therapy for COVID-19 patients with confirmed VTE[105,106].
Cytokine storm/cytokine release syndrome
Many severe infectious and noninfectious diseases, including COVID-19, are associated with cytokine overproduction, activating lots of signals and communication pathways[48,49]. The inflammation starts in the lungsACE2 receptor, which is localized in the pneumocytes, local pulmonary macrophages, and dendritic cells, and it spreads through the circulation to organs expressing ACE2, with significant effects on the cardiovascular system[50].
Oudit[51] have shown that an increased level of Ang II determines infiltration and activation of neutrophils in the myocardium, which release inflammatory cytokines (IL-6, IL-1β and MCP-1) and are a source of ROS, with a negative inotropic effect on murine myocardial contraction[51].
SARS-CoV-2 activates the innate immune system and triggers the JAK–STAT pathwaythe pattern of recognition receptor, with overproduction of IFNs[38,52].IFN type I increases the inflammatory factors and activates the cytokine storm[38,52,53]. Rapid replication of the virus determines the activation and differentiation of T helper (Th)1 cells, producing cytokines such as IL-6, granulocyte–macrophage colonystimulating factor and IFN-γ, and increases the number of Th1 and Th2 cells,macrophages and natural killer cells[54]. The virus has developed new mechanisms through nonstructural protein to avoid the immune system, and suppresses the effects of IFNs, which lead to virus dissemination and promotion of cytokine realizing syndrome[50,54-57]. The first cytokines produced in the early phase of the infection are IL-6, tumor necrosis factor (TNF)-α, IL-1, IL-8 and MCP-1[56,58].
In the severe form of COVID-19, chemokines CCL3, CXCL8, CXCL9 and CXCL10 are released into the blood circulation, as well as proinflammatory cytokines TNF-α,IFN-γ, IFN-α, IL-12, IL-1β, IL-6, IL-33, IL-18 and transforming growth factor β, leading to an important inflammatory response[18,53,58,59]. Latest studies indicate that a higher level of inflammatory biomarkers such as IL-6, IL-8 and TNF-α determine MI and correlate with high mortality[60,61].
妇产科急腹症是妇科急诊,患者临床中有急性腹痛症状出现[1-2]。因为发病突然,病因复杂,诊断难度比较大。急腹症的早期诊断可以有效的提升患者的救治效率,如果错过治疗时机,患者的生命会受到威胁[3-4]。现在我国医疗领域已经开始广泛应用,特别是临床妇产科的检查中。妇产科中彩超主要是被作为女性盆腔静脉曲张、不孕症的观察,胎儿先心病、脐带疾病及其胎盘功能的评估,对肿瘤良恶性进行分辨等,彩超的辅助诊断效果突出。根据研究显示,该疾病临床的误诊率比较高。此次我院就妇产科急腹症患者接受彩超诊断的临床价值进行研究分析,现报告如下。
IL-6 plays the main role in inflammation. There are two mechanisms through the JAK–STAT3 signaling pathway for activating and promoting inflammation[59]. The first mechanism of action is the-signaling pathway that uses membrane IL-6 receptor, which activates the innate and acquired immune system[62,63]. The second mechanism is through the-signaling pathway, which uses soluble IL-6 receptors for activating cells without IL-6 membrane receptors, such as endothelial cells[62-64].
本文依据办公建筑的实际尺寸与室内物品布置,对该办公室建立了物理模型(图1),并进行了适当简化,其尺寸为Z×X×Y(长×宽×高)=8 m×6 m×3.5 m,室内有16人,16张桌子.办公室西墙有2个窗户,东墙两侧分别有1个铁质结构和木质结构的门,北墙有1个木质结构门通往另一个房间.室内采用4台机组送风,侧送侧回,送风温度为24 ℃.风口中心距地面2.5 m,风口与地面中心线垂直断面对称分布,以东北侧风口为例,该风口中心距东侧墙体2 m,距北侧墙体1.5 m.详细室内物品规格见表1.
In SARS-CoV 2 infection, decreased ACE2 expression causes lower levels of Ang 1–7 and an increase in angiotensin II level[17,41,43]. This effect results in vasoconstriction,inflammation, proliferation, fibrosis, apoptosis, andheart injury or aggravation of pre-existing cardiovascular problems[43].
Hypercoagulability
这款闪光灯的机身设计和电池组与功率更强劲的Modus 600RT相同,但售价更低。该款产品共有佳能、尼康、索尼、富士以及M4/3卡口可供选择。
医护人员以PPT展示的方式,进行相关理论知识讲解,同时辅以情景再现模式,为受训人员展现急救过程。并且,使用高仿真急救人体模型,供受训公众演练心肺复苏等技术,鼓励其积极参与。
The central role in thrombogenesis is played by tissue factor (TF)[50,68]. TF is a transmembrane protein expressed on the surface of macrophages, cardiomyocytes and smooth muscle cells[48,49]. The monocytes in atherosclerotic plaques tend to express more TF than the circulating ones, which stimulates cytokines such as IL-6, plateletderived growth factor and MCP-1, and leads to thrombus formation. In severe infection, cytokines, especially IL-6, determine TF exposure and systemic activation of coagulation[68-70]. TF binds to factor VIIα, leading to thrombin formation, which converts fibrinogen into fibrin and determines coagulation[70]. Thrombin also binds to another class of specific receptors, protease-activating cell receptors (PARs), which are expressed in many cell types, including endothelial cells, monocytes, platelets, smooth muscle cells, and fibroblasts. Their activation is a key promoter of both coagulation and inflammation[70]. Four PAR types are identified; type 2 determines overproduction of ROS with negative inotropic action and adhesion molecules by macrophages, and induces neutrophil infiltration and expression of TNF-α and IL-1[69,71].
Severe hypoxia activated in SARS-CoV-2 infection leads to multiple effects such as endothelial inflammation with metabolic changes that affect ATP production and an increase in mitochondrial ROS, which causes platelet hyperactivation and apoptosis with release of proinflammatory and procoagulant factors[75,76].
取釉质再矿化实验组和釉质脱矿抑制实验组中的所有釉质块,去除抗酸指甲油后,进行干燥处理。用SEM双面碳导电胶将釉质块固定于样品台上,然后置于离子溅射仪中真空喷金镀膜。镀膜完成后,SEM下(×1 000)观察所有样本釉质表面形貌。
Neutrophil-derived extracellular traps are an extracellular web of chromatin and antimicrobials produced by neutrophils as an innate mechanism to combat pathogens.They can trigger the processes of inflammation and thrombosis by activating endothelial cells and platelets[72-74].
Hypoxia promotes thrombogenesis through a direct mechanismearly growth response factor 1 induction, but also through and an indirect mechanism mediated by inflammatory cytokines (TNF-α and IL-1)[75-78]. Hypoxia also activates hypoxiainducible transcription factors that promote coagulation targeting factors such as plasminogen activator inhibitor 1, but also the pyrin domain containing 3 inflammasome pathway with an increase of IL-1β, which causes venous thromboembolism(VTE)[79].
Platelets play a crucial role in coagulation and are the first blood cells that respond to endothelial damage. Coronavirus disease causes platelet hyperactivation due to Pselectin increased membrane expression, which interacts with its counter-receptors on neutrophils or other inflammatory cells, thereby activating thrombogenesis.[80]. After autopsy of patients with acute MI, many megakaryocytes and inflammatory cells are found in the microvascular system, along with venous thrombosis and platelet-rich thrombi[81]. Recent studies have shown that the antiphospholipid antibodies interact with complement factors, platelets, and endothelial cells, promoting coagulation;ongoing and future research will validate the role of antiphospholipid syndrome in COVID-19[82].
CLINICAL IMPLICATIONS
Various potential therapeutic strategies addressing specific pathophysiological mechanisms are currently used to prevent and/or alleviate the MI caused by COVID-19. Some pharmacological agents target mechanisms with definite evidence of causing cardiovascular damage, hence being part of standard of care therapy and recommended by existing guidelines, while others address hypothetical mechanisms,hence being under study.
Is it safe to continue using ACE inhibitors or angiotensin receptor blockers in COVID-19 patients?
Considering that one potential mechanism of acute MI is mediated by ACE2[83], the question arises whether therapy with ACE inhibitors (ACEIs) or angiotensin receptor blockers (ARBs) should be continued or stopped. Existing evidence-based consensus and position statements[84-87] issued by prominent cardiovascular and hypertension societies recommend against modifying this therapy if it is already underway, and to prescribe it for newly diagnosed patients as usual, given the absence of consistent evidence regarding their potential risk[88,89]. A randomized clinical study of 659 patients hospitalized with mild to moderate COVID-19 and ACEIs or ARBs therapy prior to hospitalization has shown that there was no significant difference in the mean number of days alive and out of the hospital between the patients assigned to discontinue or continue this therapy[90]. Also, a large meta-analysis of > 28000 hypertensive patients with COVID-19 on ACEIs or ARBs has found a beneficial effect of using renin–angiotensin–aldosterone system inhibitors in these patients[91].Nevertheless, additional studies are warranted to evaluate the role of ACE2 polymorphisms in conferring an increased risk of adverse outcomes, as recently disclosed by a systematic review and meta-analysis that evaluated the clinical outcomes in COVID-19 patients on ACEIs or ARBs[92].
Potential therapies for COVID-19 patients with cytokine storm mechanism
IL-6 receptor antagonists such as tocilizumab and sarilumab may represent an interesting alternative for patients with significantly elevated IL-6, ferritin, D-dimer and high-sensitivity troponin I (TnI) levels[89]. A Randomized, Embedded,Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia (REMAPCAP) has investigated the effectiveness of tocilizumab and sarilumab on survival and organ support in critically ill COVID-19 patients and it has shown improved outcomes and survival[93]. Other clinical trials are underway[94-96].
Another potential therapeutic is colchicine, due to its anti-inflammatory effect through inhibition of cytokine production and neutrophil activity, and it does not have an immunosuppressive effect compared with tocilizumab and sarilumab[97]. Several small randomized controlled trials have already shown a positive impact of adding colchicine to the standard treatment in COVID-19 patients[98-100]. Randomized trials with larger populations are in progress[101-103]. Given the high prevalence of thromboembolic anomalies and coagulopathy in patients with COVID-19, use of thromboprophylaxis may be necessary.
What are the current recommendations for antithrombotic therapy in VTE prophylaxis in patients with COVID-19?
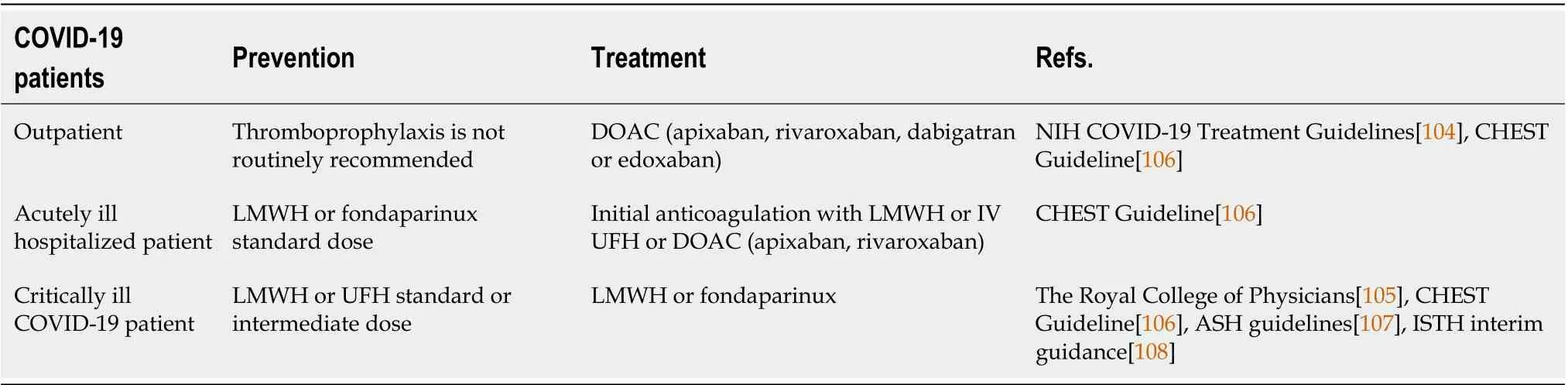
In nonhospitalized patients with mild COVID-19, anticoagulants and antiplatelet therapy are not recommended routinely[104], but should be considered depending on risk assessment[105]. For those with confirmed VTE, the CHEST guidelines recommend a direct oral anticoagulant (DOAC) with apixaban, rivaroxaban,dabigatran or edoxaban (before dabigatran and edoxaban an initial parenteral anticoagulation is needed). When a DOAC is not used, vitamin K antagonists are recommended over low-molecular-weight heparin (LMWH)[106] (Table 1).
Only a few case reports have demonstrated the presence of the genome of SARSCoV-2 in cardiac samples[4,5]. The COVID-19 virus was detected in the interstitial and endothelial cells and not necessarily in the myocytes, which emphasized the presence of lymphocyte and monocyte infiltration, and a particularly high level of monocytes causes myocardial ischemia[24,25]. Varga[27] have suggested that viral attack determines endothelium injury. This issue causes endotheliitis with the recruitment of inflammatory cells, apoptosis and pyroptosis, and subsequent microcirculatory distress[26,27]. Hence, the latest Position Statement[28] issued by the Working Group on Atherosclerosis and Vascular Biology, together with the Council of Basic Cardiovascular Science of the European Society of Cardiology acknowledges the key role of the endothelium in COVID-19-associated cardiovascular pathophysiology, and recommend that endothelial biomarkers and tests of function to be considered for early detection of cardiovascular complications.
In acutely ill hospitalized patients with COVID-19 with confirmed VTE, the CHEST guidelines recommend initial parenteral anticoagulation with LMWH or IV UFH or initial direct oral anticoagulation with apixaban or rivaroxaban (dabigatran and edoxaban can be used after initial parenteral anticoagulation)[106].
In critically ill patients with COVID-19 anticoagulant thromboprophylaxis is recommended. The CHEST guidelines are in favor of anticoagulation with LMWH or UFH over fondaparinux or a DOAC[106]. If there is any contraindication to pharmacological thromboprophylaxis, mechanical thromboprophylaxis may be considered, but it is not recommended to add it to pharmacological treatment[106].
Downregulation of ACE2 causes an increased level of angiotensin II, which induces production of inflammatory cytokines such as interferon-γ, interleukin (IL)-6, and the chemokine monocyte chemoattractant protein (MCP)-1, promoting inflammation[45-47]. MCP-1 can be an ROS source, promoting negative remodeling after MI[44,46].
In COVID-19 patients discharged from hospital, we may consider extending thromboprophylaxis for those with increased postdischarge risk of VTE and low bleeding risk[105,106,109]. The Royal College of Physicians recommends a duration of 14–28 d of thromboprophylaxis with LMWH[105]. The Anticoagulation Forum suggests using anticoagulants such as betrixaban maximum 35–42 d, rivaroxaban maximum 31–39 d or enoxaparin maximum 6–14 d[109].
水平三角形的第(4)浪之后,进入一气呵成的第(5)浪,至2015年6月7559点结束。当初将2013年6月底的低点划分作第(4)浪终点,将(4)浪D看作(5)浪1——这是常见的错误——因此才有后来的(5)浪5预期。7559点结束第(5)浪,这是循环浪III的顶点,因此后面的调整属于循环浪IV。其中,第一个浪A可能会于2019年结束,特别是当国证A指跌至上升通道的下轨时,配合波浪理论,将成为循环浪IV第一个买点。
In patients with recurrent VTE and COVID-19 despite anticoagulation with DOAC or vitamin K antagonist therapy, the CHEST guidelines recommend switching treatment to LMWH. In patients with recurrent VTE despite anticoagulation with LMWH they suggest increasing the dose of LMWH by 25%–30%[106].
Regarding antiplatelet therapy for COVID-19 patients, there are no data that would suggest any benefit of using antiplatelet agents to prevent thrombosis and we should consider the risk associated with the use of them given that a thrombocytopenic status may exist in patients with COVID-19[66,67]. Furthermore, the CHEST guidelines recommend against the use of antiplatelet agents for VTE prevention[106].
Many studies have shown that there is a high prevalence of arterial and venous thromboembolism in hospitalized patients with COVID-19 despite standard thromboprophylaxis[14]. Hence, is it possible that a higher dose of anticoagulant might be necessary? The recommendation of the intensity of anticoagulant thromboprophylaxis is not based on direct evidence of the effects of intermediate or therapeutic dose in primary prevention because of the lack of well-designed randomized clinical studies. A collaboration between three randomized clinical trial platforms ATTACC(Antithrombotic Therapy to Ameliorate Complications of COVID-19), REMAP-CAP(Randomized Embedded Multi-factorial, Adaptive Platform Trial) and ACTIV-4a(Accelerating COVID-19 Therapeutic Interventions and Vaccines) is ongoing in order to clarify this issue[113].
Potential therapies for COVID-19 patients with thrombocytopathy and endotheliopathy
As mentioned before, currently there is no specific recommendation for using antiplatelet agents in COVID-19 patients. However, according to the present understanding of the mechanisms of thrombocytopathy and endotheliopathy,targeting therapeutics to both endothelium and platelets may be effective. Considering the effects of aspirin such as antithrombotic and anti-inflammatory actions and inhibition of virus replication[114], clinical trials on the protective effect of aspirin in COVID-19 patients are underway[115,116].
(7)通过上面粒子群和遗传算法不断迭代寻优,分别得到群体最佳个体gbest_PSO和gbest_GA,信息共享,比较gbest_PSO和gbest_GA好坏,选择较好的作为PSO和GA下一代进化依据,进而获得全局最佳值global_best。
In addition to this, antithrombotic agents with vasodilatory action on vascular smooth muscle cells and anti-inflammatory action, such as prostacyclin and NO, may become a potential therapeutic alternative in patients with thrombocytopathy and endotheliopathy[80]. Clinical trials on administration of prostacyclin or NO in COVID-19 patients are in progress[117,118]. Similarly, dipyridamole, a phosphodiesterase 3 inhibitor with antiplatelet and anti-inflammatory action, could have beneficial effects in COVID-19 patients[119]. The potential therapeutic benefits are being investigated[120,121].
A recent systematic review and meta-analysis has shown that the use of statins in patients with COVID-19 has a beneficial effect on improving clinical outcomes[122].However, we must consider that elevated liver enzymes are common in patients with moderate to severe COVID-19, even though its impacts is still unknown and statin therapy should be discontinued in these patients[123,124]. Multiple clinical trials on using statins in COVID-19 patients are ongoing[116,125-127].
Experimental therapies
Various pharmacological agents aiming to limit viral entry into cells are currently under study. Previous data[128-130] have endorsed recombinant human ACE2 as an attractive therapeutic target for the current COVID-19; the molecule acting as a decoy receptor, hence curbing viral entry[83]. The efficacy of recombinant ACE2 is being investigated in a small pilot trial including patients with severe COVID-19 (Clinicaltrials.gov NCT04287686).
An alternative way of blocking SARS-CoV-2 cell invasion is inhibition of TMPRSS2 activity. Some potential therapeutic strategies targeting TMPRSS2 are already tackling COVID-19 clinically, while others are just being tested in the laboratory[131]. The former includes serine protease inhibitors such as camostat mesylate[19], which is presently considered for off-label treatment of SARS-CoV-2-infected patients (Clinicaltrials.gov NCT04321096).
CONCLUSION
MI is an important cardiovascular manifestation in COVID-19 patients associated with increased severity and high risk of mortality. At this point, the pathophysiology underlying COVID-19-related MI is not fully understood, but clinical evidence has shown that not only a direct mechanism is involved, but also SARS-CoV-2 might affect the cardiovascular system in an indirect manner through interaction with ACE2,production of cytokines, thrombocyte and endothelium dysfunction, and hypercoagulation. Elucidating the mechanisms underlying MI could help develop effective therapeutic strategies.
猜你喜欢
杂志排行
World Journal of Clinical Cases的其它文章
- Lung injury after cardiopulmonary bypass: Alternative treatment prospects
- Anemia in cirrhosis: An underestimated entity
- High tumor mutation burden indicates a poor prognosis in patients with intrahepatic cholangiocarcinoma
- Does delaying ureteral stent placement lead to higher rates of preoperative acute pyelonephritis during pregnancy?
- Management of retroperitoneal sarcoma involving the iliac artery: Single-center surgical experience
- COVID-19 pandemic changed the management and outcomes of acute appendicitis in northern Beijing: A single-center study
