枸橼酸铁铵和脂多糖对星形胶质细胞LCN2表达的影响及机制
2021-11-17唐硕徐华敏谢俊霞
唐硕 徐华敏 谢俊霞
[摘要] 目的 探討枸橼酸铁铵(FAC)和脂多糖(LPS)对原代培养的星形胶质细胞脂质运载蛋白-2(LCN2)表达的影响及可能的机制。
方法 实验分为对照组、FAC组、LPS组、FAC+LPS组、MG132+LPS组,对照组用细胞培养液处理,FAC组和LPS组分别用FAC和LPS处理24 h,FAC+LPS组先用FAC预处理4 h后再用LPS处理24 h,MG132+LPS组先用MG132预处理4 h后再用LPS处理24 h。应用蛋白质免疫印迹(Western blot)方法检测细胞内核因子κB(NF-κB)和LCN2的表达。
结果 与对照组相比,单独FAC处理不影响细胞内磷酸化的核因子κB(P-NF-κB)及LCN2的蛋白表达水平(F=11.76、68.18,q=0.469、0.655,P>0.05),LPS处理能够上调P-NF-κB及LCN2蛋白的表达(q=5.859、16.170,P<0.01);与LPS组相比,FAC预处理对LPS诱导的P-NF-κB及LCN2蛋白表达上调没有影响(q=1.516、1.151,P>0.05),而MG132预处理则能够显著抑制LPS诱导的P-NF-κB及LCN2蛋白表达上调(q=4.939、15.710,P<0.05)。
结论 细胞内高铁对LPS诱导的P-NF-κB和LCN2蛋白表达上调无明显影响,MG132能够下调LPS诱导的P-NF-κB和LCN2蛋白表达上调,蛋白酶体、NF-κB通路可能参与了LPS诱导的LCN2表达上调的抑制作用。
[关键词] 星形细胞;NF-κB;脂笼蛋白质类;铁;脂多糖类
[中图分类号] R338.2
[文献标志码] A
[文章编号] 2096-5532(2021)05-0633-04
doi:10.11712/jms.2096-5532.2021.57.103
[开放科学(资源服务)标识码(OSID)]
[网络出版] https://kns.cnki.net/kcms/detail/37.1517.R.20210510.1103.001.html;2021-05-10 17:36:51
EFFECT OF FERRIC AMMONIUM CITRATE AND LIPOPOLYSACCHARIDE ON THE EXPRESSION OF LIPOCALIN-2 IN PRIMARY CULTURED ASTROCYTES AND ITS MECHANISM
TANG Shuo, XU Huamin, XIE Junxia
(Department of Phy-siology and Pathophysiology, School of Basic Medicine, Qingdao University, Qingdao 266071, China)
[ABSTRACT] Objective To investigate the effect of ferric ammonium citrate (FAC) and lipopolysaccharide (LPS) on the expression of lipocalin-2 (LCN2) in primary cultured astrocytes and its possible mechanism.
Methods Astrocytes were divided into control group, FAC group, LPS group, FAC+LPS group, and MG132+LPS group. The astrocytes in the control group were treated with cell culture media, those in the FAC group and the LPS group were treated with FAC or LPS for 24 h, those in the FAC+LPS group were given FAC pretreatment for 4 h followed by LPS treatment for 24 h, and those in the MG132+LPS group were given MG132 pretreatment for 4 h followed by LPS treatment for 24 h. Western blot was used to measure the protein expression of phosphorylated nuclear factor-kappa B (P-NF-κB) and LCN2 in astrocytes.
Results Compared with the control group, FAC treatment alone did not affect the protein expression levels of P-NF-κB and LCN2 in astrocytes (F=11.76,68.18;q=0.469,0.655;P>0.05), and LPS treatment significantly upregulated the protein expression of P-NF-κB and LCN2 (q=5.859,16.170;P<0.01). Compared with the LPS group, FAC pretreatment had no significant effect on the upregulated protein expression of P-NF-κB and LCN2 induced by LPS (q=1.516,1.151;P>0.05), and MG132 pretreatment significantly inhibited the upregulated protein expression of P-NF-κB and LCN2 induced by LPS (q=4.939,15.710;P<0.05).
Conclusion High iron state in astrocytes has no significant effect on the upregulated protein expression of P-NF-κB and LCN2 induced by LPS, and MG132 can downregulate the upregulated protein expression of P-NF-κB and LCN2 induced by LPS. Proteasome and the NF-κB pathway may be involved in inhibition of the upregulated protein expression of LCN2 induced by PLS.
[KEY WORDS] astrocytes; NF-kappa B; lipocalins; iron; lipopolysaccharides
帕金森病(PD)作为一种常见的神经退行性疾病,主要以黑质(SN)多巴胺(DA)能神经元的缺失以及嗜酸性路易小体(LBs)的形成为特点[1]。尽管PD的主要发病机制尚未完全阐明,但包括神经炎症在内的多种因素均可能参与了PD的发病。导致神经退行性变的神经炎症活动主要由固有免疫细胞(如活化的小胶质细胞和星形胶质细胞)介导,这些细胞能够产生活性氧中间体、一氧化氮和炎性细胞因子等[2-5]。因此,阐明神经炎症机制和调控神经胶质细胞激活对于保护PD病人SN区DA能神经元至关重要。SN铁沉积是PD病人和PD动物模型的重要特征[6-7]。脂质运载蛋白-2(LCN2)是一种先天性免疫蛋白,在生理和炎症条件下作为重要的铁调节蛋白发挥作用[8]。脂多糖(LPS)能够与脂多糖结合蛋白(LBP)结合,由CD14分子介导LPS向细胞内传递,导致核因子κB(NF-κB)激活,诱导炎性细胞因子表达[9]。MG132是蛋白酶体抑制剂,同时还可以抑制蛋白酶体介导的NF-κB活化[10-11]。研究发现,LCN2基因的启动子含有NF-κB及增强子结合蛋白(C/EBP)的结合位点,提示其表达可能受到NF-κB的调控[12]。然而,蛋白酶体、NF-κB通路是否参与LPS诱导的LCN2表达尚不清楚。因此,本研究观察了铁负载试剂枸橼酸铁铵(FAC)和LPS以及MG132诱导的原代星形胶质细胞中磷酸化的核转录因子(P-NF-κB)和LCN2表达的变化。现将结果报告如下。
1 材料与方法
1.1 实验材料
DMEM/F12培养液购自美国Hyclone公司,青霉素和链霉素双抗购于碧云天公司,多聚赖氨酸、LPS、FAC均购于美国Sigma公司,MG132购于美国Selleck公司,聚偏二氟乙烯(PVDF)膜购于美国Millipore公司,BCA蛋白浓度测定试剂盒、分离/浓缩胶缓冲液、RIPA裂解液购于中国康为世纪公司,兔源β-actin抗體购于博奥森公司,LCN2抗体购于美国R&D公司,P-NF-κB和NF-κB购于美国CST公司。
1.2 细胞培养及实验分组
将新生24 h的Wistar大鼠乳鼠脱颈处死,取中脑,除去脑膜和血管,用枪头吹打形成单细胞悬液。将悬液置于大离心管中,在4 ℃下以1 000 r/min离心5 min,弃上清,重悬于含有体积分数0.10胎牛血清、100 kU/L青霉素和100 mg/L链霉素的DMEMD/F12细胞培养液中,吹打混匀。将细胞接种到培养瓶中,置37 ℃、含体积分数0.05 CO2的无菌细胞培养箱中培养48 h。2 d后,更换新鲜培养液以除去未贴壁细胞,将贴壁细胞继续培养10 d,每3 d更换一次新鲜培养液。当细胞铺满瓶底90%以上时,将培养瓶置于恒温(37 ℃)摇床上,以200 r/min的转速摇17~20 h,以去除小胶质细胞和少突胶质细胞,清洗细胞,再用胰蛋白酶消化细胞,收集细胞接种于6孔板,进行后续的实验。将细胞分为对照组(A组)、FAC组(B组)、LPS组(C组)、FAC+LPS组(D组)和MG132+LPS组(E组),对照组用细胞培养液培养,FAC组和LPS组分别用FAC和LPS处理24 h,FAC+LPS组先用FAC预处理4 h后再用LPS处理24 h,MG132+LPS组先用MG132预处理4 h后再用LPS处理24 h。
1.3 蛋白质免疫印迹(Western blot)方法检测P-NF-κB和LCN2蛋白表达
细胞处理结束以后,吸除培养液,每孔加入100 μL裂解液,冰上静置30 min,用刮子将细胞刮下来,置于提前准备好的1.5 mL的EP管中,在4 ℃下以12 000 r/min离心30 min。吸取80 μL的细胞上清置于新的EP管中,用BCA试剂盒测定蛋白浓度,按照每孔20 μg蛋白计算上样体积,电泳(80 V、1 h,120 V、1 h),然后将蛋白湿转到PVDF膜上(30 mA、90 min)。使用50 g/L奶粉封闭2 h,加入β-actin一抗(1∶10 000)、LCN2一抗(1∶1 000)、P-NF-κB一抗(1∶1 000)、NF-κB一抗(1∶1 000),置4 ℃恒温摇床上过夜,16 h后,用TBST洗膜3次,每次10 min。分别加入山羊抗兔(1∶10 000)、兔抗山羊(1∶10 000)的二抗,室温下置摇床上1 h,用TBST洗膜3次,每次10 min;使用化学发光液进行显影。
1.4 统计学分析
应用Graphpad Prism软件进行统计学分析。计量资料以±s表示,多组比较采用单因素方差分析(One-Way ANOVA),然后用Turkey法进行组间两两比较。以P<0.05为差异有统计学意义。
2 结 果
2.1 FAC和LPS诱导的原代星形胶质细胞中P-NF-κB蛋白表达的变化
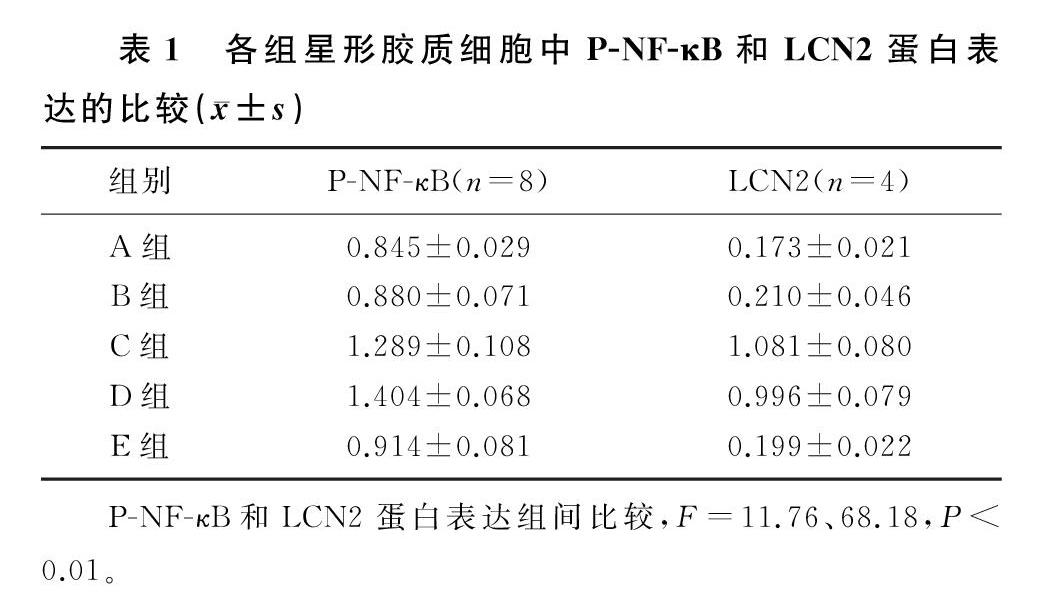
各组P-NF-κB蛋白表达水平比较差异具有统计学意义(F=11.76,P<0.01)。FAC处理后,细胞中P-NF-κB蛋白表达与对照组比较无明显变化(q=0.469,P>0.05);LPS处理后,P-NF-κB蛋白表达水平升高,与对照组相比差异具有统计学意义(q=5.859,P<0.01);FAC+LPS处理后,P-NF-κB蛋白表达水平与LPS组相比较差异无显著性(q=1.516,P>0.05),与对照组和FAC组相比差异具有统计学意义(q=7.376、6.907,P<0.01);MG132+LPS处理后,P-NF-κB蛋白表达与LPS组相比明显下降,差异具有统计学意义(q=4.939,P<0.05)。见表1。
2.2 FAC和LPS诱导的原代星形胶质细胞LCN2蛋白表達的变化
各组LCN2蛋白表达水平比较差异具有统计学意义(F=68.18,P<0.01)。FAC处理后,细胞中LCN2蛋白表达与对照组比较无明显变化(q=0.655,P>0.05);LPS处理后,LCN2蛋白表达升高,与对照组相比差异具有统计学意义(q=16.170,P<0.01);FAC+LPS处理后,LCN2蛋白表达水平与LPS组相比差异无显著性(q=1.512,P>0.05),与对照组和FAC组相比差异具有统计学意义(q=14.650、13.990,P<0.01);MG132+LPS处理后,LCN2蛋白表达与LPS组相比明显下降,差异具有统计学意义(q=15.710,P<0.01)。见表1。
3 讨 论
PD是第二大常见的神经退行性疾病,以中脑SN的DA能神经元选择性死亡为特征[1]。研究已经证明,PD发病与年龄老化、环境因素、遗传因素、氧化应激和自由基形成等有关,近年来越来越多的证据表明,神经炎症和铁在PD的进展中起关键作用[13-15]。目前的研究表明,PD病人的SN中铁选择性升高,并且铁的积累程度与PD的疾病严重程度相关[15-17]。在动物模型中,将不同浓度的FeCl3(分别含1、5和50 μg的Fe3+)单侧注射到成年大鼠的SN中,两种较低剂量的铁对DA水平和大鼠的行为反应没有影响,但是注射50 μg的Fe3+会导致DA能神经元的显著降低[18-19]。有文献报道,LPS的全身注射可促进小鼠的小胶质细胞和星形胶质细胞活化并增高促炎细胞因子水平[20]。PD病人的尸检结果显示,其DA能神经元大量丧失,小胶质细胞活化,一氧化氮、肿瘤坏死因子、白细胞介素和其他促炎细胞因子水平增高[21-22]。
有文献报道,在PD病人大脑中观察到LCN2表达的显著上调,并且SN和纹状体中的LCN2表达上调要明显高于海马和大脑皮质,LCN2水平增高将加重神经毒性和神经炎症,导致DA能神经元的破坏和异常运动行为[23]。还有研究显示,与野生型小鼠相比,在LCN2-/-小鼠中,颅内出血(ICH)引起较低的铁蛋白上调、小胶质细胞活化、脑肿胀、脑萎缩和神经功能缺损,FeCl2引起的病变程度以及脑肿胀和血-脑脊液屏障(BBB)破坏的程度也较轻,表明LCN2在ICH后增强脑损伤和铁毒性中起作用[24]。有文献报道,LCN2-/-小鼠的脑梗死体积、神经系统评分、BBB通透性、神经胶质激活和炎性递质表达等均明显低于野生型小鼠[25]。大量的研究表明,LCN2在中枢神经系统中起着重要作用,但是目前对LCN2的产生机制却知之甚少。本文研究结果显示,高铁状态对P-NF-κB和LCN2蛋白的表达没有影响,LPS则可促进P-NF-κB和LCN2的表达,用MG132预处理可以下调LPS对P-NF-κB和LCN2的诱导作用。提示MG132可能是通过抑制NF-κB途径而起到抑制LCN2表达的作用,LPS可能通过P-NF-κB上调星形胶质细胞LCN2的表达,参与PD神经炎症的进展。
[参考文献]
[1]HUANG B X, LIU J X, MENG T, et al. Polydatin prevents lipopolysaccharide (LPS)-induced Parkinsons disease via re-
gulation of the AKT/GSK3β-Nrf2/NF-κB signaling axis[J]. Frontiers in Immunology, 2018,9:2527.
[2]MCGEER E G, KLEGERIS A, MCGEER P L. Inflammation, the complement system and the diseases of aging[J]. Neuro-
biology of Aging, 2005,26(1):94-97.
[3]HIRSCH E C, HUNOT S. Neuroinflammation in Parkinsons disease: a target for neuroprotection[J]? The Lancet Neurology, 2009,8(4):382-397.
[4]MENZA M, DOBKIN R D, MARIN H, et al. The role of inflammatory cytokines in cognition and other non-motor symptoms of Parkinsons disease[J]. Psychosomatics, 2010,51(6):474-479.
[5]COOKSON M R. The biochemistry of Parkinsons disease[J]. Annual Review of Biochemistry, 2005,74:29-52.
[6]WARD R J, ZUCCA F A, DUYN J H, et al. The role of iron in brain ageing and neurodegenerative disorders[J]. The Lancet Neurology, 2014,13(10):1045-1060.
[7]HARE D J, ADLARD P A, DOBLE P A, et al. Metallobiology of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine neuroto-
xicity[J]. Metallomics, 2013,5(2):91-109.
[8]LEE S, LEE W H, LEE M S, et al. Regulation by lipocalin-2 of neuronal cell death, migration, and morphology[J]. Journal of Neuroscience Research, 2012,90(3):540-550.
[9]TSUKAMOTO H, TAKEUCHI S, KUBOTA K, et al. Lipopolysaccharide (LPS)-binding protein stimulates CD14-dependent Toll-like receptor 4 internalization and LPS-induced TBK1-IKKE-IRF3 axis activation[J]. The Journal of Biological Chemistry, 2018,293(26):10186-10201.
[10]CUAZ-PROLIN C, BILLIET L, BAUG E, et al. Antiinflammatory and antiatherogenic effects of the NF-kappaB inhibitor acetyl-11-keto-beta-boswellic acid in LPS-challenged ApoE-/- mice[J]. Arteriosclerosis, thrombosis, and vascular biology, 2008,28(2):272-277.
[11]KIM S J, PARK J H, KIM K H, et al. Effect of NF-κB decoy oligodeoxynucleotide on LPS/high-fat diet-induced atherosclerosis in an animal model[J]. Basic & Clinical Pharmacology & Toxicology, 2010,107(6):925-930.
[12]SHEN F, HU Z H, GOSWAMI J, et al. Identification of common transcriptional regulatory elements in interleukin-17 target genes[J]. The Journal of Biological Chemistry, 2006,281(34):24138-24148.
[13]GYONEVA S, SHAPIRO L, LAZO C, et al. Adenosine A2A receptor antagonism reverses inflammation-induced impairment of microglial process extension in a model of Parkinsons disease[J]. Neurobiology of Disease, 2014,67:191-202.
[14]DELEIDI M, GASSER T. The role of inflammation in spora-
dic and familial Parkinsons disease[J]. Cellular and Molecular Life Sciences: CMLS, 2013,70(22):4259-4273.
[15]YOU L H, LI F, WANG L, et al. Brain iron accumulation exacerbates the pathogenesis of MPTP-induced Parkinsons disease[J]. Neuroscience, 2015,284:234-246.
[16]DEXTER D T, WELLS F R, LEE A J, et al. Increased nigral iron content and alterations in other metal ions occurring in brain in Parkinsons disease[J]. Journal of Neurochemistry, 1989,52(6):1830-1836.
[17]VYMAZAL J, RIGHINI A, BROOKS R A, et al. T1 and T2 in the brain of healthy subjects, patients with Parkinson
disease, and patients with multiple system atrophy: relation to iron content[J]. Radiology, 1999,211(2):489-495.
[18]MARTIN-BASTIDA A, LAO-KAIM N P, LOANE C, et al. Motor associations of iron accumulation in deep grey matter nuclei in Parkinsons disease: a cross-sectional study of iron-related magnetic resonance imaging susceptibility[J]. Euro-
pean Journal of Neurology, 2017,24(2):357-365.
[19]BEN-SHACHAR D, YOUDIM M B. Intranigral iron injection induces behavioral and biochemical “Parkinsonism” in rats[J]. Journal of Neurochemistry, 1991,57(6):2133-2135.
[20]HOOGLAND I C, HOUBOLT C, VAN WESTERLOO D J, et al. Systemic inflammation and microglial activation: syste-
matic review of animal experiments[J]. Journal of Neuroinflammation, 2015,12:114.
[21]MCGEER P L, ITAGAKI S, AKIYAMA H, et al. Rate of cell death in Parkinsonism indicates active neuropathological process[J]. Annals of Neurology, 1988,24(4):574-576.
[22]MOGI M, HARADA M, KONDO T, et al. Interleukin-1 beta, interleukin-6, epidermal growth factor and transforming growth factor-alpha are elevated in the brain from parkinsonian patients[J]. Neuroscience Letters, 1994,180(2):147-150.
[23]KIM B W, JEONG K H, KIM J H, et al. Pathogenic upregulation of glial lipocalin-2 in the Parkinsonian dopaminergic system[J]. The Journal of Neuroscience, 2016,36(20):5608-5622.
[24]NI W, ZHENG M Z, XI G H, et al. Role of lipocalin-2 in brain injury after intracerebral hemorrhage[J]. Journal of Ce-
rebral Blood Flow and Metabolism, 2015,35(9):1454-1461.
[25]JIN M, KIM J H, JANG E, et al. Lipocalin-2 deficiency attenuates neuroinflammation and brain injury after transient middle cerebral artery occlusion in mice[J]. Journal of Cerebral Blood Flow & Metabolism, 2014,34(8):1306-1314.
(本文編辑 马伟平)
