N, P and S Self-doped Bamboo Charcoal as an Anode Material for Lithium-ion Batteries
2022-02-14CHENQianlinWUHong
CHEN Qianlin, WU Hong
(College of Chemistry and Chemical Engineering, Guizhou University, Guiyang 550025, China)
Abstract: The preparation process of nitrogen, phosphorus and sulfur self-doped bamboo charcoal is simple, safe and environmentally friendly, which has certain guiding significance for other biomass materials to prepare composite materials. In this research, bamboo (rich in N, P and S components) is used as a biomass-derived carbon precursor, and KOH is used as an activator, then porous bamboo charcoal (BDC-800) is prepared by activation and carbonization in a high-temperature nitrogen atmosphere at 800 ℃ while also achieving N, P and S doping. BDC-800 has a high specific surface area of 1 911 m2/g, a large pore volume of 1.21 cm3/g, and a large number of hierarchical porous structures. BDC-800 as an anode material in a lithium-ion battery shows a high storage capacity of 681.4 mAh/g at a rate of 0.50 C (1 C=372 mAh/g), good cycling stability (390.1 mAh/g at 2.00 C after 700 cycles) and excellent rate performance (754.1, 697.8, 580.2 and 403.2 mAh/g at 0.25, 0.50, 1.00 and 2.00 C, respectively). The excellent electrochemical performance of BDC-800 is attributed to its high specific surface area and hierarchical porous structure, as well as the capacitive contribution to the total charge caused by the doped N, P and S and the abundance of surface defects.
Key words: lithium-ion battery; anode; bamboo charcoal; self-doped; capacitive contribution
Energy storage technology occupies an increa-singly important position in our lives and industry. Among this technology, lithium-ion batteries (LIBs) have the advantages of a high working voltage, good cycling performance, high energy density, and environmental friendliness; thus, LIBs have become the most promising portable energy storage tech-nology[1]. LIBs are widely used in computers, watches, electric vehicles and other products, which has helped promote the rapid development of lithium batteries[2]. Graphite is the most successful commercial negative electrode material for LIBs, but it has a low theoretical capacity (372 mAh/g) and a slow charge/discharge rate. Therefore, graphite cannot meet the rapidly deve-loping market demand[3-4]. To meet market demand, researchers have been exploring different carbon materials to replace graphite.
Biomass is a carbon-rich precursor with an abundance of sources, easy access and environmental friendliness. It has high potential for use in the preparation of carbon anode materials[5-6]. The variety of available biomass, the uniqueness of their struc-tures, and their abundance of N, P, S and other components, make biomass an ideal precursor for carbon materials[7]. Ramie[8], such as loofah[9], rice husk[10], banana peel[11]and nut shells[12-13]biomass-derived carbon have shown excellent electroc hemical performances as negative electrode materials for LIBs. Bamboo is an ordinary plant, and China has abundant bamboo resources. Notably, bamboo has a short growth cycle, is easy to obtain, and is rich in N, P and S components[14]. However, untreated bamboo charcoal has poor electrochemical performance[15]. S. K. et al. grew SiO2on commercial bamboo charcoal in situ to synthesize a SiO2/C composite material, which showed high storage capacity; unfortunately, the preparation process was complicated[16]. SnO2has been grown on the surface of bamboo charcoal fibers, which are then coated with glucose carbon to obtain a BCF/SnO2/C composite material with excellent storage capacity[17]. However, tin-based anode materials demonstrate large volume changes during charge and discharge cycles, leading to the destruction of the electrode material structure and resulting in the electrode material having poor cycling stability. Porous carbon materials can alleviate the crushing effect of tin-based electrode materials, thereby improving cycle stability[18-19]. Nitrogen self-doped bamboo charcoal prepared from bamboo leaves has been used as a negative electrode material for lithium batteries to obtain good electrochemical performance[20]. Sulfur-doped bamboo charcoal has been used as a negative electrode material for potassium-ion batteries, showing good electrochemical performance[15].
When bamboo is activated and pyrolyzed at high temperature, potassium compounds can promote the further decomposition of bamboo charcoal, thereby increasing the specific surface area and porosity[21-22]. An electrode material with a high specific surface area is conducive to the rapid transport of ions and electrons. Additionally, the large pore volume allows the electrolyte to be fully saturated, allowing Li+to diffuse quickly[23]. Heteroatom (N, P, S, B, etc.) doping can improve the surface chemical properties of carbon materials and increase the number of active sites for the adsorption of Li+on the surface of carbon materials. Therefore, heteroatom doping can increase the charge/discharge rate and storage capacity of carbon anode materials[24-25]. In addition, biomass-derived carbon electrodes have two storage mechanisms for Li+: diffusion control of Li+insertion and extraction between carbon material layers and surface induction capacitance provided by the surface defects and pores of the carbon material[26-27].
This paper reports a method for preparing N, P and S self-doped bamboo charcoal. Bamboo (rich in N, P and S components) is used as the precursor of biomass carbon, and KOH is used as the activator. Bamboo is activated and carbonized at different temperatures (600, 700 and 800 ℃) and simul-taneously realizes N, P and S doping (Fig.1). BDC-800 has a specific surface area of 1 911 m2/g and a pore volume of 1.21 cm3/g, with a large number of micropores and mesopores. BDC-800 as an anode material in a lithium-ion battery shows a high storage capacity of 681.4 mAh/g at a rate of 0.50 C (1 C=372 mAh/g), good cycling stability (390.1 mAh/g at 2.00 C after 700 cycles) and excellent rate perfor-mance (754.1, 697.8, 580.2 and 403.2 mAh/g at 0.25, 0.50, 1.00 and 2.00 C, respectively). In addition, the carbon production yield (14.1% and 2.6%, respectively) of bamboo at 800 ℃ and 900 ℃ under the catalysis of KOH is explored. The unique microstructure and surface chemistry of BDC-800 is also investigated. The surface-induced capacitance behavior of BDC-800 is caused by surface defects and pores. As the scanning rate increases, the contribu-tion rate of capacitance to the total charge also increases. Among the other contributors, the capa-citance contributes to the total charge when the scan rate is 0.6 mV/s, exhibiting a rate that reaches 52.7%.
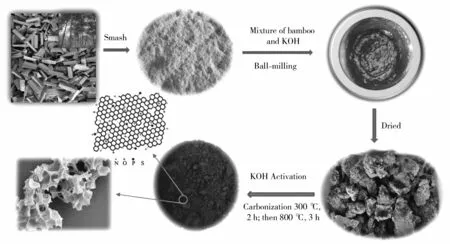
Fig.1 Schematic illustration for the preparation of BDC-800
1 Experimental section
1.1 Preparation of the bamboo-derived charcoal (BDC)
First, fresh bamboo was cleaned with ultrapure water (bamboo was taken from Zunyi, China), then the fresh bamboo was broken down into powder using a breaking machine. The bamboo powder was placed in a blast oven (80 ℃) and dried to a constant weight. The mass ratio of bamboo powder to KOH was 1 ∶1. An appropriate amount of ultrapure water was added, and then the bamboo mixed with KOH was wet ball milled for 12 h. The bamboo mixed with KOH was placed in a blast oven (80 ℃) and dried to a constant weight. Then, the bamboo powder added with KOH was placed into a flowing nitrogen tube furnace at a heating rate of 5 ℃/min. Next, the mixture of bamboo powder and KOH was carbonized at 300 ℃ for 2 h to remove the crystal water and volatile substances in the bamboo. After heating to a certain temperature (600, 700 and 800 ℃) the samples were carbonized for 3 h to obtain three bamboo charcoal samples, named BDC-600, BDC-700 and BDC-800, respectively. The bamboo charcoal samples were treated with 2 mol/L HCl solution in a water bath at 60 ℃ for 24 h and then washed with ultrapure water until reaching a neutral pH. Finally, the bamboo carbon samples were placed in a vacuum oven (80 ℃) and dried to a constant weight to obtain self-doped bamboo charcoal anode materials.
1.2 Materials characterization
X-ray diffraction (XRD, Rigaku, Japan) was performed by using a Cu Kα radiation source (λ=1.540 6 Å,1 Å=10-10nm) at 40 kV and 40 mA. Raman spectroscopy (Thermo DXR2xi) was conducted with laser excitation at 532 nm to characterize the structure of the carbon materials. Scanning electron microscopy (ΣIGMA+X-Max20 Zeiss, Germany) and transmission electron microscopy (TEM, JEM 2010 JEOL, Japan) were used to observe the morphology and microstructure of the carbon materials. A NOVA-1000 automatic analyzer (USA) was used to calculate the specific surface area by nitrogen adsorption/desorption isotherms, and the corresponding pore size distribution (PSD) was obtained by density functional theory (DFT) calculations. X-ray photoel ectron spectroscopy (XPS, ESCALAB 250XI) was performed using a monochromatic Al Ka X-ray source to measure the surface composition and chemical bonding properties of bamboo charcoal.
1.3 Cell assembly and electrochemical tests
The electrochemical performance of bamboo charcoal doped with N, P and S as anode materials for LIBs was studied. A button battery (CR-2430) was assembled in an argon-filled glove box. The prepared active substance (bamboo charcoal, 80% by mass), conductive carbon black (acetylene black, 10% by mass) and polyvinylidene fluoride (PVDF, 10% by mass) were mixed; then, N-methyl-2-pyrrolidone (NMP) was added and stirred evenly. After preparing the slurry, it was evenly coated on a copper foil current collector, and the pole pieces were dried in a vacuum oven at 80 ℃ for 12 h. The area of the circular pole piece is 1.54 cm2, and the amount of active substance loaded is 1.16 mg. A Celgard 2 400 polypropylene membrane was used as the separator, lithium metal was used as the counter electrode, and 1 mol/L LiPF6mixed with an organic solvent (ethylene carbonate (EC): dimethyl carbonate (DMC)=1 ∶1 by volume) was used as the electrolyte. The button batteries were tested on a LANHE blue battery test system (CT3001A) for charge/discharge cycle testing over a potential range of 0.01~3.00 V. Additionally, a CHI760E electrochemical workstation was used for cyclic voltammetry (CV). Electrochemical impedance spectroscopy (EIS) was performed at an amplitude of 5 mV over a frequency range of 0.01~105Hz.
2 Results and discussion
2.1 Materials characterization
KOH has very high reactivity during biomass pyrolysis, decreasing the yield of solid biomass products as the carbonization temperature increases[28-29]. As shown in Tab.1, the carbon production yield of bamboo carbonized at 800 and 900 ℃ for 3 h in a N2flow is 24.4% and 23.6%, respectively. The carbon production yields of bamboo after the addition of KOH are 14.1% and 2.6%, respectively. Regarding the production of biomass-derived carbon from bamboo, the decrease in yield is attributed to the further decomposition of solid products, which is promoted by KOH catalysis[30]. Since the carbon production rate during carbonization at 900 ℃ under the catalysis of KOH is low, it is of minor significance to continue exploring the carbon materials produced at 900 ℃; therefore, the products carbonized at 600, 700 and 800 ℃ are explored further.
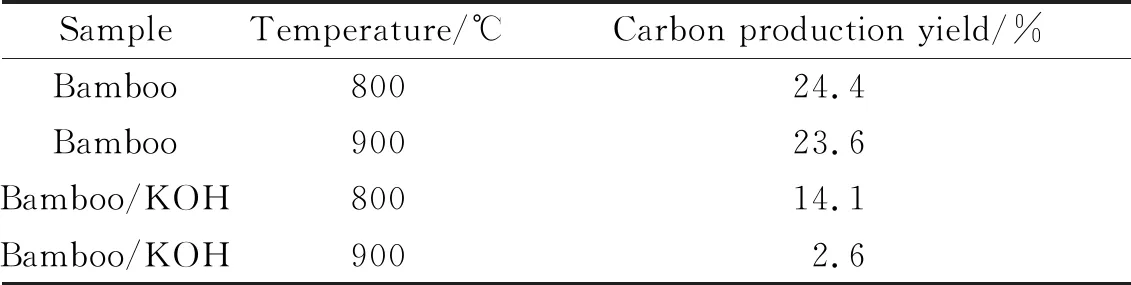
Tab.1 Carbon production yield of bamboo carbonized at 800 and 900 ℃ in a nitrogen atmosphere
Fig.2 shows the microstructures of BDC-600, BDC-700 and BDC-800, which were obtained by XRD and Raman spectroscopy. In the XRD patterns of the three samples, 2θdiffraction peaks appear at approximately 23° and 44°, corresponding to the (002) and (100) planes of the graphite carbon material (Fig.2(a)), respectively; this result indicates that the three samples are amorphous carbon[26]. Compared with the BDC-600 and BDC-700 samples, the diffraction peaks of the (002) plane of BDC-800 are shifted to the left, which indicates that the average interlayer spacing of the (002) plane of BDC-800 increases[31]. The increase in the interlayer spacing of the carbon material is conducive to the rapid transport of Li+on the surface and inside the electrode material[32]. Fig.2(b) shows the Raman diagrams of the three samples. Two peaks appear at approximately 1 340 cm-1(D band) and 1 590 cm-1(G band). The D band represents the absence of an ordered carbon structure defects. The G band represents an ordered carbon structure[33]. TheID/IGratio is used to measure the degree of disorder of carbon materials, and theID/IGvalues of BDC-600, BDC-700 and BDC-800 are 0.96, 0.98 and 1.05, respectively. The results showed that the structural defects of bamboo charcoal increased with the increase of pyrolysis temperature, which was attributed to the enhanced catalytic action of KOH, which promoted the structural defects of bamboo charcoal[34-35]. At the same time, the substitution of some carbon atoms by N, P and S promoted the structural defects of bamboo charcoal, which were the active sites of bamboo charcoal. The increase of active sites for adsorption of Li+in bamboo charcoal could lead to a larger reversible capacity[36-38].
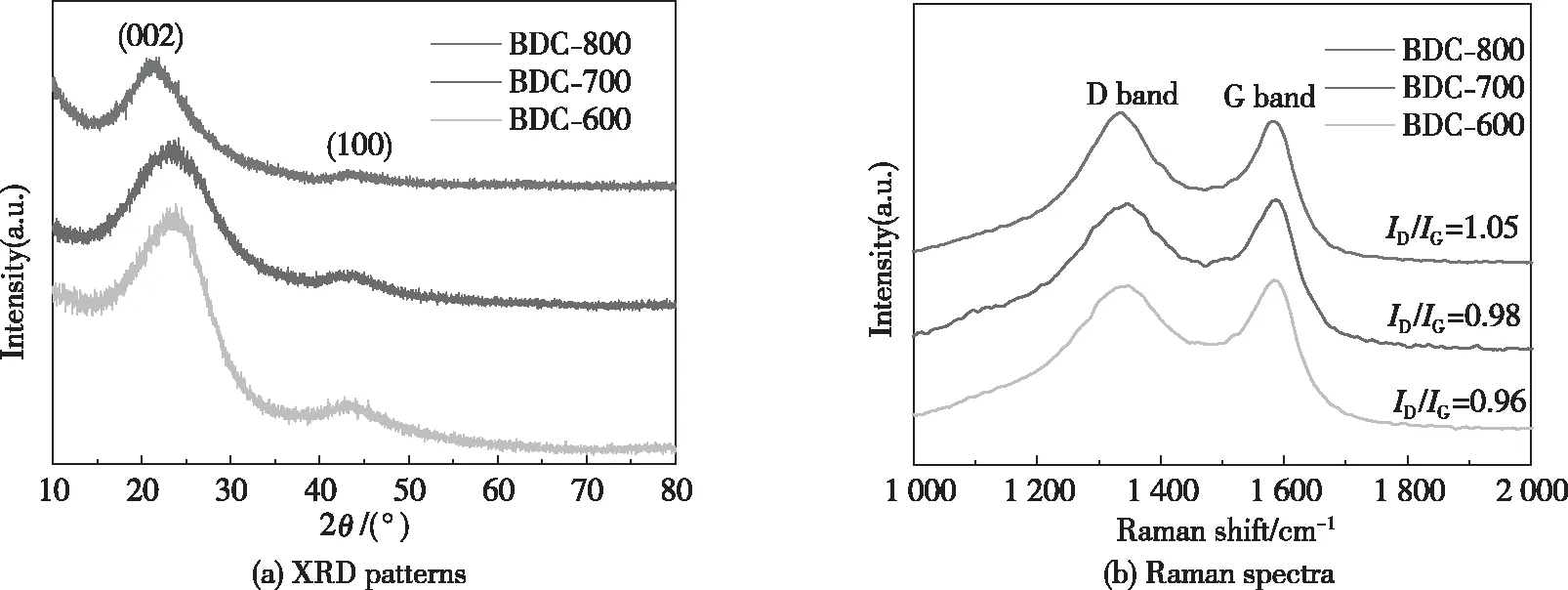
Fig.2 XRD and Raman of BDC-800, BDC-700 and BDC-600
Fig.3(a) shows the microscopic morphology of bamboo, and Fig.3(b) shows the microscopic mor-phology of bamboo charcoal obtained by the pyrolysis of bamboo.
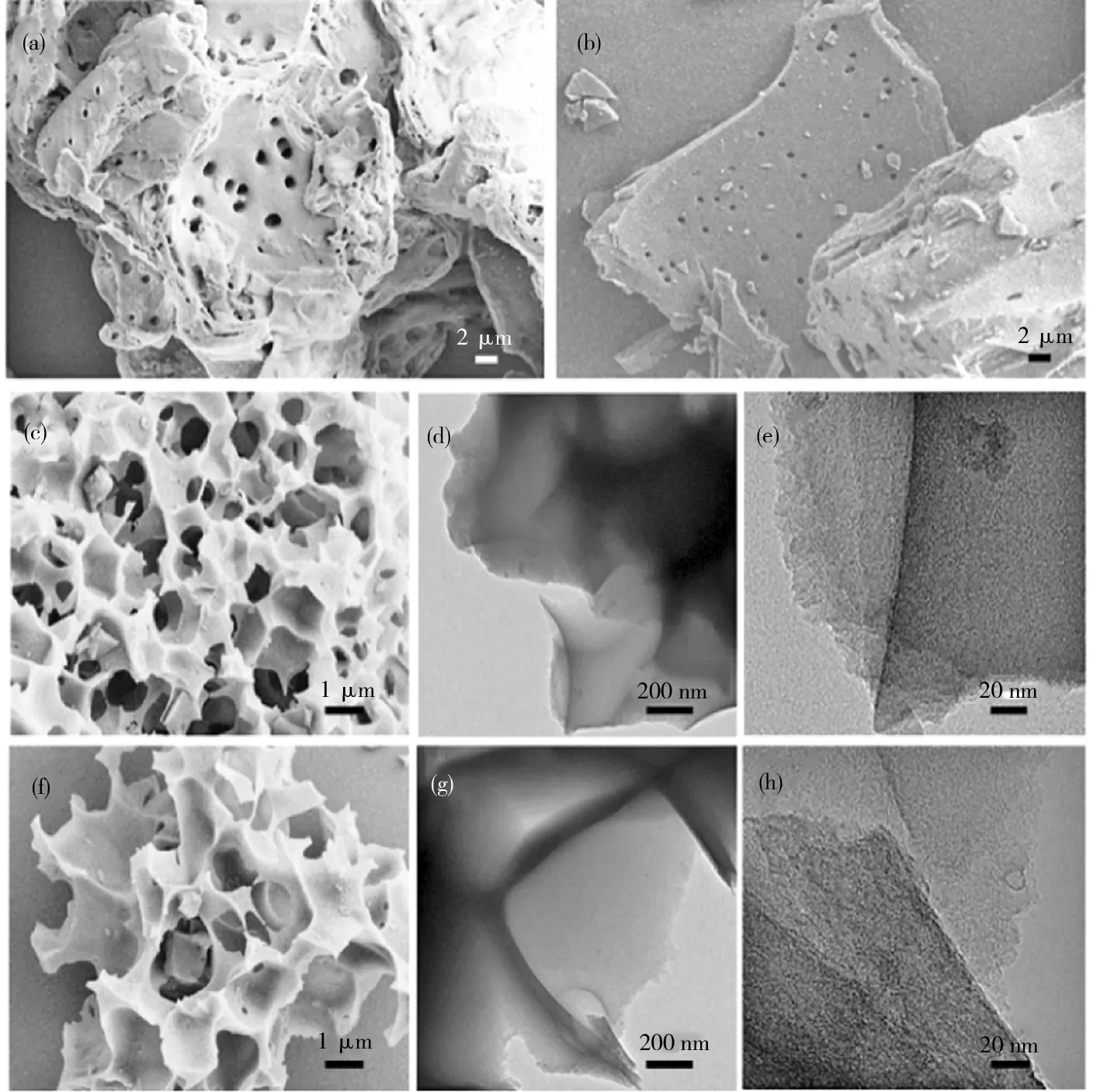
(a) SEM image of bamboo; (b) SEM image of bamboo carbonized; (c) SEM image of BDC-600; (d)—(e) TEM image of BDC-600; (f) SEM image of BDC-700; (g)—(h) TEM image of BDC-700.
As shown in Fig.4, the morphology and micro-structure of the BDC-800 sample, as well as the distribution of O, N, P and S in the carbon material, were further studied. There was no obvious change in the morphology of bamboo before and after pyrolysis. Fig.3(c), Fig.3(f) and Fig.4(a) show the morphologies of bamboo after activation and pyrolysis by the addition of KOH; notably, BDC-600, BDC-700 and BDC-800 exhibit interconnected pore struc-tures. As the pyrolysis temperature increases, the pore size expands. Furthermore, the increase in temperature enhances the catalytic effect of KOH, thus enhancing the pore formation process[29]. The interconnected porous structure shortens the Li+and electrolyte migration path, reduces the diffusion resistance, and promotes the rapid transport of Li+and electrons inside and on the surface of the electrode material[25]. Fig.4(b) shows the TEM images of the edge of the BDC-800 carbon material. The carbon material has disorderly stacked graphite-like band fringes, and its pitch is 0.413 nm (Fig.4(c)). Fig.4(e)—(i) shows the elemental mapping (EDS) of BDC-800, revealing that N, P, S and O are uniformly distributed on the carbon material.
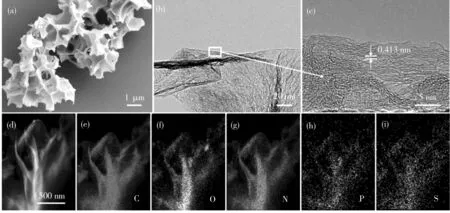
(a) SEM image; (b) TEM image; (c) HRTEM image; (d)—(i) EDS mapping images.
As shown in Fig.5, the specific surface area and pore structure of BDC-600, BDC-700, and BDC-800 were studied by nitrogen adsorption/desorption isotherms. Fig.5(a) shows that the nitrogen adsor-ption/desorption isotherms of BDC-600 and BDC-700 have type I characteristics, indicating that these samples have a characteristic microporous structure; the microporous structure plays a role in storing charges[34]. The nitrogen adsorption/desorption isotherm of BDC-800 has the comprehensive characteristics of a type I/IV material, which means that BDC-800 has both microporous and mesoporous composite structures.
The mesoporous structure in BDC-800 allows the electrolyte to fully infiltrate the material and promotes the rapid diffusion of Li+[23]. Fig.5(b) shows that the pore sizes of BDC-600 and BDC-700 are less than 2 nm, and the pore size of BDC-800 is in the range of 5.5 nm. The specific surface areas of BDC-600, BDC-700 and BDC-800, which were calculated according to Brunauer-Emmett-Teller (BET) theory, are shown in Tab.2. The bamboo charcoal specific surface area and porosity increase as the temperature increases during the activation and pyrolysis of bamboo; this result is due to the enhanced pore formation under the catalysis of KOH[29].
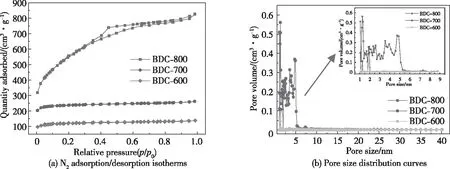
Fig.5 N2 adsorption/desorption and pore size of BDC-600, BDC-700 and BDC-800
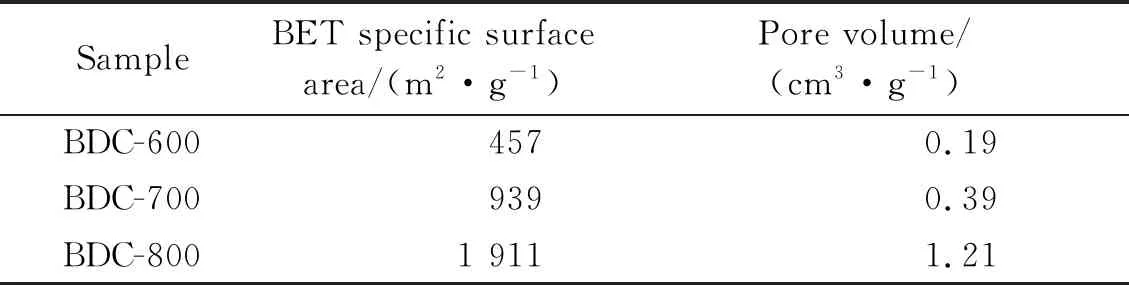
Tab.2 BET specific surface areas and pore volumes of the obtained bamboo-derived carbonized materials
The reaction process of KOH etched bamboo charcoal at different temperatures. When the pyrolysis temperature reached 400 ℃, the bamboo charcoal and KOH started the activation reaction (1). The decomposition of KOH was completed at about 600 ℃, and the formed K was inserted into the carbon layer. When the pyrolysis temperature reached 700 ℃, K2CO3began to decompose to produce K2O and CO2(2). At the same time, the generated CO2reacted with bamboo charcoal (3), resulting in escaped CO to increase the specific surface area porosity of bamboo charcoal[39]. When the pyrolysis temperature reached 800 ℃, K2CO3and K2O were thermally reduced with bamboo charcoal to metal K (4) and (5). At the same time, the bamboo charcoal was further etched to increase the specific surface area and pore volume of bamboo charcoal[29, 40].
6KOH+2C→2K+3H2+2K2CO3
(1)
K2CO3→K2O+CO2
(2)
CO2+C→CO
(3)
K2CO3+2C→2K+3CO
(4)
C+K2O→2K+CO
(5)
With the increase of pyrolysis temperature, the specific surface area and porosity of bamboo charcoal increased, which was completely consistent with the results of nitrogen adsorption/desorption isotherm. The electrode material with high specific surface area is conducive to the rapid transmission of Li+and electrons[23].The larger pore volume allows the electrolyte to fully permeate the electrode material, thus reducing the diffusion resistance of Li+in the electrode material and improving the storage capa-city[41-42]. Compared with BDC-600 and BDC-700 electrode materials, BDC-800 electrode material has the highest specific surface area and pore volume, so BDC-800 electrode material shows better electroche-mical performance.
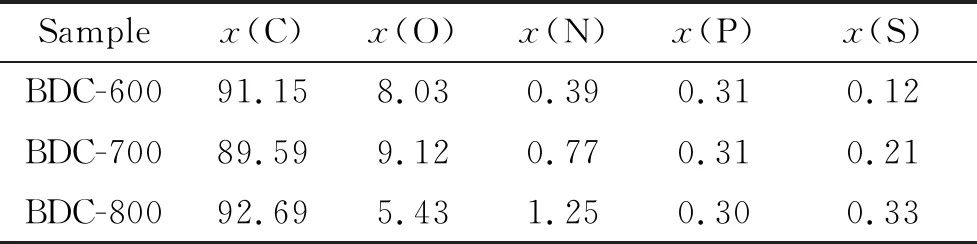
As shown in Fig.6, XPS was used to analyze the surface composition and chemical bond properties of BDC-600, BDC-700 and BDC-800 (Tab.3), N, P and S were all doped in the three kinds of bamboo charcoal. The amount of doped N and S increases as the pyrolysis temperature increases; in contrast, the amount of doped P decreases as the pyrolysis temperature increases. Due to the high thermal stability of graphite, nitrogen and nitrogen oxides, as the pyrolysis temperature increases, the amount of doped N increases[43-44]. The phosphorus-doped structural formulas (C-P, P-O, C-P-O) have poor thermal stability, and the relative content of P decreases with an increasing temperature[45]. The bond energies of S-O (521.7 kJ/mol) and S=O (523 kJ/mol) are higher than that of C-C (347 kJ/mol).
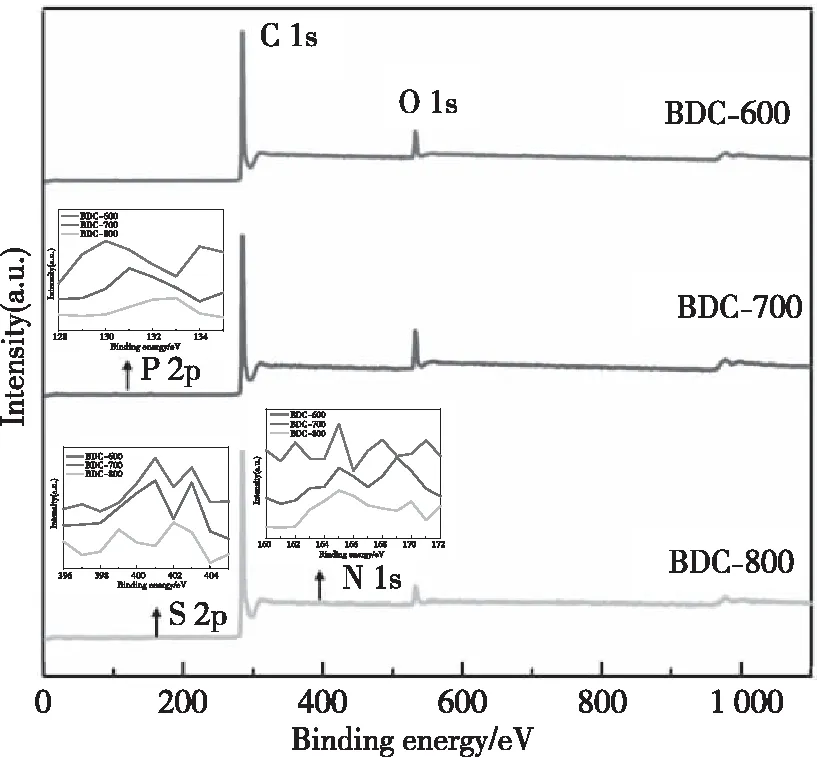
Fig.6 The total XPS spectra for all samples
Tab.3 Chemical composition analysis from XPS unit:%
As the pyrolysis temperature increases from 600 to 800 ℃, the relative content of S increases[43]. Porous carbon with residual oxygen can improve the wettability of the carbon electrode and increase its storage capacity, but it will reduce the rate performance to a certain extent[46-47]. With the increase of N, P and S contents, the structural defects of porous bamboo charcoal increased, which was completely consistent with Raman’s results. The structural defects and pores of porous bamboo charcoal lead to the capacitive control behavior and the capacitive diffusion control can contribute to the reversible capacity[19, 26-27]. The increase of active sites for adsorption of Li+in bamboo charcoal increases the reversible capacity of the bamboo charcoal electrode material.
Fig.7(a) shows that there are three peaks in the high-resolution C 1s spectrum of BDC-800: C=C/C-S (284.6 eV)[48], C-C (284.8 eV)[49], and C-O/C-N/C-P (285.5 eV)[26,36]. Fig.7(b) shows that the high-resolution N 1s spectrum of BDC-800 has four peaks: pyridinic N (398.6 eV), pyrrolic N (400.1 eV), graphitic N (401.7 eV) and oxidized N (403.3 eV)[35-38]. Hydrocarbons have a negative impact on the electrochemistry of carbon materials. Fortunately, N doping can inhibit the formation of hydrocarbons[50]. The electronegativity of N(3.04) is higher than that of C(2.55). Furthermore, N doping is beneficial to the adsorption of Li+, which increases the conductivity and storage capacity of the carbon material[20]. Fig.7(c) shows that there are three peaks in the high-resolution P 2p spectrum of BDC-800. These peaks are at 130.2, 134.2 and 133.1 eV and correspond to C-P, P-O and C-P-O[51-53], respectively. P acts as a bridge between C and O to inhibit unstable quinone and promotes the formation of carboxyl groups to maintain the electrochemical stability of the carbon surface[45]. The P group has excellent cation exchange properties, which enhances the ability of carbon materials to adsorb Li+, thus increasing their conductivity and storage capacity[54]. Fig.7(d) shows that the high-resolution S 2p spectrum of BDC-800 has two peaks: 164.1 eV (S 2p3/2) and 165.0 eV (S 2p1/2) correspond to thiophene sulfur (C-S-C) and 168.6 eV and 169.8 eV correspond to sulfoxide (C-SOx-C,x=2~4)[35,49,55]. The adsorption energy of S-doped sites is lower than that of oxygen sites; thus, S-doped sites are more conducive to Li+adsorption than O sites[15]. The self-doping of N, P and S improves the chemical properties of the bamboo charcoal surface and improves the conductivity and storage capacity of the bamboo charcoal[56-57].
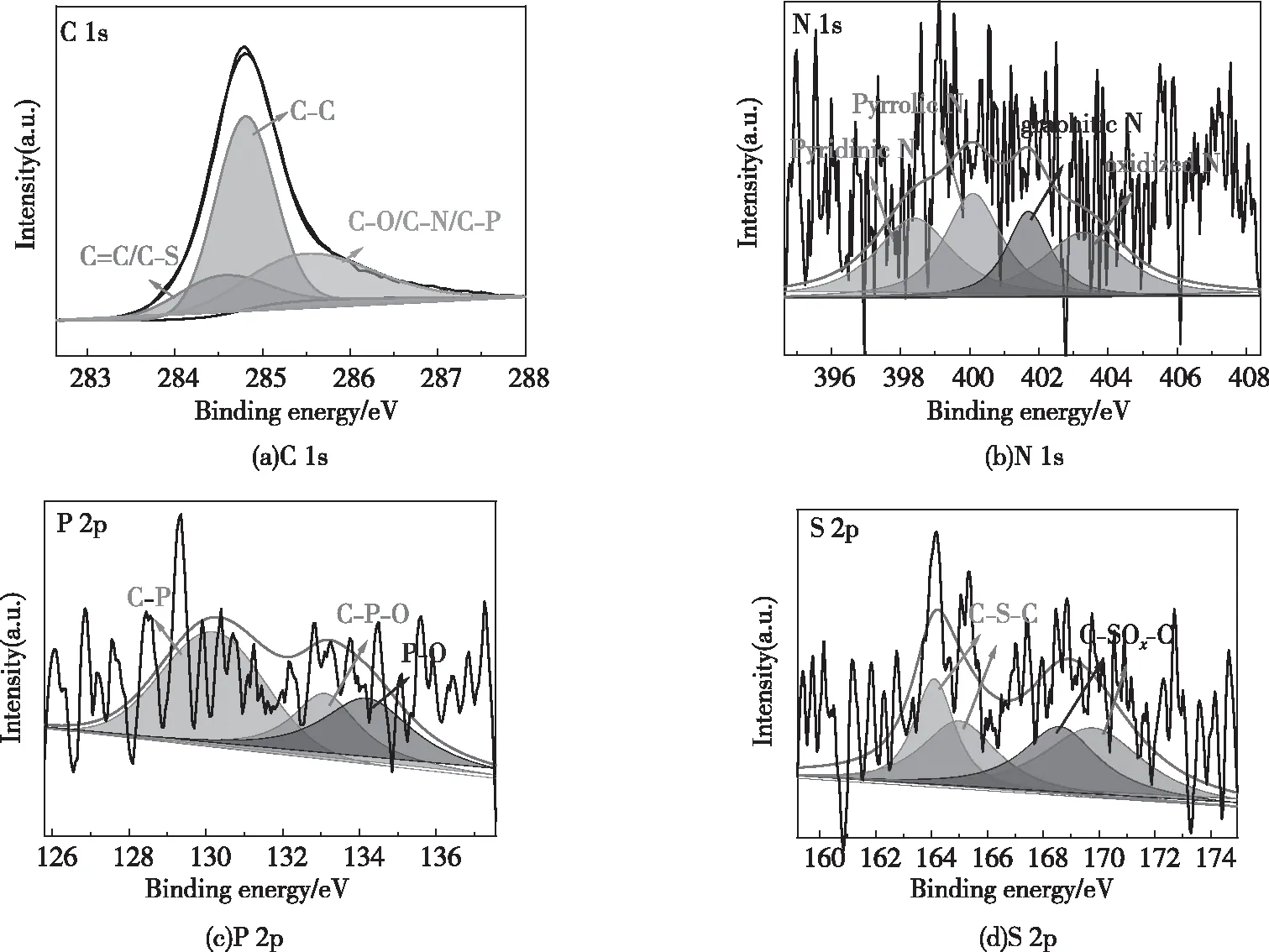
Fig.7 XPS spectra of BDC-800
2.2 Electrochemical characterization and battery performance
Fig.8(a) shows that in the potential range of 0.01~3.00 V, the battery is charged/discharged at a rate of 0.20 C for the first 3 cycles to activate the electrode material and then the battery is charged/discharged at a rate of 0.50 C until the end of the 150thcycle. The BDC-600, BDC-700 and BDC-800 samples retain storage capacities of 301.4, 368.6 and 681.4 mAh/g, respectively. The storage capacity of bamboo charcoal increases with an increasing pyrolysis temperature, which is attributed to the increase in the specific surface area and porosity of the carbon material. The self-doping of N, P and S improves the chemical properties of the bamboo charcoal surface, and the number of active sites to store Li+on the bamboo charcoal increases[24-25]; in addition, the surface defects and pores of the carbon material contribute to its capacitive behavior[26]. Compared with previously reported biomass carbon materials, BDC-800 has excellent electrochemical performance (Tab.4). The Fig.8(b) shows the first charge/discharge curves of BDC-600, BDC-700, and BDC-800. The platform observed in the charge and discharge curve is consistent with the result of the CV curve.
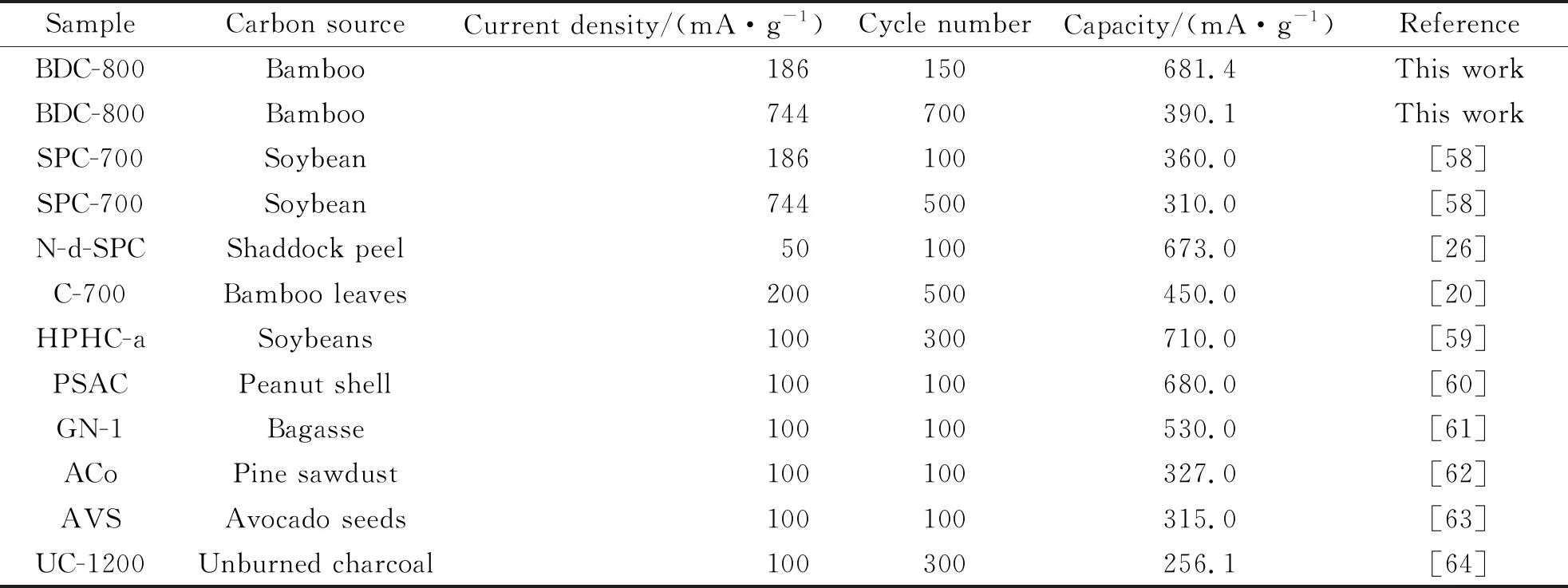
Tab.4 Comparison of the electrochemical performance of biomass-derived carbon materials for LIBs (1 C=372 mAh/g)
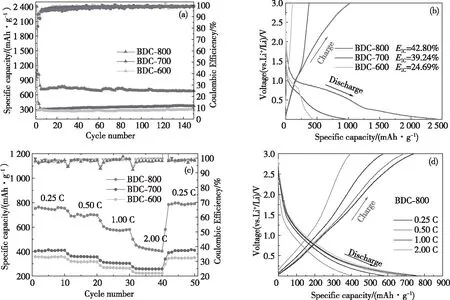
(a) Cycling performances of all samples at 0.50 C with the first 3 cycles at 0.20 C; (b) BDC-600, BDC-700, BDC-800 first charge and discharge curve; (c) Rate performances of all sample at various rates from 0.25 to 2.00 C; (d) Discharge/charge curves of BDC-800 at various rates from 0.25 to 2.00 C.
The first discharge specific capacity of BDC-600 electrode material is 433.4 mAh/g, the charge specific capacity is 107.0 mAh/g, and the first coulomb ef-ficiency is 24.69%.The first discharge specific capa-city of BDC-700 electrode material is 956.1 mAh/g, the charging specific capacity is 375.2 mAh/g, and the first coulomb efficiency is 39.24%.The first discharge specific capacity of the BDC-800 electrode material is 2 406.0 mAh/g, the charge specific capacity is 1 029.7 mAh/g, and the first Coulomb efficiency is 42.8%. During the first discharge process, the larger specific surface product leads to the formation of a larger SEI layer, which consumes more lithium ions and increases the discharge specific capacity. At the same time, due to the increase of active sites on the N, P and S doped surface, the specific discharge capacity of the porous structure electrode material is more favorable to increase[19]. The pore volumes of BDC-600, BDC-700 and BDC-800 are 0.19, 0.39 and 1.21 cm3/g, respectively (This is shown in Tab.2). The larger pore volumes can store more lithium ions, thus improving the storage capacity of electrode materials. The experimental results verified that the synergistic effect of higher specific surface area and pore volume, as well as higher content of N, P and S doped porous bamboo charcoal improved the first Cou-lomb efficiency of porous bamboo charcoal.
Fig.8(c) shows the rate performance of the BDC-600, BDC-700 and BDC-800 electrode materials. When BDC-800 is charged/discharged at rates of 0.25, 0.50, 1.00 and 2.00 C, the specific discharge capacities of the electrode materials are 754.1, 697.8, 580.2 and 403.2 mAh/g (Fig.8(d)), respectively, indicating that BDC-800 has good rate performance. When the charge/discharge rate is restored to 0.25 C, the specific discharge capacities of the BDC-600, BDC-700 and BDC-800 electrode materials are 350.8, 411.3 and 790.9 mAh/g, respectively. Compared with BDC-600 and BDC-700, the BDC-800 sample has a higher specific capacity. Notably, there is no constant voltage plateau. This curve is caused by the capacitive control behavior driven by the surface of the carbon material, indicating that there is capacitive control behavior during the charge/discharge cycles[65].
The electrochemical performance of N-, P-, and S-doped porous bamboo charcoal was studied at a scan rate of 0.1 mV/s, showing the potential change of Li+/Li insertion and extraction in the potential range of 0.01~3.00 V (Fig.9(a)). The cyclic voltammetry curves of the BDC-800 lithium battery almost overlap, indicating that BDC-800 has good cyclic reversibility. It delivers a typical CV behavior of carbon materials in lithium batteries[66].
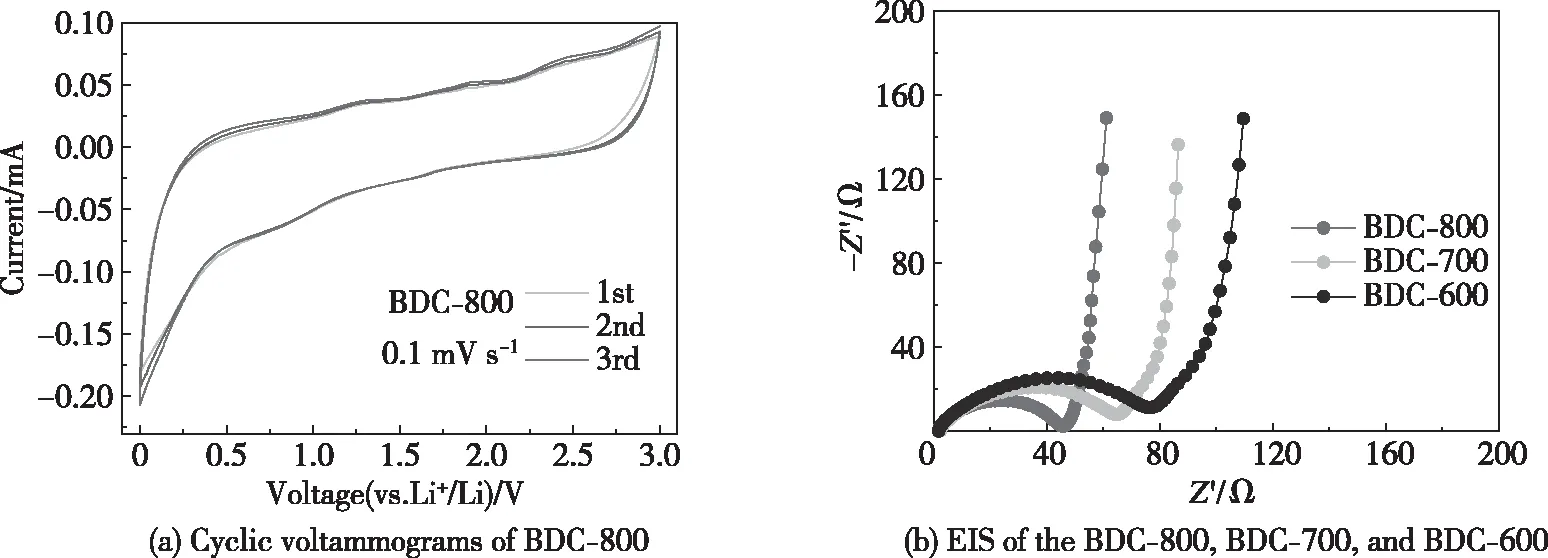
Fig.9 Cyclic voltammograms and EIS results
Fig.9(b) shows the EIS results of the BDC-600, BDC-700 and BDC-800 samples. The Nyquist plots are composed of a semicircle and diagonal lines, and the equivalent circuit model from these results is illustrated. In the equivalent circuit model,Rsis the resistance of the electrolyte;Rctis the charge transfer resistance, which is represented by the semicircle;Zwrepresents the Warburg impedance, and CPE represents the capacitance of the double-layer capacitor and passivation film. The charge transfer resistances of the BDC-600, BDC-700 and BDC-800 samples are 76, 64 and 46 Ω, respectively, which indicates that the charge transfer resistance decreases as the pyrolysis temperature increases. This is because the pore structure of the carbon material increases, the pore size becomes larger, and the interlayer spacing of the carbon material increases as the pyrolysis temperature increases. These changes shorten the migration path of Li+and the electrolyte[34], as well as promoting the doping of N, P and S. Impurities improve the electrochemical properties of a carbon material surface and increase the conductivity of the carbon material[56-57].
High-rate charge/discharge is very important for battery applications in industry and life. Fig.10 shows that within the potential range of 0.01~3.00 V, BDC-800 has 700 charge/discharge cycles at a high rate of 2.00 C. The first 3 charge/discharge cycles are at 0.20 C to activate the electrode material and then the charge/discharge cycling is conducted at a high rate of 2.00 C until the end of the 700thcycle; notably, BDC-800 still retains a storage capacity of 390.1 mAh/g. Generally, when an electrode material is charged/discharged at a high rate, the electrode structure is destroyed, resulting in a rapid decrease in the storage capacity of the electrode[67].
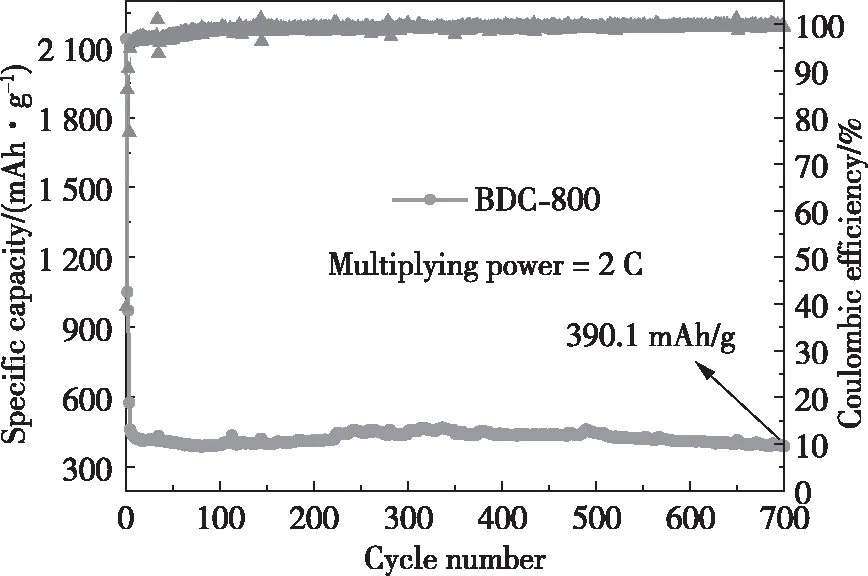
Fig.10 Cycling performance of BDC-800 at a high rate of 2.00 C
The structure change of BDC-800 electrode material during charge/discharge cycle is given. As can be seen from Fig.11, the shape of BDC-800 after 150 charge/discharge cycles still maintains the shape before the charge/discharge cycles. The results show that the storage capacity of the BDC-800 electrode material hardly attenuates after being charged/discharged at a high rate, indicating that BDC-800 has good cycling stability. Due to the expanded interlayer spacing, high specific surface area and hierarchical porous structure of BDC-800, the rapid transport of Li+is promoted on the surface and inside this carbon material[34]. The BDC-800 electrode material has high potential in high-rate charge/discharge applications.
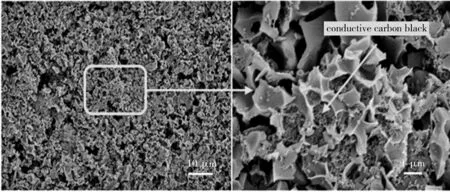
Fig.11 Morphology of BDC-800 electrode material after 150 cycles of charge and discharge
Biomass-derived carbon anode materials have two Li+storage mechanisms: diffusion behavior and capacitive behavior[68]. The contribution of capacitive behavior to the storage capacity during charge/discharge cycles is analyzed. As shown in Fig.12(a), within the potential range of 0.01~3.00 V, the BDC-800 lithium battery undergoes an increasing scan rate from 0.2 to 1.0 mV/s. Under these conditions, the CV curve of BDC-800 has a similar expansion, and the redox peak current increases as the scan rate increases, indicating that BDC-800 can quickly respond to CV scans. According to the pseudo-capacitance theory calculation proposed by P. Simon et al.[69], Fig.12(b) shows the current peak value of the redox reaction during the charge/discharge process (Fig.12(a)), and the slope of the scan rate curve (bvalue) is 0.84 and 0.80 (when 0.50 (a) CV curves at different scan rates from 0.2 to 1.0 mV/s within a potential range of 0.01~3.00 V; (b) Fitted lines and log(peak current) vs. log(scan rate) plots at different oxidation/reduction states; (c) Capacitive contribution shown by the shaded region at 0.6 mV/s; (d) Capacity contribution at different scan rates (0.2, 0.4, 0.6, 0.8 and 1.0 mV/s). Fig.12(c) shows the BDC-800 sample at a scan rate of 0.6 mV/s. The capacitance area presents a contour similar to the CV curve, indicating that the demonstrated capacitance is pseudo-capacitance. As shown in Fig.12(d), when the scan rate is 0.2, 0.4, 0.6, 0.8 and 1.0 mV/s, the contribution of the capacitance behavior to the total charge is 42.5%, 46.8%, 52.7%, 56.9%, and 62.3%, respectively. As the rate increases, the contribution rate of the capacitance behavior to the total charge increases, which is one of the reasons for the excellent electrochemical performance of BDC-800. In summary, N-, P-, and S-doped bamboo charcoal with a porous structure (BDC-800) was successfully prepared by a simple method. KOH was used as an activator. As the temperature was increased during the activation and pyrolysis of bamboo, the specific surface area and porosity of the bamboo charcoal increased; moreover, the bamboo charcoal had a large number of hierarchical porous structures. The specific surface area of BDC-800 was 1 911 m2/g, and the pore volume was 1.21 cm3/g. At the same time, N, P and S doping improved the chemical properties of the carbon material surface and increased the number of active sites for storing Li+. BDC-800 as an anode material in a lithium-ion battery showed a high storage capacity of 681.4 mAh/g at a rate of 0.50 C (1 C=372 mAh/g), good cycling stability (390.1 mAh/g at 2.00 C after 700 cycles) and excellent rate performance (754.1, 697.8, 580.2 and 403.2 mAh/g at 0.25, 0.50, 1.00 and 2.00 C, respectively). In addition, the oxidation-reduction reaction process of the BDC-800 electrode material was controlled by two behaviors: diffusion and capacitance behaviors. The capacitance behavior was caused by the surface defects and pores, and as the scanning rate increased, the contribution of the capacitance behavior to the total charge increased. BDC-800 had excellent electrochemical performance when used as a negative electrode material for LIBs. This performance was attributed to its high specific surface area and rich hierarchical porous structure, as well as the capacitive behavior caused by the doped N, P and S and the abundance of surface defects. The BDC-800 electrode material has the potential to become a next-generation lithium-ion battery anode material.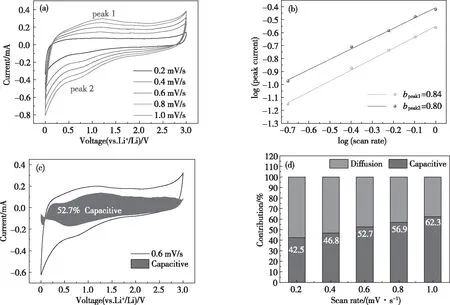
3 Conclusions
