Hollow mesoporous polyaniline nanoparticles with high drug payload and robust photothermal capability for cancer combination therapy
2021-12-08YinfengZhangFangFangYongjieChenMinLiLiLiWenyueLiJinfengZhang
Yinfeng Zhang,Fang Fang,Yongjie Chen,Min Li,Li Li,Wenyue Li,Jinfeng Zhang,
1 International Medical Center,Beijing Friendship Hospital,Capital Medical University,Beijing,100050,China
2 Key Laboratory of Molecular Medicine and Biotherapy,School of Life Sciences,Beijing Institute of Technology,Beijing 100081,China
3 College of Pharmacy,Chongqing Medical University,Chongqing 400016,China
4 School of Electrical,Computer and Energy Engineering,Arizona State University,Tempe,Arizona,USA
Keywords:Hollow mesoporous polyaniline nanoparticles Drug delivery Nanocarriers Chemo-photothermal therapy Cancer combination therapy
ABSTRACT In recent years,synergistic chemo-photothermal therapy has revealed promising potential in treatments against various kinds of cancer.However,the development of superb photothermal agents with high drug loading capacity is still highly required.In this work,a hollow mesoporous polyaniline nanoparticle(HPANI NP) has been developed for encapsulating chemotherapeutic drug doxorubicin (DOX) with an remarkable drug loading content as high as 37.5%.Additional PEG modification endowed the drugloaded HPANI NPs with improved water-dispersibility and bioavailability.Such PEG-HPANI-DOX NPs exhibited strong NIR absorbance and robust photothermal conversion capacity,exhibiting highly efficient synergistic cancer treatment.More interestingly,the responsively released DOX molecules could emit strong red fluorescence,which could be employed to monitor the cellular endocytosis and drug release profile of PEG-HPANI-DOX NPs.Finally,the as-fabricated NPs showed good biocompatibility and low toxicity,serving as a promising nanoagent for highly efficient drug delivery and cancer combination therapy.
1.Introduction
Cancer as one of the most malignant diseases threatens the lives of humans globally [1].Up to date,conventional available cancer treatment modalities,including surgery resection,radiotherapy,and chemotherapy have been tremendously utilized in clinic.Although these approaches are effective to a certain extent,the therapeutic effect of each modality is still unsatisfactory because of its own limitations and drawbacks.For example,surgical resection is intrinsically invasive,which is difficult to completely eradicate solid tumors and may even lead to the potential risk of tumor metastasis and recurrence.Furthermore,current chemotherapy and radiotherapy always suffer from poor tumor uptake of therapeutic agents,severe side effects on normal tissue,as well as unfavorable multidrug resistance [2–5].Therefore,development of minimally invasive treatment modalities against cancers simultaneously exhibiting high therapeutic efficacy remains a great challenge.Encouragingly,the rapid developments of photothermal therapy(PTT)have opened new opportunities for cancer diagnosis and treatment in recent decades[6–8].As one of the most potential cancer therapeutic modalities,PTT demonstrated several charming advantages such as non-invasiveness,high therapeutic efficiency,good biosafety and favorable selectivity without systemic side effects,which could absorb near-infrared (NIR) light(700–1300 nm)to produce heat for the thermal-ablation of tumors[9–11].
Up to now,numerous photothermal agents have been exploited for PTT,such as inorganic nanomaterials,including Au nanoparticles (NPs) [12,13],Pd nanostructures [14,15],carbon nanomaterials [16–18],sulfides [19–21],transition metal oxides [22],and transition metal carbides [23].However,these inorganic nanomaterials shows adverse biodegradability and potential long-term biotoxicity,hindering their further clinical application [24–26].In addition,small organic molecules such as indocyanine green(ICG) [27],porphyrin [28],and perylene-diimide [29] have also been developed as photothermal converters,owing to their reduced health risks,potential biodegradability and fast body clearance.Nevertheless,the therapeutic efficacy and thermal stability of these small molecule-based agents are significantly compromised because of the poor photostability and undesirable photothermal performance [30,31].In recent years,polymer NPs have aroused growing interests for PTT applications due to their state-of-the-art light-harvesting property,excellent photothermal conversion efficiency,good biocompatibility,as well as satisfactory photostability [32–36].Although promising in cancer treatment,the complete scavenging of tumor by PTT monotherapy is still impeded by the poor absorption and the light scattering in biological tissues [11,37] as well as tumor thermoresistance [38,39].Thus,PTT-only nanoagents may not be the ideal choice for ideal cancer therapy.Recently,the combination of PTT with chemotherapy holds great promise for tumor theranostic applications because it not only greatly promote the therapeutic performance but also substantially ameliorate the side effects and suppress drug resistance[40–42].However,polymer NPs based drug delivery system exhibits extremely poor drug loading content (usually less than 10%) [43–47],which would greatly compromise their ultimate therapeutic outcomes.Therefore,development of biocompatible PTT agents with robust photothermal efficacy and favorable drug loading capacity for combinational chemo-photothermal therapy is urgently required.
In this work,we constructed a hollow mesoporous polyaniline nanoparticle(HPANI NP)as a drug carrier for loading chemotherapeutic drug,doxorubicin (DOX) to achieve photothermal-chemo combination therapy.An important advantage of the asfabricated HPANI-DOX NPs is the high drug loading capacity determined to be 37.5%,which synergistically combines the robust photothermal capability of polyaniline NPs,exhibiting a remarkable cancer-cell killing ability.Moreover,successfully loading of DOX molecules into the HPANI NP further endows the combinatorial therapeutic NPs with fluorescence imaging capability,which will be beneficial to track and monitor the theranostic agent in various biological environments.
2.Experimental
2.1.Materials
Aniline,ethylbenzene (EB,99.8%),sodium dodecyl sulfate(SDS,99%),poly(vinyl alcohol) (PVA,Mw≈25,000 g.mol-1,88% (mole)hydrolyzed,Polysciences Inc.),ammonium persulfate (APS,98%),poly(maleicanhydride-alt-1-octa-decene) (C18PMH),doxorubicin(DOX),mPEG-NH2(5 K)were purchased from Sigma-Aldrich.Fetal bovine serum(FBS),Dulbecco’s modified Eagle’s medium(DMEM),Dulbecco’s phosphate buffered saline (PBS,10X,pH 7.4),trypsin-EDTA,and penicillin–streptomycin were purchased from Thermo Fisher Scientific.
2.2.Fabrication of the HPANI NPs and PEG-HPANI-DOX NPs
HPANI NPs were prepared according to the reported work[48].Firstly,0.49 ml of monomer aniline (5.38 mmol) and 1.23 ml of hydrophobic ethylbenzene(EB,10.04 mmol)were mixed and then added into the deionized water (6 ml) which containing 63 mg of SDS served as a surfactant.After stirring for 1 h (r.t.),the mixture was further ultrasonicated for 250 s to undergo emulsification under ice cooling.Afterwards,aqueous solutions of PVA (10%,by mass) as the stabilizer and APS solution (5.38 mmol,1.67 ml) as the oxidant were respectively added dropwise into the resulting emulsion (r.t.).The color of the emulsion ultimately turned dark green after the polymerization process,in which the as-prepared sample was defined as HPANI NPs.The HPANI NPs powder were obtained after drying at 90 °C.
To encapsulate drug molecules into the HPANI NPs,a mixture of HPANI NPs and DOX solution was firstly prepared at 37°C for 24 h,where the mass ratios of the as-prepared HPANI NPs with DOX were regulated ranging from 5:2 to 5:12 to achieve the optimal balance between the DOX encapsulation efficiency and loading content.The suspension was then centrifuged for 40 min and washed three times to get rid of excess free DOX molecules.The resultant HPANI-DOX NPs were re-suspended in PBS.Next,the PEG-HPANI-DOX NPs were fabricated through the reprecipitation method,in which the amphiphilic polymer C18PMH-PEG was prepared via the reaction of C18PMH and mPEG-NH2according to the previously reported method [10].After adding C18PMH-PEG solution (5 mg.ml-1,500 μl) to the HPANI-DOX NPs suspension(5 ml),the mixture was ultrasonicated for another 40 min.The PEG-HPANI-DOX NPs were formed after centrifugations and washes,which were driven by non-covalent hydrophobic forces.Finally,the as-prepared NPs were freeze-dried and stored at 4 °C refrigerator for future use.
2.3.Characterization of the HPANI NPs and PEG-HPANI-DOX NPs
The structure of the as-prepared HPANI NPs were measured by transmission electron microscopy (TEM,Hitachi H-7700) with the dried HPANI NPs on a carbon film.The Micromeritics Tristar 2420 system was applied to investigate the nitrogen adsorption–desorption isotherms of the PANI NPs to indicate the presence of mesopores.A Malvern Zetasizer instrument was used to characterize the Dynamic light scattering analysis (DLS) and zeta potential.UV–vis-NIR absorption spectra and fluorescence spectra were individually conducted on a PE Lamda 19 UV–vis-NIR Spectrophotometer and a Cary Eclipse Fluorescence Spectrophotometer.
2.4.Photothermal features of the HPANI NPs and PEG-HPANI-DOX NPs
The NIR laser (808 nm) was used to induce heat conversion of different NP dispersions and the temperatures were recorded every 30 s.The HPANI NPs dispersions with different concentrations(0.1,0.25,0.5,1 mg.ml-1)were continuously irradiated with a NIR laser(808 nm,1.5 W.cm-2) for 300 s.Meanwhile,0.5 mg.ml-1HPANI NPs dispersions were irradiated with 808 nm laser with different power densities(0.5,1.5,2.5 W.cm-2)for 300 s.Besides,the water,free DOX,HPANI NPs,HPANI-DOX NPs,and PEG-HPANI-DOX NPs solutions were respectively irradiated with the laser (808 nm,1.5 W.cm-2) for 300 s.The Real-time infrared thermal images of deionized water as well as the PEG-HPANI-DOX NPs dispersions were recorded with a Ti29 thermal IR camera (FLUKE).
2.5.Cellular endocytosis and fluorescence imaging
A549 human lung cancer cells were cultured in a standard incubator (37 °C,5% CO2) with DMEM culture medium containing 1%penicillin/streptomycin and 10% FBS.After 24 h incubation,the A549 cells seeded on 24-well plates were treated with the free DOX,HPANI NPs,HPANI-DOX NPs,and PEG-HPANI-DOX NPs,respectively.Subsequently,incubating the treated cells for another 4 h and rinsing with PBS.After staining the cell nuclei with 4,6-diamidino-2-phenylindole (DAPI) for 5 min,the fluorescence images were obtained by a fluorescent microscope(Nikon ECLIPSE 80i).
2.6.In vitro cytotoxicity by MTT assay
The cytotoxicity of different samples in cells was investigated by the methyl thiazolyl tetrazolium (MTT) assay.The A549 cells were incubated in 96-well plates with a density of 0.9 × 104cells each well.After 24 h,treating the cells with different samples(free DOX,the PEG-HPANI NPs,and the PEG-HPANI-DOX NPs)at a serial concentrations of DOX from 1.25 to 20 μg.ml-1.After incubating for 12 h,the PEG-HPANI-DOX NPs-incubated cells were irradiated with or without laser (808 nm,1.5 W.cm-2,6 min).The only laser irradiation group was used as the control group.Subsequently,after 12 h incubation in the dark,the cells were added with DMEM containing 10% MTT stock solution,and followed by treating with DMSO after another 4 h.Finally,the absorbance of each well at 490 nm was tested by a microplate reader (BioTek Powerwave XS) to assess the in vitro cytotoxicity.Furthermore,the cell viabilities of PMHC18-PEG and the HPANI NPs-treated cells at different concentrations were evaluated through the same approach.
3.Results and Discussion
3.1.Preparation and characterization of the HPANI NPs and PEGHPANI-DOX NPs
In this work,a monodispersed hollow mesoporous polyaniline NPs (HPANI NPs) encapsulated with DOX (named as HPANI-DOX NPs) and modified with poly (maleic anhydride-alt-1-octade cene)-polyethy-lene glycol (C18PMH-PEG) (termed as PEG-HPANIDOX NPs)were developed for combinational chemo-thermal therapy,which exhibited high DOX payload and robust photothermal capability (Fig.1).Firstly,the HPANI NPs were fabricated through the oxidative polymerization of aniline monomer in direct emulsion with ammonium peroxydisulfate (APS) as the oxidant and poly(vinyl alcohol) (PVA) as the stabilizer according to the previously reported method [48].TEM and SEM images show that each HPANI NP has well-defined spherical morphology and a hollow cavity (Fig.2a,Figs.S1 and S2a in Supplementary Material).The sizes of these NPs are ranging from~300 to 400 nm in diameter.To confirm the hollow mesoporous structural features,we investigated the nitrogen adsorption/desorption isotherms and the pore size distribution of HPANI NPs.As described in Fig.2b,the typical hysteresis during the desorption process in the isotherms revealed the presence of mesopores.Consistent with the morphology observed in the TEM image,the HPANI NPs displayed an average pore size about 2.5 nm simultaneously containing large pore size of 10–20 nm,and favorable specific surface areas~31.4 m2.g-1,which were elaborated by the Barrett-Joyner-Halenda (BJH)approach and the Brunauer-Emmett-Teller (BET) method,proving the great potential of the as-prepared HPANI NPs to be utilized as robust drug delivery carriers.
Beyond that,the HPANI NPs loaded with DOX and modified with PEG to further improve their water-dispersibility and bioavailability.As demonstrated in Fig.2c,the PEG-HPANI-DOX NPs with PEG coating displayed a slightly larger hydrodynamic diameter~383.7 nm.Whereas,the size of HPANI NPs (340.2 nm)and HPANI-DOX NPs (347.5 nm) just described neglectable changes,which could be attributed to the loading of most DOX were in the hollow cavities and pore channels.In addition,the DLS measurements during 14 days confirmed the excellent sizestability of the PEG-HPANI-DOX NPs (Fig.S3).Moreover,the zeta potentials of the free DOX molecules,C18PMH-PEG,HPANI NPs,HPANI-DOX NPs,and PEG-HPANI-DOX NPs were +11.2 mV,-13.5 mV,+17.8 mV,+18.9 mV,+10.5 mV,respectively,indicating the successful loading with DOX and surface modification with C18PMH-PEG of the HPANI NPs (Fig.2d).
To further verify the encapsulation of DOX,the UV–vis-NIR and fluorescence spectra of different as-obtained components have been tested.As exhibited in Fig.3a and b,the appearance of representative absorbance peaks and fluorescence peaks of the free DOX(red) in the HPANI-DOX NPs (green)and the PEG-HPANI-DOX NPs(black)confirmed the successful DOX incorporation.The characteristic photographs in Fig.3a revealed the outstanding water dispersibility of each ingredients.More interestingly,the strong red fluorescence emissions also endowed the as-prepared HPANIDOX NPs and PEG-HPANI-DOX NPs with excellent fluorescence imaging capabilities.Additionally,the DOX releasing from the PEG-HPANI-DOX NPs displays both pH-and laser-responsive release profiles (Fig.3c and d).At pH 5.0,an enhanced release of DOX was obviously detected when compared with that at pH 7.4,which was probably attributed to protonation of the amine group on the DOX.This would weaken the non-covalent interactions between DOX and drug carrier.Meantime,laser irradiation further accelerated the release of DOX because of the conversion of NIR light to local thermal energy which would speed up the molecular motion.Such pH and light dually-responsive characteristics will be in favor of enhancing the anticancer therapeutic efficiency.The optimal drug loading content and drug encapsulation efficiency of the as-prepared HPANI-DOX NPs were 37.5% and 75%,respectively,when the weight ratio of the HPANI NPs and DOX is 5:4(Table 1),which is much higher than that of most drug delivery systems based on conventional carriers (typically less than 10%),making the as-fabricated hollow mesoporous NPs a promising candidate for highly effective cancer therapy.

Table 1Drug loading contents and drug encapsulation efficiencies of the as-prepared HPANIDOX NPs with various mass ratios of HPANI NPs and DOX

Fig.1.Schematic illustration of the fabrication of PEG-HPANI-DOX NPs as drug carriers with high drug payload and robust photothermal capability.

Fig.2.(a) TEM image of the as-prepared HPANI NPs;(b) Nitrogen adsorption/desorption isotherms of the HPANI NPs,where the inset is the corresponding pore-size distribution curve;(c)Dynamic light scattering(DLS)measurements;(d)Zeta potentials of different NPs in DI water.The error bars represent standard deviations(SD),n=3.
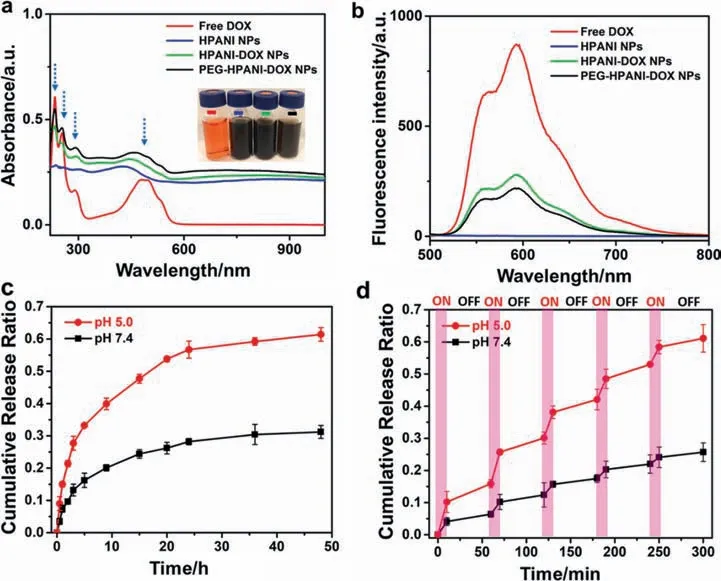
Fig.3.(a) UV–vis-NIR absorption and(b)fluorescence spectra of free DOX(red),HPANI NPs(blue),HPANI-DOX NPs(green) and PEG-HPANI-DOX NPs(black) solutions,the inset is a representative photograph of the above-mentioned samples;DOX release from the PEG-HPANI-DOX NPs overtime in PBS at different pH values (5.5 and 7.4) (c)without NIR irradiation or (d) under 808 nm laser irradiation.Pink Rectangles represent the stage of laser irradiation.
3.2.Photothermal properties of the the HPANI NPs and PEG-HPANIDOX NPs
To confirm the HPANI NPs could be applied as photothermal agents,the temperature variation of HPANI NPs depond on the concentration and laser power density were investigated.As shown in Fig.4a and b,the HPANI NPs displayed a significant concentration-dependent and laser power density-dependent temperature enhance preformance,which is conducive to the utilization of PEG-HPANI-DOX NPs as a photoactive agent to control temperature variation by simplifying the concentration or laser irradiation adjustment.Synchronously,to study the photothermal features of the DOX-loaded HPANI NPs,photothermal heatingcurves of water,free DOX,and HPANI NPs,the HPANI-DOX NPs,and PEG-HPANI-DOX NPs aqueous dispersions were respectively monitored for 300 s upnder laser irradiation (Fig.4c).In comparison to the water and free DOX with neglectable temperature increasement,all HPANI NPs-based formulations exhibited a prominent temperature enhancement up to~65°C with laser irradiation,proving the excellent photothermal conversion capability stemming from the polyaniline polymer.Moreover,as elaborated in Fig.4d,the infrared thermal images of the PEG-HPANI-DOX NPs aqueous dispersion upon laser irradiation further confirmed that the PEGylated DOX-loaded HPANI NPs still exhibited robust photothermal capability.Fig.S4 further confirmed the good thermal-stability of the PEG-HPANI-DOX NPs upon 808 nm laser irradiation for five light on/off cycles.Consequently,the desirable photothermal performance of the as-prepared PEG-HPANI-DOX NPs demonstrated their great potential to be used as effective PTT agents.
3.3.Cellular uptake and fluorescence imaging
The fluorescence imaging was used to explore the cellular internalization and drug release properties of the PEG-HPANI-DOX NPs through fluorescent microscopy.As demonstrated in Fig.5,after treatment with DOX-based samples,obvious red fluorescence was clearly detected in the nucleus,while no fluorescence signal could be observed in the HPANI NPs without DOX incubated cells.These results not only depicted the effectively cellular endocytosized of the DOX-loaded HPANI NPs,but also verified the drug release and accumulationin tumor cells via the as-designed drug delivery system.It is also worthwhile to note that the red fluorescence signals of DOX from the NPs groups are little weaker than that of the free DOX treated A549 cells,which could be attributed to the slower release of DOX molecules from the nanocarrier.Because of different cellular uptake mechanisms,compared to the small DOX molecules penetrate cells and nucleus by faster diffusion,the NPs are internalized into cells via endocytosis with longer time [49].
3.4.Cellular cytotoxicity
To assess the combinational antitumor efficiency and cytotoxicity of the as-fabricated NPs at cellular level,we exploited the cell viabilities through 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetra zolium bromide(MTT)assay.As depicted in Fig.6a,the A549 cells were treated with the only laser irradiation (808 nm,1.5 W.cm-2,6 min),free DOX molecules,the PEG-HPANI NPs with laser irradiation,and the PEG-HPANI-DOX NPs with or without laser irradiation at different DOX doses,respectively.The control group was the cells with only laser irradiation and did not contain NPs.As we can see,both free DOX,the PEG-HPANI NPs with laser and the PEG-HPANI-DOX NPs with or without irradiation showed dose-dependent cytotoxicity while the irradiation control group exhibit negligible toxicity.Additionally,the cytotoxicity of chemotherapy combined with PTT treated with the PEG-HPANIDOX NPs upon irradiation was effectively improved compared with conventional chemotherapy that only employs DOX,suggesting remarkably combinational therapeutic efficiency against cancer cells.

Fig.4.(a) Photothermal elevation of the HPANI NPs dispersed in deionized water with different concentrations (0.1,0.25,0.5,1 mg.ml-1) upon laser irradiation (808 nm,1.5 W.cm-2,300 s);(b)Photothermal heating curves of the HPANI NPs under 808 nm laser irradiation with different power densities(0.5,1.5,2.5 W.cm-2);(c)Photothermal increase curves of different components (water,free DOX,HPANI NPs,HPANI-DOX NPs,and PEG-HPANI-DOX NPs aqueous dispersions) with laser irradiation (808 nm,1.5 W.cm-2,300 s);(d) Infrared thermal images of water and the PEG-HPANI-DOX NPs with the 808 nm laser irradiation for 8 min.

Fig.5.The cellular fluorescence imaging of A549 cells treated with different formulations(free DOX,HPANI NPs,HPANI-DOX NPs,and PEG-HPANI-DOX NPs)via a fluorescent microscope.Scale bar is 50 μm.All images share the same scale bar.

Fig.6.(a) Relative cell viabilities of A549 cells with various treatments (only 808 nm laser irradiation with 1.5 W.cm-2 for 6 min,free DOX,PEG-HPANI NPs with laser irradiation,and the PEG-HPANI-DOX NPs with or without laser irradiation).The concentration of HPANI in PEG-HPANI group is equivalent to the concentration of HPANI in PEG-HPANI-DOX NPs group;(b) Relative cell viabilities of A549 cells incubated with PMHC18-PEG and the HPANI NPs.(n=5).
Finally,to prove that cell cytotoxicity is not caused by the C18PMH-PEG surfactant and HPANI NPs,MTT assay was further carried out to examine the cell viability of A549 cells treated with C18PMH-PEG or HPANI NPs.As exhibited in Fig.6b,both C18PMH-PEG and HPANI NPs-treated cells remained great cell viability even at a high sample concentration of 500 μg.ml-1,confirming the excellent biocompatibility and low toxicity of them.Overall,the PEG-HPANI-DOX NPs demonstrate good biocompatibility and remarkable cancer cell-killing ability,indicating they are promising nanoagents for effectively combinational chemo-PTT cancer therapy.
4.Conclusions
In summary,we have successfully fabricated PEG-HPANI-DOX NPs for highly effective combinational chemo-thermal therapy against A549 cancer cells.The as-prepared HPANI-DOX NPs described superior drug loading capacity (37.5%) and sufficient drug encapsulation efficiency (75%),owing to the unique hollow mesoporous structure.Meanwhile,the strong NIR absorption and satisfactory photothermal conversion capability endowed their great potential to be applied as PTT agents.As validated in the fluorescent images,the released DOX could be internalized into cellular nucleus and simultaneously emitted strong red fluorescence signals,which could be utilized to monitor the cellular uptake and real-time drug release profile of the PEG-HPANI-DOX NPs.Benefiting from the high drug payload and robust photothermal capability,the PEG-HPANI-DOX NPs could be served as an desirable multifunctional nanoagents for efficiently synergistic cancer therapy.Finally,the cytotoxicity experiments confirmed the significant cancer cell-killing ability as well as excellent biocompatibility of the PEG-HPANI-DOX NPs.We believe that the development of such hollow mesoporous polymer nanocarriers will supply new possibilities for designing novel PTT nanoagents for effective drug delivery and cancer therapy in the future.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to acknowledge financial support by the National Natural Science Foundation of China (32001010 and 21701018),Beijing Natural Science Foundation (2214078),Beijing Institute of Technology Research Fund Program for Young Scholars,and The Young Elite Scientist Sponsorship Program of Beijing Association for Science and Technology (2021–2023).J.Zhang would also like to thank Biological &Medical Engineering Core Facilities(Beijing Institute of Technology) for providing advanced equipment.
Supplementary Material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cjche.2021.03.011.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Knowledge expression,numerical modeling and optimization application of ethylene thermal cracking:From the perspective of intelligent manufacturing
- Low-temperature conversion of methane to oxygenates by supported metal catalysts:From nanoparticles to single atoms
- Recent advances in amino acid-metal coordinated nanomaterials for biomedical applications
- Coalescence dynamics of two droplets of different viscosities in T-junction microchannel with a funnel-typed expansion chamber
- Effects of piperacillin synthesis on the infterfacial tensions and droplet sizes
- Study on gas–liquid flow characteristics in stirred tank with dual-impeller based on CFD-PBM coupled model
