Rational design and synthesis of upconversion luminescence-based optomagnetic multifunctional nanorattles for drug delivery
2021-12-08XuhuaLiangYanyanZhaoMinChengFeiZhang
Xuhua Liang,Yanyan Zhao ,Min Cheng ,Fei Zhang
1 College of Biology Pharmacy and Food Engineering,Shangluo University,Shangluo 726000,China
2 Shaanxi Qinling Industrial Technology Research Institute of Special Biological Resources,Shangluo 726000,China
3 Shaanxi Tasly Plants Pharmaceutical Co.,Ltd,Shangluo 726000,China
Keywords:Optomagnetic nanocomposite Nanorattle Upconversion luminescence Iron oxide Drug delivery
ABSTRACT Optomagnetic multifunctional composite based on upconversion luminescence nanomaterial is regarded as a promising strategy for bioimaging,disease diagnosis and targeted delivery of drugs.To explore a mesoporous nanostructure with excellent water dispersibility and high drug-loading capacity,a novel nanorattle-structured Fe3O4@SiO2@NaYF4:Yb,Er magnetic upconversion nanorattle (MUCNR) was successfully designed by using Fe3O4 as core and NaYF4:Yb,Er nanocrystals as shell.The microstructures and crystal phase of the as-prepared MUCNRs were evaluated by transmission electron microscopy,Xray powder diffraction and N2 adsorption/desorption isotherms.The Kirkendall effect was adapted to explain the formation mechanism of the MUCNRs.The loading content and encapsulation efficiency of doxorubicin hydrochloride (DOX) could reach as high as 18.2% and 60.7%,respectively.Moreover,the DOX loading MUCNR (DOX-MUCNR) system showed excellent sustained drug release and strong pHdependent performance,which was conducive to drug release at the slightly acidic microenvironment of tumor.Microcalorimetry was used to quantify the interactions between the carrier structure and drug release rate directly.The heat release rates in the heat-flow diagrams are basically consistent with the DOX release rate,thereby showing that microcalorimetry assay not only provides a unique thermodynamic explanation for the structure–activity relationship of Fe3O4@SiO2@NaYF4:Yb,Er MUCNRs but also provides powerful guidance to avoid the blind selection or design of drug carriers.Therefore,our work firmly provided a comprehensive perspective for using Fe3O4@SiO2@NaYF4:Yb,Er MUCNRs as a remarkable magnetic targeted drug carrier.
1.Introduction
Designing and synthesizing a multifunctional nano medical system provides a beneficial platform for diagnosing and treating diseases[1,2].In particular,optomagnetic multifunctional composites based on upconversion luminescence (UCL) nanomaterials have become a research hotspot in material and biomedicine field because of its promising application prospects in bioimaging,analysis and detection,disease diagnosis,and targeted administration[3,4].On the one hand,rare earth-doped UCL materials possess an extensive application prospect in biomedicine field because of its strong tissue penetrating power,free damage to biological tissues,no background florescence interference,high sensitivity,high signal-to-noise ratio and high stability[5–7].For example,Phuong et al.[8] synthesized a novel folic acid functionalized NaYF4:Yb3+,Er3+nanophosphors for biomedical application.These nanophosphors are emitted in red color with the strongest band at 650 nm under 980 nm excitation.This result could provide NaYF4:Er3+,Yb3+@silica-N=FA complex for developing fluorescence label and image tool in cancer biology and medicine.Zou et al.[9] reported a novel nanotheranostic agent for dual-mode imaging-guided cancer therapy based on europium complex-grafted-oxidative dopamine.
On the other hand,with superparamagnetism,high saturation magnetization,high magnetic susceptibility,and good biocompatibility,magnetic Fe3O4NPs is widely applied in magnetic separation,magnetic resonance imaging,and targeted administration[10–12].Liu et al.[13] prepared a targeted therapeutic drug delivery system based on Fe2O3/Fe3O4@mSiO2-HA nanocomposites.The results showed that the magnetic nano-system exhibited good magnetic targeting,active hyaluronic acid targeting,and had the potential to provide a targeted delivery platform for many antitumor drugs.Guan et al.[14]reported a dual-functional platform that was realized through an amide reaction between high-fluorescence carbon quantum dots (CD) and amino-functional Fe3O4@SiO2to obtain multifunctional magnetic fluorescent nanocomposite particles Fe3O4@SiO2-CDs.Magnetic targeting experiments showed that bifunctional magnetic nanoparticles have an outstanding magnetic targeting effect on tumors.
Moreover,multifunctional nanocomposites with hollow structure are often used in drug carrier,cell imaging,photodynamics,photothermal treatment and other fields because of its spherical morphology and relatively high specific surface area and cavity(pore) volume [15,16].Currently,many studies on the design and synthesis of hollow nanoparticles have been proposed,and great research progresses have been achieved.Template method,which includes hard and sacrificial template methods,is the most common synthesis approach.The former often uses a certain size of silicon balls,carbon spheres,and polystyrene microsphere as template,and one layer of the desired nanocrystals is grown uniformly on the nanosphere surface [17–19].Finally,internal templates are eliminated through a certain mean,thereby obtaining hollow nanoparticles.The morphology and size of hollow nanoparticles can be controlled by templates.However,this method has some disadvantages,such as low yield of hollow nanoparticles,poor mechanical properties,and easy fracture [20].Furthermore,hard template method claims a very complicated process of template removal,and changing structure and surface properties of hollow nanoparticles is easy[21].Therefore,the sacrificial template method based on Kirkendall effect has attracted wide attention from researchers.It is accepted as an effective method to synthesize inorganic hollow nanospheres [22].Nevertheless,it still has great challenges to synthesize multifunctional and size-controllable porous structured nanoparticles with specific surface properties.
In this study,a kind of rattle-structured optomagnetic multifunctional nanocomposites was designed by Kirkendall effect.Magnetic Fe3O4@SiO2nanoparticles were the nucleus of this composite.Its external shell was composed of UCL NaYF4:Yb,Er nanocrystals.A large cavity can be found between the nucleus and the shell.Therefore,a nanorattle integrated with targeted drug delivery,drug tracking,and drug-controlled release was formed.In the synthesis,magnetic Fe3O4NPs were first coated with a layer of SiO2and later with another layer of Y2O3:Yb,Er nanocrystalline.The role of silica coating is to prevent dissolution damage of Fe3O4in the follow-up reactions and acid environment in living bodies.The Fe3O4@SiO2@NaYF4:Yb,Er magnetic upconversion nanorattles (MUCNRs) were prepared in the ion exchange process by using Y2O3:Yb,Er nanocrystals as the template.The formation mechanism,superparamagnetism and UCL properties of this composite were discussed thoroughly in this study.Key attentions were paid to drug-loading and controlled release performance by using doxorubicin hydrochloride (DOX) as the model drug.
2.Materials and Methods
2.1.Materials
FeCl3.6H2O and FeSO4.7H2O were supplied by Hengxing Chemical Reagent Co.Ltd.Y(NO3)3.6H2O (99.99%),Yb(NO3)3.5H2O(99.99%),and Er(NO3)3.6H2O (99.99%) were purchased from Xiya Chemical Technology Co.,Ltd.DOX was purchased from Shanghai Hualan Chemical Co.,Ltd.Cetyl trimethyl ammonium bromide(CTAB) and tetraethyl orthosilicate (TEOS) were purchased from Yili Chemical Co.,Beijing.All other reagents were of analytical reagent grade.
2.2.Preparation of Fe3O4@SiO2 NPs
Superparamagnetic Fe3O4NPs were prepared by coprecipitation method.The detailed process was introduced as follows:6.1 g FeCl3.6H2O and 4.2 g FeSO4.7H2O were added into a round bottom flask with 100 ml distilled water and dissolved by electromechanical stirring under the protection of N2.The mixture was heated to 90 °C.Subsequently,10 ml stronger ammonia water was injected quickly and stirred mechanically.The mixture was first kept in constant-temperature (90 °C) bath for 30 min and then cooled to room temperature.Black magnetic particles were separated by magnet and then rinsed and precipitated by distilled water for several times until the washing liquid became neutral.All black particles were scattered in 100 ml distilled water through ultrasonic dispersion.Under rapid mechanical stirring,2 ml citric acid solution (0.5 g.ml-1) was added in.The mixture was heated to 95 °C,reacted for 90 min,cooled in air to room temperature,separated and precipitated,rinsed by distilled water for several times,dried and precipitated in vacuum,and stored in a dryer.
The improved Stöber method was applied to prepare Fe3O4@-SiO2NPs [23].The process was introduced as follows:20 ml distilled water,1 ml 25% ammonium hydroxides,and 80 ml ethyl alcohol were added in a 250 ml round bottom flask.Next,0.1 g Fe3O4NPs was added in and scattered through ultrasonic dispersion.After uniform dispersion,the mixture was stirred mechanically under 25 °C for 30 min.Next,0.4 ml TEOS was added in slowly while stirring the mixture strongly.With stirring for another 6 h,the mixture was separated.Products were collected and rinsed by ethyl alcohol and water for three times and then dried for 12 h under 80 °C.
2.3.Preparation of Fe3O4@SiO2@Y2O3:Yb,Er NPs
The preparation of Fe3O4@SiO2@Y2O3:Yb,Er NPs was completed in two steps.First,a layer of amorphous Y(OH)CO3:Yb,Er was synthesized on the surface of Fe3O4@SiO2NPs.Second,amorphous Y(OH)CO3:Yb,Er was calcined and transformed into Y2O3:Yb,Er nanocrystals.The specific operation process was:10 ml of RE(NO3)3solution (0.1 mol.L-1,RE=78% Y+20% Yb+2% Er) and 6 g urea were dissolved into 250 ml distilled water and stirred continuously for 20 min.Later,0.05 g Fe3O4@SiO2NPs was added in and stirred mechanically for 30 min.Next,the mixture was heated to 90 °C and stirred mechanically for another 2 h.Products were separated and collected,and then rinsed by ethyl alcohol and water.Next,products were dried under 80°C for 12 h and then calcined under 550°C for 2 h(heating rate=2°C.min-1),thus obtaining Fe3O4@SiO2@Y2O3:Yb,Er NPs.
2.4.Preparation of MUCNRs
A total of 0.1 g NaF and 3 ml HF (0.1 mol.L-1) were dissolved into 8 ml distilled water and stirred for 5 min.Next,0.05 g Fe3O4@-SiO2@Y2O3:Yb,Er NPs was added in and stirred mechanically for 5 min.Later,the mixture was heated to 80 °C and reacted for 2 h.The reaction solution was cooled to room temperature in air.Products were separated and collected and then rinsed by ethyl alcohol and water for three times.All products were dried in vacuum under 80 °C for 12 h.In this way,the MUCNRs of Fe3O4@-SiO2@NaYF4:Yb,Er could be obtained.
2.5.Characterization
The crystal structures of the samples were investigated by XRD(Bruker)with CuKα radiation(λ=0.15405 nm).Transmission electron microscopy (TEM) images were obtained using FEI Tecnai G2 F20 S-Twin with a field emission gun that operated at 200 kV.The specific surface area and porous structural characteristics were tested by N2adsorption/desorption isotherms by Micromeritics ASAP 2020 M apparatus.The up-conversion emission spectra were obtained by using a 980 nm laser diode as the excitation source.The magnetic properties of the nanocomposites were investigated by superconducting quantum interference device magnetometer at 300 K.The thermodynamic change in the drug loading and release process was detected by a Setaram C80 calorimeter using membrane-mixing cells at 37 °C under atmospheric pressure.
2.6.Cytotoxicity test of Fe3O4@SiO2@NaYF4:Yb,Er MUCNRs
In this study,L929 fibroblast cells were used as detection cells to test the cytotoxicity of sample materials.L929 fibroblast cells were cultured in RPMI media without folic acid (FFRPMI).This media contains 10% heat inactivated fetal calf serum (HIFCS),100 IU.ml-1penicillin,and 100 μg.ml-1streptomycin.The culture environment was set to 37°C,5%CO2,and 95%air atmosphere.The cytotoxicity of Fe3O4@SiO2@NaYF4:Yb,Er MUCNRs were tested by MTT method [24,25]:200 μl L929 fibroblast cells were inoculated into 96-well culture plates (5000 cells/well) and cultured clinging to walls overnight under 37 °C and 5% CO2.Fresh FFRPMI media with different MUCNRs concentrations (200 μl) were changed.Each group of experiment set 5 repeated wells and cultured continuously for 24 h.Next,200 μl FFRPMI media were changed and 20 μl freshly prepared 5.0 mg.ml-1MTT solution was dipped in.Samples were cultured for another 3 h,and media in wells were then eliminated.Meanwhile,150 μl DMSO was added in and oscillated for 10 min on a shaking table to realize the full dissolution of crystals.The absorbance of different holes at 490 nm was measured by enzyme-linked immuno sorbent assay.
But I tell you what. If you want to buy five of them, I won t complain too loudly about that. You can t expect me to give up all my wonderful sculptures to some stranger who left a 60 foot crane in my back yard.
2.7.Study on drug loading and controlled-release performance
Drug loading and controlled-release performance of Fe3O4@-SiO2@NaYF4:Yb,Er MUCNRs were investigated by using DOX as model drugs.A total of 10 mg Fe3O4@SiO2@NaYF4:Yb,Er MUCNRs were collected and scattered into 3 ml 1.0 mg.ml-1DOX PBS through ultrasonic dispersion (pH=7.4).The mixture was stirred under room temperature until reaching an equilibrium state.Later,the mixture was centrifuged,and the DOX-loaded samples were gained,which was recorded as DOX-Fe3O4@SiO2@NaYF4:Yb,Er MUCNRs.Supernatant and washing liquid were collected,and absorbance at 492 nm was measured.The drug encapsulation efficiency (EE,%) and drug loading content (DLC,%) were calculated through Eqs.(1) and (2),respectively.

Drug releasing experiment in vitro could be carried out as follows:the DOX-loaded MUCNRs were immersed into 2 ml PBS with pH=7.4 and pH=5.The mixture was stirred slowly for a certain period in the dark under 37 °C and then centrifuged.The absorbance of supernatant was tested at 492 nm,and the released DOX content was calculated by the standard curve.If the supernatant concentration exceeds the linear range of standard curves,then the supernatant was diluted for certain times to control the concentration within the linear range of the standard curve.Each group of experiments was repeated for three times.
3.Results and Discussion
3.1.Morphology and synthetic mechanism of Fe3O4@SiO2@NaYF4:Yb,Er MUCNRs
The synthetic process of Fe3O4@SiO2@NaYF4:Yb,Er MUCNRs was shown in Fig.1.This process was mainly divided into four steps:
First,a layer of SiO2was coated onto the surface of Fe3O4NPs through the improved Stöber method to protect Fe3O4NPs.TEM images of Fe3O4NPs and Fe3O4@SiO2NPs were shown in Fig.2.Fe3O4was non-uniform with particle size of no more than 10 nm because the nucleation and growth processes of the particles were affected by the equilibrium of hydrolysis.In addition,each Fe3O4@SiO2NPs contained several Fe3O4NPs.As shown in Fig.2C,the Fe3O4@SiO2NPs possessed a relatively narrow particle size distribution,which had an average particle size of(63±18)nm.A thick SiO2coating could prevent the complete corrosion of SiO2by excessive HF in the ion exchange process,and the residual SiO2layer could protect Fe3O4NPs and assure the magnetisms of the final products.
Second,a layer of Y(OH)CO3:Yb,Er was coated onto the Fe3O4@SiO2NPs surface by a uniform precipitation process.Under 90 °C,two chemical reactions (a and b) of urea occurred,and metastable-state supersaturated solution was formed gradually.This metastable-state intermediates would develop the chemical reaction (c) immediately and generate abundant Y(OH)CO3:Yb,Er to cover on the surface of Fe3O4@SiO2NPs.As shown in Fig.3,all the diffraction peaks of Fe3O4@SiO2@Y(OH)CO3:Yb,Er were consistent with Fe3O4@SiO2NPs,indicating that the surface Y(OH)CO3:Yb,Er belongs to the amorphous form.
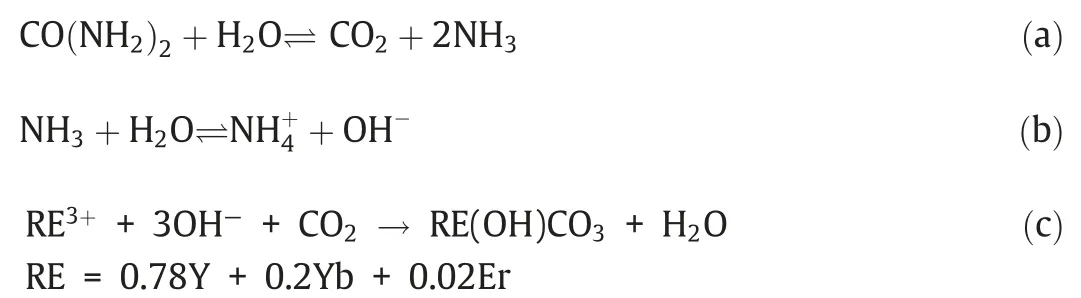
Third,the prepared Fe3O4@SiO2@Y(OH)CO3:Yb,Er was calcined under 550 °C for 2 h to obtain Fe3O4@SiO2@Y2O3:Yb,Er (heating rate=2 °C.h-1).In this process,the decomposition reaction (d)occurred,and Y2O3:Yb,Er nucleus began to form.According to previous report,this nucleation process and the growth process of crystal nucleus were uneven [26].Thus,the final outer layer of Y2O3:Yb,Er was formed by gathering nanocrystals in different sizes.Y2O3:Yb,Er possessed a porous secondary structure because of the spaces among different nanocrystals.The XRD pattern of Fe3O4@SiO2@Y2O3:Yb,Er was showed in Fig.3.In addition to the weak diffraction peak of Fe3O4,all other diffraction peaks could be attributed to peaks of cubic phase Y2O3,indicating that the Y2O3:Yb,Er nanocrsytal has pure cubic-phase structure.

Fourth,the outer layer of Y2O3:Yb,Er was transformed into α-NaYF4:Yb,Er through ion exchange,and nanorattle-structured Fe3-O4@SiO2@NaYF4:Yb,Er MUCNRs could be formed by Kirkendall effect.In the reaction,the temperature,reaction time,and HF dosage had to be controlled strictly.Moreover,the porous secondary structures of Fe3O4@SiO2@Y2O3:Yb,Er were vital to the ion exchange process.Diffusion rate of ions in this polycrystalline structure was significantly higher than that in Y2O3single-crystal structure,which accelerated the ion exchange reaction and formed a hollow structure.Hence,Y2O3:Yb,Er polycrystalline structures,which were formed by gathering of nanocrystallines with different sizes,became an excellent template to synthesize the heterostructure of α-NaYF4:Yb,Er.TEM images in Fig.4 showed evident nanorattle structure of the nanoparticles after ion exchange,and the particle size was approximately 130 nm.The external α-NaYF4:Yb,Er polycrystalline layer was about 25 nm,and its internal structure was composed of Fe3O4@SiO2nucleus.Since excessive HF could trigger uneven etching of internal SiO2layer,the particle size of the internal Fe3O4@SiO2nucleus was uneven.However,the SiO2layer was etched uncompletely,and it could meet requirements of protecting Fe3O4.Fig.3 showed that the XRD diffraction peaks of Fe3O4@SiO2@NaYF4:Yb,Er MUCNRs all belonged to those of α-NaYF4and Fe3O4.However,no diffraction peak of Y2O3was identified,indicating the complete transformation of Y2O3:Yb,Er into α-NaYF4:Yb,Er in the ion exchange process.

Fig.1.The schematic illustration of synthetic procedure of the Fe3O4@SiO2@NaYF4:Yb,Er MUCNRs.

Fig.2.TEM images of (a) Fe3O4 NPs,(b) Fe3O4@SiO2 NPs and (c) particle size distribution of Fe3O4@SiO2 NPs.

Fig.3.XRD patterns of the Fe3O4 NPs,Fe3O4@SiO2@Y(OH)CO3:Yb,Er NPs,Fe3O4@SiO2@Y2O3:Yb,Er NPs and Fe3O4@SiO2@NaYF4:Yb,Er MUCNRs.

Fig.4.TEM images of Fe3O4@SiO2@NaYF4:Yb,Er MUCNRs.
Controlling the concentration of HF in ion exchange is also crucial because it could react with SiO2.SiO2could be sacrificed completely if a severe dose of HF,which could further trigger reaction with Fe3O4NPs and destroy magnetism of products,was observed.Therefore,a small overdose of HF was chosen in this study for the complete reaction of Y2O3.Although this HF concentration could decrease the thickness of the SiO2layer to some extent,it was not etched completely.The residual SiO2layer could still meet the requirements of protecting Fe3O4NPs.
In addition,maintaining similar crystal structure between parent and final products was crucial to keep the heterogeneous structure and morphology of nanoparticles [27].α-NaYF4:Yb,Er nanocrystals had similar crystal structure with the cubic-phase Y2O3nanocrystals,which could help Y2O3nanocrystals to maintain the original spherical morphology when they were transformed into α-NaYF4:Yb,Er nanocrystals through ion exchange.However,reaction conditions should be controlled strictly to prevent the transformation from α-NaYF4:Yb,Er to β-NaYF4:Yb,Er.If the reaction temperature and reaction time were increased,α-NaYF4:Yb,Er would be transformed into β-NaYF4:Yb,Er which had more thermodynamic stability.This would lead to changes in the spherical morphology of the final products.Fig.5(c) and (d) showed that when the reaction time was prolonged to 4 h or the reaction temperature was increased to 130 °C,the synthesized nanoparticles would develop a diffraction peak of β-NaYF4.This circumstance was disadvantageous in maintaining the nanoparticle structure and morphology.
Fig.5.XRD patterns of the Fe3O4 NPs (a),Fe3O4@SiO2@NaYF4:Yb,Er MUCNRs (b),products synthesized in the presence of HF and NaF solution with continuous stirring at 80 °C for 4 h (c) and 130 °C for 2 h (d).
3.2.Test of specific surface area and porous structural characteristics
The specific surface area and porous structural characteristics were tested by N2adsorption/desorption method.The adsorption/desorption isotherm was shown in Fig.6,which presented the structural characteristics of a typical IV-shaped curve.Moreover,the adsorption and desorption curves enclosed an evident H1hysteresis loop,indicating that the product after ion exchange was a typical porous material.According to calculation,the specific surface area,pore volume,and average pore size were 82.775 m2.g-1,0.35 cm3.g-1,and 7.8 nm,respectively.Good porous structure and relatively high specific surface and porous volume all demonstrated the considerable application potential of Fe3O4@-SiO2@NaYF4:Yb,Er MUCNRs in drug delivery.
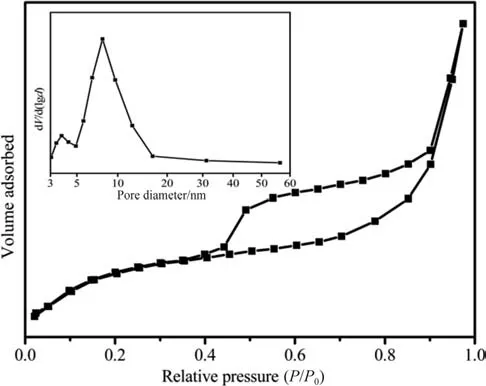
Fig.6.N2 adsorption/desorption isotherms and pore size distribution curves(inset)of the as-obtained Fe3O4@SiO2@NaYF4:Yb,Er MUCNRs.
3.3.Research on UCL properties
Rare-earth ion-doped UCL materials had been studied extensively because of their unique optical properties that enable high penetration depth in tissues under NIR laser excitation and minimize autofluorescence and photo damage.Under 980 nm nearinfrared light excitation,Fe3O4@SiO2@NaYF4:Yb,Er MUCNRs presented strong red light emission.As shown in Fig.7,the MUCNRs had three emission peaks at 522,542 and 656 nm,which were generated by the transitions of Er3+from three excited-state energy levels of2H11/2,4S3/2,and4F9/2to the ground state level (4I15/2)[28].Due to the excellent UCL properties,Fe3O4@SiO2@NaYF4:Yb,Er MUCNRs realized drug tracking and monitoring in the drug delivery process.
3.4.Magnetic properties of the as-prepared NPs
The hysteresis loops of Fe3O4,Fe3O4@SiO2NPs,Fe3O4@SiO2@-NaYF4:Yb,Er MUCNRs,and DOX-MUCNRs,as well as magnetic separation pictures of DOX-MUCNRs under the action of an external magnetic field,were shown in Fig.8.The hysteresis loops of all samples had no coercive forces and remanence but showed good superparamagnetism.The saturation magnetization intensities of Fe3O4NPs and Fe3O4@SiO2NPs were 72.4 A.m2.kg-1and 33.5 A.m2.kg-1,respectively.The saturation magnetization intensities of Fe3O4@SiO2@NaYF4:Yb,Er MUCNRs before and after loading of DOX were 15.8 A.m2.kg-1and 12.3 A.m2.kg-1,respectively.In addition,DOX-MUCNRs responded well to the external magnetic field after being dispersed in PBS.Thus,the magnetism test results demonstrated that the composites had promising application prospects in magnetic targeting and bioseparation.

Fig.7.The UC emission spectrum of Fe3O4@SiO2@NaYF4:Yb,Er MUCNRs under 980 nm NIR laser excitation.

Fig.8.The magnetic hysteresis loops of Fe3O4 NPs,Fe3O4@SiO2 NPs,Fe3O4@SiO2@NaYF4:Yb,Er MUCNRs and DOX-Fe3O4@SiO2@NaYF4:Yb,Er MUCNRs.Inset is the separation process of DOX-Fe3O4@SiO2@NaYF4:Yb,Er MUCNRs by a magnet.
3.5.In vitro cytotoxicity of MUCNRs
Nontoxicity refers to the premise to use nanoparticles in drug delivery.In the present study,the cytotoxicity of Fe3O4@SiO2@-NaYF4:Yb,Er MUCNRs was investigated by MTT method.As shown in Fig.9,the cell viability of L929 fibroblast cells exceeded 90%even at a high-dose concentration (400 μg.ml-1) and changed slightly at different concentrations,indicating that the cytotoxicity of MUCNRs composite can be neglected.Thus,the MUCNRs was a kind of nontoxic carrier materials.
3.6.Study on drug loading and controlled-release performance

Fig.9.In vitro cell viability data of cultured L929 fibroblast cells after incubation with Fe3O4@SiO2@NaYF4:Yb,Er MUCNRs for 24 h using standard MTT method.
Based on above analysis,Fe3O4@SiO2@NaYF4:Yb,Er MUCNRs had relatively large specific surface area,cavity volume,appropriate size distribution,and good dispersion.Therefore,Fe3O4@SiO2@-NaYF4:Yb,Er MUCNRs possessed considerable application potentials in drug delivery.This study investigated the drug loading and controlled-release performance by using DOX as a model drug.
In this study,10 mg Fe3O4@SiO2@NaYF4:Yb,Er MUCNRs were scattered into PBS solution that contained 3 ml 0.1 mg.ml-1DOX(pH=7.4),and the mixture was stirred under room temperature for 24 h.EE(%) and DLC(%) amounted to 60.7% and 18.2%,respectively.This drug loading behavior could be analyzed through the interaction of DOX and MUCNRs.Considering that the isoelectric point of DOX was approximately 8.3,DOX was in the protonation state when pH was 7.4.In the experiment,the Zeta potentials of Fe3O4@SiO2@NaYF4:Yb,Er MUCNRs were tested as -5.2 mV when pH=7.4.Therefore,protonation DOX could form electrostatic force with the drug carrier,thereby promoting drug delivery.The sustained drug release efficiency was shown in Fig.10.The MUCNRs system showed excellent sustained drug release performance and strong pH dependence.Drug release was relatively slow when pH=7.4,but it was accelerated significantly when pH=5.0,and the releasing amount reaches 63%in 12 h.According to test results,the Zeta potentials of Fe3O4@SiO2@NaYF4:Yb,Er MUCNRs were+35 mV,and DOX could be further protonized when pH=5.0.As a result,DOX and MUCNRs produced electrostatic repulsive force to promote drug release.As a pH-responsive drug controlledrelease system,this drug releasing system could release drugs quickly in a slightly acid microenvironment of tumors to achieve targeted cancer treatment.Therefore,Fe3O4@SiO2@NaYF4:Yb,Er MUCNRs could be used as a kind of high-quality anti-tumor drug carrier material.

Fig.10.Cumulative DOX release from the DOX-Fe3O4@SiO2@NaYF4:Yb,Er MUCNRs systems in the release media of PBS with different pH values (7.4 and 5.0).
3.7.Thermodynamic behavior of drug loading and release performance
Heat is the basic form of energy output when substances interact.The thermal changes in drug loading and release process can be directly quantified by microcalorimetry,which could provide a thermodynamic explanation to illustrate the interactions between drug and carrier[29,30].As shown in Fig.11a,the positive heat flow signal of DOX-Fe3O4@SiO2@NaYF4:Yb,Er MUCNRs indicated that the drug loading process was exothermic with an enthalpy of-12.84 kJ.mol-1,which was consistent with the theory of exothermic bonding.Meanwhile,the negative heat flow signal in Fig.11b indicated that the drug release process was endothermic because the interactions between DOX and Fe3O4@SiO2@NaYF4:Yb,Er MUCNRs were disrupted.When pH was 5.0,the heat-flow plot presented a sharp peak along with rapid heat absorption,which reached its peak value at 3 h and flattened at approximately 24 h.The release process provided an enthalpy of 15.3 kJ.mol-1,which is much smaller than that of pH 7.4 (23.5 kJ.mol-1).This result suggested that although the abundant hydrogen bonds endowed the carrier with enough affinity to DOX,the electrostatic repulsive force enabled a large amount of loaded DOX to quickly release,thereby leading to a “burst release”.Obviously,the heat release rates in the heat-flow diagrams are consistent with the DOX release rate,which shows that microcalorimetry assay not only provides a unique thermodynamic explanation for the structure–activity relationship of Fe3O4@SiO2@NaYF4:Yb,Er MUCNRs but also provides powerful guidance to avoid the blind selection or design for drug carriers.

Fig.11.Heating rate (dH/dt) vs time of (a) DOX loading process and (b) releasing process of the MUCNRs.

Fig.12.(a) The emission intensity of DOX-Fe3O4@SiO2@NaYF4:Yb,Er MUCNRs as a function of release time at pH 5.0,(b) upconversion emission spectrum of Fe3O4@SiO2@NaYF4:Yb,Er MUCNRs and UV–vis absorption spectrum of DOX.
3.8.UCL intensity changes during the drug release process
Moreover,the relationship between UCL intensity and drug releasing amount of DOX-Fe3O4@SiO2@NaYF4:Yb,Er MUCNRs was investigated under the excitation of 980 nm (Fig.12a).After DOX loading,the UCL of MUCNRs was quenched obviously,and the intensity of red light remains basically constant.The DOXFe3O4@SiO2@NaYF4:Yb,Er MUCNRs system was scattered in the PBS solution of pH=5.0 for drug releasing,and the solution was centrifuged in the preset time (6,12,24,and 48 h) to test the fluorescence of nanoparticles under the excited light of 980 nm.With the releasing of DOX,the green fluorescence of this drug-carrying system was enhanced gradually.In other words,the quenching effect weakened gradually,whereas the red fluorescence intensity remained unchanged.This phenomenon was related with the FRET phenomenon caused by the superposition of UV–vis absorption peak of DOX and UCL of NaYF4:Yb,Er within the green wave band(Fig.12b).With the slow releasing of drug,the quenching effect and the green fluorescence gradually weakened and enhanced,respectively.Therefore,such relationship between the UCL and the drug-releasing amount of nano carrier could be used as a bioprobe for the real-time detection of drug-releasing amount.
4.Conclusions
This study designed and synthesized a multifunctional nanodrug carrier with a nanorattle-structure.This nano-drug carrier was composed of the external α-NaYF4:Yb,Er nano polycrystalline structure,the internal magnetic Fe3O4@SiO2nucleus,and the large hollow structure between nucleus and shell.The formation mechanism could be explained by Kirkendall effect.The synthesized Fe3-O4@SiO2@NaYF4:Yb,Er MUCNRs has a large cavity volume and specific surface area,as well as good dispersity and biocompatibility,which were very applicable in drug carrying.According to drug loading and releasing results of DOX,the MUCNRs showed excellently sustained drug release performance and strong pH dependence,that is,the drug release rate increased in the acid microenvironment of certain cancerous tissues.The EE (%) and DLC (%) of MUCNRs could reach as high as 60.7% and 18.2% when pH was 7.4.However,the drug carrier system could release DOX quickly and continuously when pH was 5.Under 980 nm nearinfrared excitation,Fe3O4@SiO2@NaYF4:Yb,Er MUCNRs presented strong UCL properties.After loading with DOX,green fluorescence showed very significant quenching effect,whereas the red fluorescence remained basically constant.With the gradual releasing of DOX,the upconversion green fluorescence of the drug carrier system was enhanced gradually.Such relation can be used for drug tracking and real-time detection of drug releasing behavior.Magnetic test results showed that Fe3O4@SiO2@NaYF4:Yb,Er MUCNRs had good superparamagnetism.Moreover,the saturation magnetization intensities before and after the loading of DOX were 15.8 and 12.3 A.m2.kg-1,respectively.Therefore,these results provided a comprehensive perspective for using Fe3O4@SiO2@NaYF4:Yb,Er MUCNRs as a remarkable magnetic targeted drug carrier.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the Key Research and Development Plan of Shaanxi Province (2020GY-313),the Specialized Research Fund of Education Department of Shaanxi Province (19JK0255),the Specialized Scientific Research Fund Projects of Academician Shengyong Zhang (18YSZX001),and the Science and Technology Innovation Team of Shangluo University (20SCX02).
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Knowledge expression,numerical modeling and optimization application of ethylene thermal cracking:From the perspective of intelligent manufacturing
- Low-temperature conversion of methane to oxygenates by supported metal catalysts:From nanoparticles to single atoms
- Recent advances in amino acid-metal coordinated nanomaterials for biomedical applications
- Coalescence dynamics of two droplets of different viscosities in T-junction microchannel with a funnel-typed expansion chamber
- Effects of piperacillin synthesis on the infterfacial tensions and droplet sizes
- Study on gas–liquid flow characteristics in stirred tank with dual-impeller based on CFD-PBM coupled model
