Controllably tailoring external surface sites of nanosheet HZSM-5 for maximizing light olefins in catalytic cracking of n-decane
2021-12-08TiantianZhuHairuiLiangBofengZhangYajieTianGuozhuLiu
Tiantian Zhu,Hairui Liang,Bofeng Zhang,Yajie Tian,Guozhu Liu,
1 Key Laboratory for Green Chemical Technology of Ministry of Education,School of Chemical Engineering and Technology,Tianjin University,Tianjin 300072,China
2 Henan Province Engineering Research Center of Catalytic Reaction,College of Chemistry and Chemical Engineering,Henan University,Kaifeng 475004,China
Keywords:Nanosheet HZSM-5 Acid properties Surface modification Catalysis Deactivation Fixed-bed
ABSTRACT A series of triphenylethoxysilane (TPEOS)-modified nanosheet HZSM-5 catalysts (ZN-x,x=4%,8% and 16%,mass) were synthesized by chemical liquid deposition to selectively change external acidity distributions.TPEOS modification was found to passivate some external Brønsted and Lewis acid sites by 37.8%,in which Brønsted acid sites (BAS) were found more easily sacrificed by breaking the surface Al-O bond of bridging hydroxyl groups and forming Si-O-Si bonds.The selectivity of ZN-8 catalyst for light olefins (ethylene,propylene and butene) in n-decane catalytic cracking is up to 26% (450 °C,WHSV=10.95 h-1),which is ca.78% higher than that of parent one.The better performance was attributed to the appropriate external acid density in ZN-8,which inhibits bimolecular hydrogen transfer reaction of light olefins on the adjacent acid sites,resulting in more olefins,few coke precursors and thus an excellent catalytic stability.
1.Introduction
Light olefins,such as ethylene and propylene,are important petrochemical raw materials and have shown growing global demand in recent years [1–3].Currently,catalytic cracking technology to produce light olefins has attracted much attention owing to its lower energy cost and relatively high propylene yield[4,5].
Among various cracking catalysts,HZSM-5 zeolites with rich acidity,good shape selectivity and hydrothermal stability have been deemed ideal catalysts in current studies [1,6].However,its microporous channels restrict the diffusion of reactants and/or products,which would sacrifice catalytic activity and easily produce coke [2,7].To overcome these drawbacks,the construction of hierarchical [8–11] and nanosized zeolites [12–15] has been extensively studied to increase light olefin yields.It is noteworthy that nanosheet ZSM-5 first synthesized by Ryoo et al.[16]exhibited more excellent diffusion abilities for reactants with large volumes owing to the enhanced accessibility of acid sites,based on their ultrashort diffusion length and large external surface area (ca.50% of total surface area).When employing nanosheet HZSM-5 as a catalyst in the catalytic cracking of n-heptane,Xiao et al.[17] demonstrated a 96.6% n-heptane conversion with more than 1.5 times propylene selectivity than that under commercial HZSM-5 zeolites.It should be noted that the acid amount on the external surface of nanosheet HZSM-5 zeolite was more than half of the total acid amount [18,19].Therefore,olefins produced from the catalytic cracking were easily adsorbed on the adjacent external acid sites and consumed by deep hydrogen transfer reactions.This not only limits the light olefin yields in products,but also leads to many side reactions,such as bimolecular oligomerization,cyclization,and aromatization reactions,which accelerate coke accumulation and cause inactivation of the nanosheet catalysts [7,20,21].Obviously,modification of external surface acid will be one of the most important methods to further maximize light olefins in the catalytic cracking of hydrocarbons.
Recently,grafting silica on catalysts by chemical liquid/vapor deposition methods was effective in regulating the acid amounts and distribution of zeolite catalysts [22–29].Our previous work demonstrated that although decreased n-decane conversion after TEOS-pillared nanosheet HZSM-5,light olefin selectivity in the product was enhanced due to the weakened acid densities[21].Small molecular(TEOS)was demonstrated to deactivate the acid sites in the micropores and external surface of zeolites simultaneously,while the larger silane,like SiOCH3(C3H7)3,could selectively inactivate the external acid sites of HZSM-5 [30,31].Hence,with modification by silane with larger volume than zeolite micropores,we could selectively change the external acidic sites of nanosheet HZSM-5.Ascribing to excellent diffusion properties and appropriate external surface acid distribution,the bimolecular reactions(like hydrogen transfer) could be inhibited,thus leading to an enhanced light olefin production in the catalytic cracking of hydrocarbons.To the best of our knowledge,up to now little work has been done on this issue.
Here,triphenylethoxysilane(TPEOS,0.95 nm× 1.12 nm× 1.18 nm) was selected as the silane reagent,which could selectively passivate the external acid sites of nanosheet HZSM-5 zeolite.A series of nanosheet catalysts with partial deactivation of external acid sites were prepared by using chemical liquid deposition(CLD) of TPEOS with contents of 4%,8% and 16% (mass) SiO2.The structures of the prepared catalysts were characterized by XRD,SEM,TEM,FTIR and N2adsorption-desorption measurements.The acid properties were measured by Py/DTBPy-IR and NH3-TPD.To explore the effect of the modification of external acid sites on reaction activity and product selectivity,catalytic cracking of ndecane under modified nanosheet HZSM-5 zeolites was performed under a fixed-bed reactor at different temperatures from 350°C to 500 °C with a WHSV of 10.95 h-1.The catalytic stability and coke deposition of different catalysts and a proposed reaction mechanism are also discussed in the manuscript.

Fig.1.XRD patterns of ZN-x catalysts.
2.Experimental
2.1.Synthesis of nanosheet ZSM-5 catalysts
All chemical reagents are listed in the supplementary materials.The detailed experimental process is as follows.First,nanosheet ZSM-5 zeolites with Si/Al=50 were hydrothermally synthesized according to the literature [16].
Synthesis of structure direct agent (SDA): First,1-bromodocosane and N,N,N′,N′-tetramethyl-1,6-diaminohexane were dissolved in toluene and acetonitrile,respectively.The toluene mixture was then dropped into the acetonitrile mixture in a 500 ml three-necked round-bottom flask at 70°C in a N2atmosphere and refluxed for 12 h.The resultant products were filtered,washed with diethyl ether,and dried at 60 °C overnight.The obtained products were mixed with 1-bromohexane and acetonitrile solution and stirred at 85 °C for 10 h under a N2atmosphere.After the reaction,the obtained products were filtered,washed with diethyl ether,and dried at 60°C to obtain the structure directing agent (SDA) of [C22H45N+(CH3)2C6H12N+(CH3)2C6H13](Br-)2,denoted C22-6-6.
Synthesis of nanosheet ZSM-5 zeolites:According to the procedure of Emdadi et al.[32],TEOS,Al2(SO4)3.18H2O,NaOH,H2SO4,and H2O were mixed and vigorously stirred at room temperature for 20 h and then C22-6-6was added into the mixture solution and continued stirring for 4 h at the same conditions.The molar ratio of the mixture was 30Na2O:1Al2O3:100SiO2:10C22-6-6:18H2-SO4:4000H2O.Finally,the gel solution was transferred into a stainless steel autoclave for hydrothermal crystallization at 150 °C for 5 days.After crystallization,the products were filtered,washed with distilled water,dried at 100 °C overnight,and calcined at 550 °C for 6 h to obtain nanosheet ZSM-5 zeolites.The asprepared samples were ion-exchanged with NH4Cl solution(1 mol.L-1),reacted at 85 °C for 3 h,dried at 100 °C for 12 h,and calcined at 550 °C for 6 h.Nanosheet HZSM-5 zeolites with a Si/Al ratio of 50 were obtained by repeating ammonia exchange three times.
2.2.Silylation of as-synthesized catalysts
Then,triphenylethoxysilane(TPEOS)with a large diameter was selected as the silanizing reagent to graft silane on the outer acid sites of the as-prepared nanosheet HZSM-5 by means of chemical liquid deposition.Modified catalysts with controllable external acid sites were synthesized following the procedure of Zheng et al.[27].First,0.5 g of as-prepared nanosheet HZSM-5 was mixed with 0.05 g triphenylethoxysilane (TPEOS) corresponding to 4%(mass) SiO2and then 12.5 ml of hexane was added to dissolve the mixture to form a homogeneous solution,which was stirred magnetically at 70 °C for one hour.The resultant products werewashed repeatedly with hexane three times,dried at 100°C for 5 h,and then calcined at 550°C for 4 h.The modified nanosheets were denoted as ZN-4,ZN-8,and ZN-16,where the numbers 4,8 and 16 refer to the theoretical addition of the percent content of SiO2,respectively.As a reference,the parent nanosheet HZSM-5 zeolites were also calcined at 550°C for 4 h to maintain the same condition.
2.3.Structure and acidity characterization
The surface morphology and inner structure were characterized by scanning electron microscopy(SEM,Hitichi S-4800)with a 3 kV accelerating voltage and transmission electron microscopy (TEM,JEOL JEM-F200) with a 200 kV accelerating voltage,respectively.The X-ray diffraction patterns (XRD Bruker D8-Focus) obtained with radiation (CuKα,λ=0.154 nm) at a scanning speed of 5 (°).min-1in the range of 5°-50° could give phase properties.N2adsorption-desorption (BET) was carried out with a Micromeritics ASAP2460 adsorption apparatus to analyze the pore characteristics.The Si/Al ratio was determined by ICP-OES(Agilent 5110) and the spent ZN-x was detected by TGA.
The acid strength and acid content of the catalysts were determined by ammonia temperature-programmed desorption(NH3-TPD,AMI-300).Fourier transform infrared spectroscopy(FTIR,VERTEX 70) of adsorbed pyridine (Py-FTIR) and 2,6-di-tertbutyl pyridine (DTBPy-FTIR) measurements were carried out to obtain both the total and external Brønsted and Lewis acid concentrations.The detailed steps of NH3-TPD and Py/DTBPy-FTIR are described in the Supplementary Materials.The concentrations of Brønsted,Lewis and external Brønsted acids were calculated according to Eq.(S2),Eq.(S3) and Eq.(S4),respectively (see Supplementary Materials).
2.4.Catalytic activity test
Fresh as-synthesized catalysts (0.2 g) were mixed with 0.8 g of SiC with the same particle size of 420 μm–840 μm particles,and catalytic cracking of n-decane was performed in the fixed-bed reactor(1.0 cm i.d.).At a certain carrier gas flow rate(14 ml.min-1),n-decane was pumped into the reactor via a constant current pump,and the reactants passed through the preheating section(300 °C) and constant temperature section in turn.The products were insulated at 200°C and directly entered the gas chromatography (GC456) with two channels,analyzing the composition of gas and liquid at the same time.For different modified nanosheets,the catalytic cracking reaction was conducted at different temperatures (350 °C,400 °C,450 °C,500 °C) and atmospheric pressures to investigate the difference in n-decane conversion and selectivity of the products.In addition,the stability of the catalysts was evaluated at 500 °C for 10 h.Furthermore,the spent catalysts were characterized by thermogravimetry (TGA701) to analyze the coke content,further demonstrating the stability of different nanosheets.The conversion of reactant and selectivity of products were calculated according to Eq.(S5) and Eq.(S6),respectively.
3.Results and Discussion
3.1.Textural properties
Fig.1 shows the XRD patterns of ZN-x catalysts.All the catalysts exhibit a typical MFI crystal phase according to the standard PDF card (JCPDS-46-0003) analyzed by Jade 6 software [16].No other crystalline phases were detected,which could be ascribed to a highly dispersed silica component on the zeolite surface or newly formed MFI crystalline phases after TPEOS modification.The enhanced Si/Al molar ratios with increasing TPEOS amount further demonstrated the successful introduction of silica species,as shown in Table 1.The relative crystallinity (Rc,Eq.(S1)) of ZN-x decreases with increasing of silane amount (as shown in Table 1),which could be ascribed to the enhanced amount of amorphous silica phases on nanosheet HZSM-5.All the modified ZN-x and parent nanosheet HZSM-5 show flower-shaped morphologies and are composed of intersecting layers from SEM images (Fig.2 and Fig.S1).The TEM results in Fig.2 and Fig.S1 reveal nanosheet layer stacks arranged in parallel.No obvious difference was detected for prepared zeolite samples before and after TPEOS modification.
Table 1Structural properties of ZN-x

Table 2Acid properties of the ZN-x catalysts
N2adsorption–desorption measurements were carried out to analyze the textural properties of prepared nanosheet HZSM-5.As shown in Fig.3(a),both ZN-x and the parent nanosheet HZSM-5 exhibit Type IV isotherms.At relative pressures higher than 0.4,a clear hysteresis loop due to capillary condensation was detected,indicating the presence of mesopores [33].The pore-size distribution was calculated by the NLDFT method,as shown in Fig.3(b).It revealed ZN-x catalysts have similar poresize distributions centered from 2 to 10 nm and ca.7.4 nm of the average pore size,which is mainly ascribed to mesopores between nanosheet layers.The specific surface area (SBET) and pore volume(Vtotal)of different catalysts are calculated and displayed in Table 1.Both SBETand Vtotaldecreased with increasing TPEOS introduction.The decreased Vtotalmainly came from mesoporous volume(Vmeso)sacrifice,following ZN (0.351 cm3.g-1) >ZN-4 (0.345 cm3.g-1)>ZN-8 (0.305 cm3.g-1) >ZN-16 (0.280 cm3.g-1).However,the micropore volume (Vmicro) even shows a slight increase in ZN-16 compared with the parent nanosheet HZSM-5.All of these results demonstrate that when using TPEOS as a silane precursor,modification would occur on the nanosheet surface but not the inner micropores owing to space limitations.Such treatment could alleviate the negative effects based on surface sacrifice for catalytic performance,which will be discussed in the catalytic reaction section.
3.2.Acid properties
The acid content and strength of different catalysts were determined by NH3-TPD.As shown in Fig.4(a),two desorption peaks at 100 °C to 350 °C and from 350 °C to 600 °C,are ascribed to NH3adsorbed on weak acids and strong acids,respectively.It is noted that the desorption peaks of nanosheet catalysts after silane CLD modification shifted to lower temperatures compared with parent nanosheet HZSM-5,indicating that both weak and strong acid strength decreased.The detailed acid strength was calculated and is shown in Table 2,in which the weak and strong acid density of ZN-16 decreased by 34% and 41%,respectively,compared with that of ZN.
Py-FTIR was adopted to analyze the protic(Brønsted)and aprotic(Lewis)acid properties in the prepared catalysts,and the results are shown in Fig.4(b).The infrared absorption bands ca.1543 cm-1and 1452 cm-1are assigned to pyridine adsorption on Brønsted and Lewis acid sites,respectively [34].Combined with the total concentration of acid sites determined by NH3-TPD,the Brønsted acid sites and Lewis acid sites of different samples are quantified according to Eq.(S2)and Eq.(S3).Pyridine adsorption results indicate the Brønsted and Lewis acid concentrations of ZN-x after CLD modification by TPEOS were decreased,which are similar to the results reported in the literature [27].And the decrease of the Brönsted acid content is significantly higher than the Lewis acid content as shown in Table 2.More importantly,the ratio of Brønsted/Lewis (B/L as summarized in Table 2.) decreased from 4.48 of ZN to 2.10 of ZN-16,indicating that Brønsted acid sites of zeolite catalysts are more sensitive to TPEOS modification.
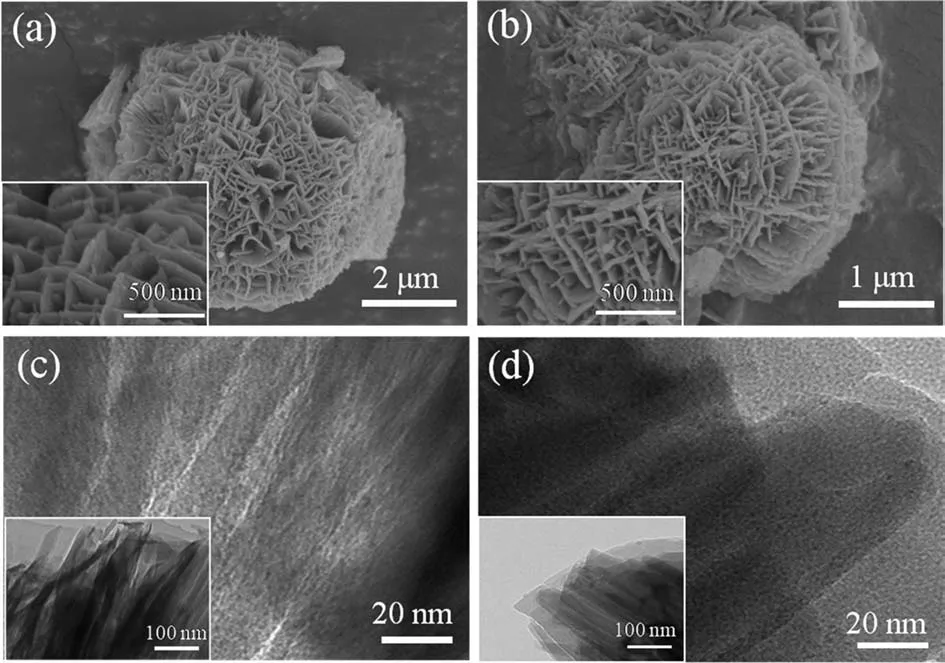
Fig.2.SEM and TEM images of (a,c) ZN and (b,d) ZN-16.

Fig.3.(a) N2 adsorption-desorption isotherms and (b) pore-size distributions of ZN-x.

Fig.4.(a)NH3-TPD profiles,(b)pyridine-adsorbed FTIR spectra(Py-FTIR)and(c)2,6-ditert-butylpyridine-adsorbed FTIR spectra(2,6-DTBpy-FTIR)of ZN,ZN-4,ZN-8 and ZN-16.
Because the kinetic diameter of 2,6-DTBpy (1.05 nm) is larger than that of the channels of HZSM-5 (straight channels 0.53 nm×0.56 nm,zigzag channels 0.51 nm×0.55 nm),only Brønsted acid sites located on the external surface or in the pore mouth region of the nanosheets can be reached by 2,6-DTBpy.The external Brønsted acid concentrations of different catalysts were determined by 2,6-DTBpy-adsorbed FTIR spectra [35].As shown in Fig.4(c),the peak at 1615 cm-1was ascribed to the 2,6-DTBpy adsorbed on external Brønsted acid sites.After grafting TPEOS,a significantly weakened external Brønsted acid (Bexternal) with increasing TPEOS amount was achieved,as shown in Table 2.Moreover,the external Brønsted acid to total Brønsted acid(Bexter-nal/Btotal) decreased from 0.32 for ZN to 0.22 for ZN-16 catalysts,which demonstrated that TPEOS CLD treatment primarily sacrificed the external Brønsted acid sites,and had a negligible effect on internal Brønsted acid sites.

Table 3Poducts distribution of n-decane catalytic cracking with different catalysts at 500 °C
The acidic properties were also reflected by the hydroxyl distribution,which could be characterized by FTIR [36].As shown in Fig.5,an infrared absorption peak centered at 3745 cm-1was ascribed to silanol groups (Si-OH) located on the surface of nanosheets [27],and the intensity was enhanced with increasing TPEOS amount.Combined with acid distribution,a proposed process of TPEOS modification is shown in Fig.6.First,owing to the spatial effect,TPEOS is adsorbed and hydrolyzed on the external acid sites and Si-O-Al bonds are generated.Then,Si-OH groups are formed after sequenced decomposition of TPEOS,and Si-O-Si bonds are generated during the calcination process through the condensation of adjacent surface -OH groups.

Fig.5.FTIR spectra of ZN,ZN-4,ZN-8 and ZN-16.

Fig.6.Proposed schematic diagram of grafting of chemical liquid deposition.
3.3.Catalytic cracking performance
3.3.1.Cracking activity and product distribution
The catalytic cracking of n-decane under as-prepared nanosheet HZSM-5 catalysts was performed on a fixed-bed reactor with a WHSV of 10.95 h-1from 350 °C to 500 °C.As shown in Fig.7(a),the n-decane conversion were increased with enhanced temperature for all catalysts,and ZN showed the highest conversion under the same reaction conditions.Usually,the catalytic cracking of hydrocarbons under zeolite catalysts depends on both acidic and textural properties of catalysts.From the characteristic results mentioned above,the decreased SBETand acidities after TPEOS modification would lead to decreased conversion.However,it is worth noting that based on the spatial effect of TPEOS,only part of surface acid sites would be deactivated with 4% decreased of n-decane conversion under ZN-16 catalysts.Certain number of acidities were preserved to participate the catalytic cracking reaction.With the increase of reaction temperature to 500 °C,such negative effects caused by sacrificing of acid sites would be weakened,leading to decrease by only 4% of conversion.
Furthermore,we focused on the selectivity of light olefinsunder four catalysts at different temperatures.Fig.7(b)shows that theselectivity was increased with increasing temperature,which was highly similar to conversion trend.Surprisingly,the selectivity of light olefins was significantly different under different catalysts,and the surface-passivated nanosheet HZSM-5 showed higherselectivity than catalysts before modification.In particular,the ZN-8 catalyst at 500 °C exhibited the highest selectivity of light olefins up to 28.3%,which increased by 34% compared with the parent nanosheet HZSM-5.Meanwhile,the corresponding light olefin yield increased by 41.6%,which might be ascribed to the fact that the weakened acidity promoted the desorption of adsorbed products,and the decreased external acid density inhibited the side reaction that would consume olefins.

Fig.7.(a) Conversion and (b) selectivities of light olefins (C=2,C=3,C=4) for catalytic cracking of n-decane under ZN-x at different temperatures.
3.3.2.Reaction mechanism
In general,the catalytic cracking of hydrocarbons over acidic zeolites follows a typical carbocation mechanism,and hydrocarbon cracking mainly occurs on zeolite acid sites,especially Brønsted acids [37,38].In detail,the reaction path of n-decane in our research mainly follows the process (seen in Fig.8):first,n-decane is protonated at the Brønsted acid sites,forming carbocations.Then,a series of short chain alkanes and alkenes and new carbocation intermediates would be formed through β-scission of carbocations.Through a continuous C-C bond breaking process,light olefins are formed.Meanwhile,the generated light olefins can be further consumed through bimolecular hydrogen transfer reactions,polymerization reactions and/or cyclization reactions to form new cyclic hydrocarbons,and aromatic hydrocarbons,which are finally converted to coke species [39].

Fig.8.Schematic diagram of regulating acid sites (Brønsted acid as an example) on the external surface of nanosheet HZSM-5 and possible reaction path of n-decane.
Based on the detailed product distribution at 500°C as shown in Table 3,we compared the depth of the hydrogen transfer reaction under different zeolite catalysts.Generally,the olefin/paraffin(o/p)ratio of the same carbon number compound in the product can be used to measure the degree of hydrogen transfer reactions,and the higher o/p ratio,the lower hydrogen transfer activity [40].Here,(Fig.9(a)) and(Fig.9(b)) are regarded as parameters highly related to the hydrogen transfer reaction.It was found that theandvalues increased over those of the TPEOS modified nanosheets,indicating that the degree of hydrogen transfer reaction decreased.ZN-8 exhibited the highestandat different temperatures,corresponding to the lowest hydrogen transfer activity.Theof all catalysts had a minimum at 400–450°C,indicating that the hydrogen transfer activity is higher in this temperature range.In addition,when using isobutene/isobutaneas a reference,which is more sensitive to the hydrogen transfer reaction,similar trends can be observed in Fig.9(c).However,theof all catalysts (seen in Fig.S2) is negatively correlated with temperature,which is different from the results of propylene and butene.The decreased trend ofis probably due to the preferential formation of ethane(compared to ethene) by protolytic cracking because of the low stability of ethyl carbenium ions at high temperature [41].Moreover,the selectivity of aromatics is another parameter to measure the extent of chain reactions because the light olefins produced by the carbocation mechanism can further undergo cyclization,polymerization and dehydrogenation to form aromatics[42].The selectivity of aromatic products,as shown in Fig.9(d),also confirms a decreasing trend after TPEOS modification.All these results indicate that the bimolecular reactions represented by hydrogen transfer were inhibited by reducing the external acid density,and the selectivity of light olefins was significantly improved.
Previous studies have confirmed that increasing the specific surface areas and pore volumes can greatly improve the diffusion rates of reactants and products and promote the desorption of intermediates at acidic sites [43].Thus,the selectivity of light olefins in products can be improved by inhibiting the secondary reaction in catalytic cracking of hydrocarbons.However,in this work,the introduction of TPEOS will lead to the loss of specific surface area and pore volume,which is unfavorable for the diffusion process.Although there was an increased diffusion limitation of reactants and products,TPEOS-modified nanosheet HZSM-5 showed enhanced yields of light olefins due to weakened acidities and decreased external acid densities.Therefore,we speculate that the regulation of acid distribution plays a decisive role in improving the selectivity of light olefins.

Fig.9.The ratio of olefins/paraffin:(a),(b)(c)i -— C4,and(d)selectivity of aromatics in n-decane catalytic cracking at various temperatures under ZN,ZN-4,ZN-8 and ZN-16 catalysts.
Based on the acid properties mentioned above,it is considered that the separation of bridging hydroxyl groups and the formation of new Si-O-Si on the surface of ZN-x after CLD of silane would significantly reduce the external Brønsted and Lewis acid densities of the nanosheet catalysts.These Brønsted acid sites are mainly located on the surface of nanosheets and the mesopores between layers.Therefore,the bimolecular reaction of olefins adsorbed on the external Brønsted acid sites of the catalysts will be inhibited,and the selectivity of olefins will be increased,as shown in Fig.8.
Based on the above analysis,we further focused on the gas/liquid (G/L) ratio of the products at different temperatures (Fig.S3),which could reflect the catalytic cracking depth under different surface-passivated nanosheet HZSM-5 catalysts.The G/L of each nanosheet HZSM-5 catalyst increased with increasing temperature,and such values increased under TPEOS-modified nanosheets.The enhanced G/L ratio from 0.27 of ZN to 0.43 of ZN-8 at 500°C can be attributed to the inhibited bimolecular reaction of gaseous products between light olefins,resulting in high gas phase yields.Therefore,it could be concluded that inactivating excessive surface acidic sites could reduce the occurrence of secondary reactions and improve the selectivity of light olefins without remarkably reducing the activity of catalysts.
3.3.3.Stability test and coke analysis
The cracking stability of n-decane under the prepared ZN-x was determined at 500 °C,and the result is shown in Fig.10(a).Each catalyst exhibits a similar initial conversion of n-decane(ca.83%),and the deactivation rates (Rd) are similar after 580 min of continuous reaction,demonstrating excellent catalytic stability.
The deactivation of nanosheet catalysts is usually attributed to carbon deposition on the pore mouth and external surface due to excess external acid sites [44].The amount of carbon deposited on the spent catalysts was obtained by thermogravimetric analysis (TGA),and the results are shown in Fig.10(b)and Fig.10(c).In general,Mass loss below 400 °C is mainly attributed to volatilization of the non-desorbed products,while the remaining mass loss is mainly ascribed to the coke [21].It can be found that the modified nanosheet catalysts present a relatively low mass loss compared to pristine catalysts,and the spent ZN-8 catalysts exhibit the lowest coke amount of 4.1% (mass),which was calculated as the mass loss between 400 °C and 800 °C.This result can be ascribed to the reduction of the highly exposed Brønsted and Lewis acid sites,which leads to the production of fewer aromatics and carbon deposition precursors.Meanwhile,the decreased strength of acidities are favorable for light olefins desorption and can restrain their secondary reaction which will generate carbon deposition [20].

Fig.10.(a) Conversion versus time of n-decane catalytic cracking at 500 °C and 10.95 h-1 WHSV.(b) Thermogravimetric curves,(c) DTG curves and (d) Raman spectra of spent catalysts,where a,b,c and d represent the ZN,ZN-4,ZN-8 and ZN-16.
Therefore,passivation of external acid sites can effectively improve the stability of the catalysts and prolong the catalyst life.
The coke types of spent catalysts were determined by Raman spectra (Fig.10(d)).The G band ca.1600 cm-1represents structured coke at the mesopores and external surface of zeolites,and the D band ca.1350 cm-1is assigned to amorphous coke located in the channel or pore mouth of micropores [17].It was found that the G bands in surface-passivated nanosheets are lower than those in parent nanosheet HZSM-5,indicating that less structured coke is located on the external surface and mesopores,which implies that a weakened aromatic reaction occurs at the surface of modified nanosheet HZSM-5.In addition,the D/G values decreased as ZN (0.26) >ZN-4 (0.27) >ZN-16(0.49) >ZN-8 (0.51),which demonstrated that decreased external acid density might limit coke evolution [45].The ZN-8 catalyst exhibited the highest D/G value,which might be related to the inhibited hydrogen transfer reaction.It always leads to deep dehydrogenation and produces graphitized coke [46].Thus,the decreased external acid density of nanosheet HZSM-5 is favorable for restricting the extent of secondary reactions such as aromatization and achieving a higher D/G value.
4.Conclusions
Nanosheet HZSM-5 (ZN-x) zeolites with different external acid densities were successfully prepared by chemical liquid deposition of TPEOS.The results show that only passivating the surface acid sites(both Brønsted and Lewis acid sites)can promote an efficient transformation of n-decane without sacrificing the surface areas and pore volumes.As the silane reagent increased,the total acid concentration,especially the external Brønsted acid of ZN-x,gradually decreased;for example,the Bexternal/Btotaldecreased from 0.32 for ZN to 0.22 for ZN-16.Silane reagents(TPEOS)with large diameters selectively inactivate surface acid sites but not internal acid sites.Owing to the selectively covered external Brønsted acid,nanosheet HZSM-5 catalysts modified by 8% (mass) TPEOS (ZN-8)show a 78% enhanced selectivity of light olefins by inhibiting bimolecular reactions such as hydrogen transfer in the catalytic cracking of n-decane.Moreover,nanosheet HZSM-5 treated with TPEOS show a restricted aromatization reaction,which leads to less coke formation and better catalytic stability.In summary,an enhanced catalytic performance for cracking of hydrocarbons to produce light olefins was realized by controllably adjusting the external acid sites of nanosheet HZSM-5 catalysts.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Financial supports by the National Natural Science Foundation of China (21776210 and 22008055) are gratefully acknowledged.
Supplementary Material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cjche.2021.04.015.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Knowledge expression,numerical modeling and optimization application of ethylene thermal cracking:From the perspective of intelligent manufacturing
- Low-temperature conversion of methane to oxygenates by supported metal catalysts:From nanoparticles to single atoms
- Recent advances in amino acid-metal coordinated nanomaterials for biomedical applications
- Coalescence dynamics of two droplets of different viscosities in T-junction microchannel with a funnel-typed expansion chamber
- Effects of piperacillin synthesis on the infterfacial tensions and droplet sizes
- Study on gas–liquid flow characteristics in stirred tank with dual-impeller based on CFD-PBM coupled model
