中草药粗提物杀灭离体鱾新本尼登虫效果
2021-11-13刘彦甍侯廷龙付耀武冯娟张其中
刘彦甍, 侯廷龙, 付耀武, 冯娟, 张其中, *
中草药粗提物杀灭离体鱾新本尼登虫效果
刘彦甍1, a, 侯廷龙1, a, 付耀武1, 冯娟2, 张其中1, *
1. 暨南大学水生生物研究所, 广东省高校水体富营养化与赤潮防治重点实验室/热带亚热带水生态工程教育部工程研究中心,广州 510632 2. 中国水产科学研究院, 南海水产研究所/农业农村部南海渔业资源开发利用重点实验室, 广州 510300
鱾新本尼登虫作为海洋鱼类的一种体外寄生虫, 是海水养殖中主要的流行病原。为了探究盐度对鱾新本尼登虫成虫和幼虫活力的影响, 以成虫和幼虫的累积死亡率为指标, 检测了成虫和幼虫在四种盐度(0、10、20和30 ppt)胁迫下的活力状况。在此基础上, 测试了五种中草药猪苓、羌活、淡竹叶、牡丹皮和紫草的酒精粗提物离体杀灭鱾新本尼登虫的活性。研究结果表明, 当水温维持在22℃时, 成虫和幼虫处于30 ppt的海水盐度下, 在实验分别开始的8小时和5小时内, 它们的累积死亡率均为0%, 可以维持较好的活力, 该结果可为鱾新本尼登虫的离体杀虫实验提供时间依据。另外, 离体杀虫实验结果表明, 浓度为16 mg·L-1和8 mg·L-1的羌活酒精粗提物分别在158.7 min和135.7 min之内可以杀灭100%的成虫和幼虫。淡竹叶、牡丹皮和紫草的酒精粗提物在浓度高于64 mg·L-1时才能杀灭所有的幼虫。猪苓提取物减少了成虫和幼虫的存活率。羌活提取物在浓度高于128 mg·L-1时可以完全抑制鱾新本尼登虫虫卵的孵化, 但是, 持续暴露在256 mg·L-1的猪苓、淡竹叶和紫草粗提物下十天, 虫卵的孵化率仍无显著变化。根据这些结果可以表明上述的几种植物粗提物可以作为水产养殖中控制鱾新本尼登虫感染的植物型药物的潜在来源。
鱾新本尼登虫; 植物提取物; 抗寄生虫活性; 活力
0 Introduction
Capsalid skin flukes (Monogenea: Capsalidae) were considered to be one of the most serious and chronic problems in finfish[1-2].has been reported as a detrimental ectoparasite on many species of marine fishes[3]. The parasite was originally found by Hargis[4]on infected opaleye,, from California. In China, many fish such as,,sp.,,, andhave been reported to be hosts of[5].has a relatively simple life cycle, which comprises four developmental stages: the parasitic adult, the filamentous egg, and the infective oncomiracidia. Adultattachs to the host skin and lay filamentous eggs into the water, which hatchs into infective oncomiracidia that can re-infect rapidly[6]. Fish heavily infected bymay stop eating and suffer skin ulcerations, which can lead to slow growth, subsequent bacterial infections, and mortality[7-8].
There are few effective methods to preventinfections. Treatment ofprimarily relies on chemical compounds, such as formalin, copper sulphate, and potassium permanganate[9-10]. However, many of these chemicals have been proven to possess limited efficacy for controlling or preventing N. girellae[11]. In addition, a freshwater bath is a simple treatment method to dislodge N. girellae[12-13]. However, freshwater treatment may be stressful to the host fish[14], and the effort only removes attached parasite stages[15].Thus, there is an urgent need to develop an alternative therapeutant to control.
Traditional Chinese herbal medicinal plants have been used to control diseases of human beings and livestock for a long time[16-17]. Medicinal plants reduce the risks to human and other organisms by decreasing the bioaccumulation of harmful residues in the environment[18], and are generally preferred. Recently, there are considerable public and scientific interest in the use of herbal compounds for treating fish diseases caused by protozoa, metazoan, bacteria, and fungi[19]. The efficacy of,,,,andagainst bacterial or viral diseases has been previously investigated[20-24]. However, there are no reports investigating the antiparasitic efficacy of these five plants against.
The present study aimed to evaluate the activity power ofadults and oncomiracidia at different salinities, and examine theefficacy of plant crude extracts of,,,,andagainst, as well as find potential alternative parasiticides for controllingin aquaculture.
1 Materials and methods
1.1 Fish and parasites
One hundredinfected with, weighing 205 ± 12 g, were obtained from a local fish farm in Zhanjiang City, Guangdong Province, China. All fish were kept in several 100-L glass aquaria equipped with re-circulated aerated tap seawater. Fish were fed daily at 2% body weight with pellet feed (Hengxing Coral Feed Co., Ltd., Zhanjiang, China).
Adults, eggs, and oncomiracidia ofwere collected as described previously[6]. Briefly,infected with(mean intensity of 22.5±3.2) were anaesthetized with 150 mg·L-1tricaine methanesulfonate (MS-222, Sigma, USA).adults were carefully removed with a needle and scalpel from the body surface of fish and transferred to wells of a 24-well tissue culture plate filled with 500 μL filtered seawater (30 ppt of salinity). The live adults could rapidly attach to the bottom of the culture plateand lay filamentous eggs into the water, which could be collected by a needle. The eggs were transferred to wells of a 96-well tissue culture plate filled with 200 μL filtered seawater for 7 to 10 days cultivation to produce oncomiracidium.
1.2 Preparation of plant extracts
Five medicinal plants (,,,, and) were purchased from the Qingping Chinese medicine market in Guangzhou, China. They were dried in an oven at 55°C. Dried plant materials were then pulverized in an electronic grinder with size 50 mesh strainer. Five dried powder samples (50 g each) were extracted three times with 500 mL of 95% ethanol. All ethanol extracts of each plant were combined, subsequently concentrated at 60°C under reduced pressure in a vacuum rotary evaporator. Dried extracts of each plant were stored at 4°C prior to use.
1.3 Activity power tests of N. girellae adults
Adults ofwere collected according to the method described above (Section 1.1). Adults could attach rapidly to the bottom of the culture plate using their attachment organ. Adults were observed for activity in a light microscope to ensure transfer had not affected parasite survival. Any opaque or dead parasites were removed from the culture plate, and then new parasites were added. Activity power tests of adults were applied in several 24-well tissue culture plates filled with 500 μL filtered seawater (0, 10, 20, and 30 ppt). Each well contained five adult parasites. There were eight replicated wells in each concentration. The experiments were carried out in a constant temperature environment (22 ± 1°C). Wells were observed under a light microscope for continuous 20 hours. Live or dead adults in each well of the 24-well tissue culture plate were recorded every two hours. Parasites were considered dead if they were no movement and did not respond to light and mechanical stimulation. Cumulative mortality (cumulative dead parasite number/total parasite number) ofadult in each well was determined every two hours.
1.4 Activity power tests of N. girellae oncomiracidia
Newly laid eggs were collected and incubated for 7 to 10 days to obtain oncomiracidia in a 96-well tissue culture plate with 200 μL filtered seawater (30 ppt of salinity) at a constant temperature environment (22 ± 1°C). Activity power tests of oncomiracidia were performed in several 96-well tissue culture plates filled with 200 μL filtered seawater (0, 10, 20, and 30 ppt). Each well contained approximately twenty oncomiracidia. There were eight replicated wells in each concentration. Wells were monitored under a light microscope every hour, for eight consecutive hours. Time of death was recorded when oncomiracidia stopped moving and failed to respond to a light stimulus. Cumulative mortality ofoncomiracidia in each well was determined.
1.5 In vitro efficacy of plant extracts against N. girellaeadults, eggs, and oncomiracidia
A stock solution was prepared by dissolving 3.072 mg of a plant extract in 1.5 mL of filtered seawater (30 ppt of salinity) containing 1% (v/v) dimethyl sulfoxide (DMSO) to a final concentration of 2048 mg·L-1. Stock solutions were stored at -20°C prior to the experiments. Twofold serial dilutions of a stock solution was made in several 1.5 mL centrifuge tubes to yield concentrations of 1024, 512, 256, 128, 64, 32, 16, and 8 mg·L-1. Two controls, 0.1% DMSO in seawater and untreated seawater, were prepared for each plant extract.
tests of adults were applied in 24-well tissue culture plates filled with 500 μL filtered seawater (30 ppt of salinity) and 500 μL test solutions in each well to get final concentrations of 512, 256, 128, 64, 32, 16, and 8 mg·L-1. Five adult parasites were added into each well. There were three replicated wells in each concentration. Trials were repeated three times with different adult populations on different dates. Wells were monitored under an optical microscope following exposure for eight hours. The lethal duration of adult in each well of the 24-well tissue culture plate was recorded. Parasites were considered dead if there was no movement and/or they did not respond to light and mechanical stimulation[6]. Each plant extract was evaluated in separate experiments.
For the eggs and oncomiracidia trial, approximate twenty eggs or oncomiracidia in 200 μL filtered seawater (30 ppt of salinity) were distributed to each well of a 96-well plate. The plant extract solutions were added to each well to get final concentrations of 128, 64, 32, 16, 8, and 4 mg·L-1with three replicates. Trials were repeated 3 times with different egg or oncomiracidium populations. Eggs were exposed to continuous immersion in each plant extract and control for 10 days, incubated at a constant temperature environment (22 ± 1°C) under natural photoperiod. Every 24 h, eggs were observed under a light microscope. Hatching rate ofeggs was calculated based on the number of eggs with an opened operculum. The lethal duration of oncomiracidia was determined for each well at 5 h post treatment.
1.6 Statistical analysis
Data in this study were statistically analyzed using the IBM SPSS Version 22.0 and expressed as mean ± standard deviation (SD). Differences between groups were determined by Duncan’s multiple range test using one-way ANOVA. Probabilities of 0.05 were considered to be statistically significant.
2 Results
2.1 Activity power ofadults
The results showed a noticeable time-response relationship, with longer incubating time resulting in greater mortality ofadults (Table 1). Dead parasites were not observed in the wellswithin 8 hours of the start of the experiment at 30 ppt of salinity. Adult parasites at 20 ppt and 30 ppt of salinity showed 22.5% and 5.0% mortality after a 10 h exposure, respectively. After 18 hours, cumulative mortality ofadults was up to 90.0% when exposed to 30 ppt seawater. All wells containing seawater showed 100% mortality of adult parasites at the end of the experiment. Live parasites were not observed in the wellswhen exposed to freshwater.
2.2 Activity power of N. girellae oncomiracidia
Shorter incubating time resulted in fewer mortality ofoncomiracidia (Table 2). No oncomiracidium was live in the wellswhen exposed to freshwater. Oncomiracidia showed 0.0% mortality at 30 ppt of salinity after a 5 h exposure. Dead oncomiracidia were not observed in the wellswithin 4 hours of the start of the experiment at 20 ppt of salinity. Oncomiracidia at 10 ppt of salinity showed 5.0% and 63.7% mortality after a 1 or 2 h exposure, respectively. All wells containing seawater showed 100% mortality ofoncomiracidia after 8 h exposure.
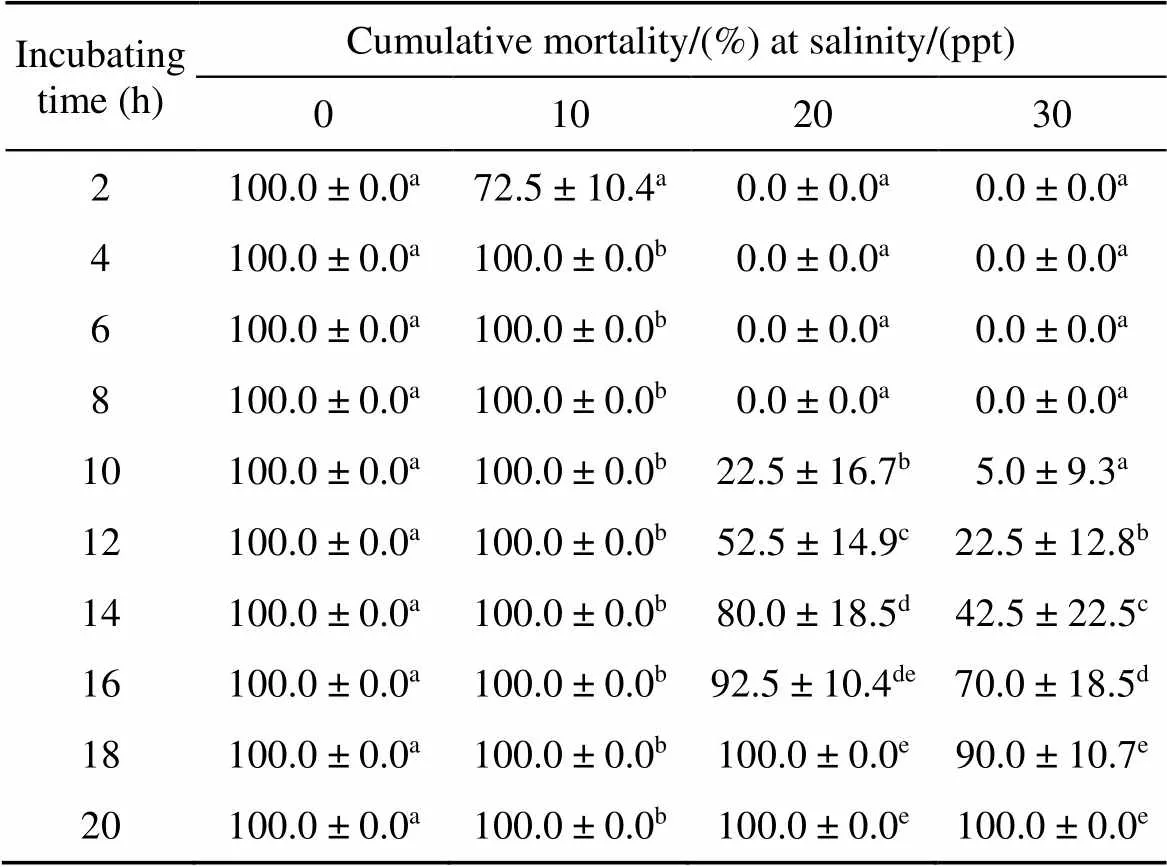
Table 1 Activity power of N. girellaeadults at different salinities at 22 ± 1°C
Values with different lowercase letters in the same column are significantly different (< 0.05).

Table 2 Activity power of N. girellaeoncomiracidia at different salinities at 22 ± 1°C
Values with different lowercase letters in the same column are significantly different (< 0.05).
2.3 In vitro efficacy of plant extracts against N. girellae adults
A noticeable anti-adults effect was observed in treatment groups (Table 3). The mean lethal duration for killing all adults became significantly shorter with increment of plant extracts concentration. For example, the mean lethal duration for killing all adults was 158.7 min byethanol extract at 16 mg·L-1, but just 4.7 min at 512 mg·L-1. Extract ofcaused 100% mortality of adults at the 512 mg·L-1concentration after 90.7 min of exposure but did not kill all adults within 8 h exposure at 256 mg·L-1. Mean death duration for killing all adults ranged from 7.3 min at 512 mg·L-1to 151.7 min at 64 mg·L-1ofethanol extract.The two controls containing 0.1%DMSO and just seawater caused no mortality of adults.
2.4 In vitro efficacy of plant extracts against N. girellae eggs
andethanol extract at ≥128 mg·L-1and 256 mg·L-1, respectively, caused no egg development (Table 4). Eggs remained clear brown in colour during 10 days exposure. There was no significant efficacy on hatching rate exposed to 256 mg·L-1of,, andethanol extract for 10 days, the hatching rate of eggs in these treatments was close to 100%. Hatching rate of controls containing 0.1% DMSO or only seawater was at a normal level.
2.5 In vitro efficacy of plant extracts against N. girellae oncomiracidia
Anti-oncomiracidia efficacy significantly increased with increase in treatment concentrations of plant extracts (Table 5). Alloncomiracidia were dead when exposed to ethanol extractofat 128 mg·L-1for 35.0 min. After 5 h exposure,ethanol extract at ≥8 mg·L-1caused 100% mortality of oncomiracidia. Mean death duration for killing all oncomiracidia ranged from 30.7 min at 128 mg·L-1to 135.7 min at 8 mg·L-1ofextract.,,andextracts at ≥64 mg·L-1killed alloncomiracidia. The two controls containing 0.1% DMSO or just seawater caused no mortality of oncomiracidia.

Table 3 Lethal duration of N. girellaeadults in different concentrations of plant extracts during 8 h exposure
Values with different lowercase letters in the same row are significantly different (< 0.05). +: plant extracts at the listed concentrations did not kill alladults within 8 h exposure.
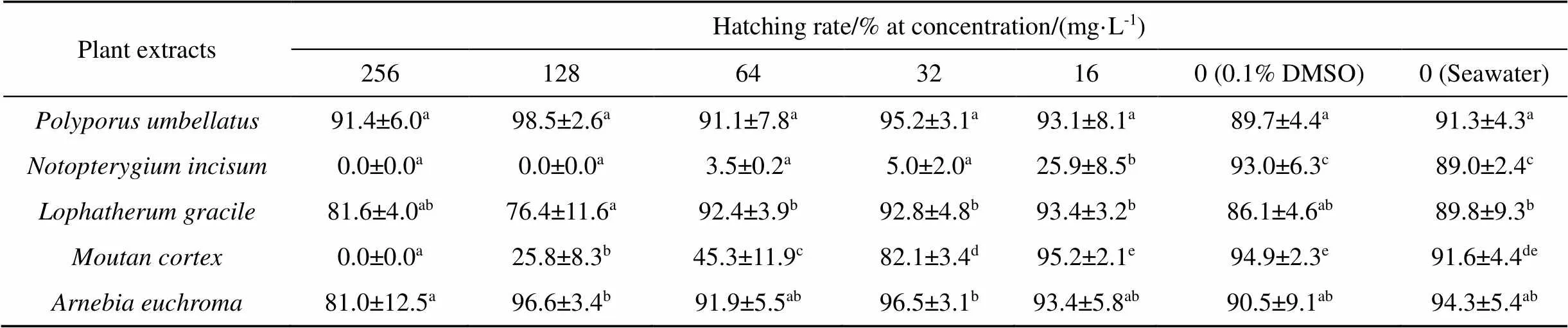
Table 4 Hatching rate of N.girellae eggs exposed to different concentrations of plant extracts for 10 days
Values with different lowercase letters in the same row are significantly different (< 0.05).
3 Discussion
Treatment that disrupt or break the life cycle of the ectoparasites by killing parasitesis considered as an effective method to manage parasite infections in aquaculture[25-26]. Necessity to this method is the accurate determination of activity power of adults and oncomiracidia, as well as hatching periods of eggs at specific environmental parameters (i.e., temperature and salinity).experiments provide a controlled and easily manipulated environment and have fewer potential concerns[27]. In our study,experiments were conducted on a 24-well plate for 20 hours and a 96-well plate for 8 hours to determine the activity power ofadults and oncomiracidia, respectively. The adults and oncomiracidia maintained good activity in the first 8 and 5 hours at 30 ppt of salinity, respectively. Based on the results of this study, we suggested that theadults or oncomiracidia should be used forexperiments (water temperature of 22 ± 1°C, 30 ppt of salinity) within 8 or 5 hours, respectively. For example, in thetest, only the anti-adults efficacy within 8 hours was calculated. However, the best testing time forexperiments at other temperatures required further study.
Phytotherapy, an alternative treatment for treating fish disease, has attracted researchers’ considerable attention[18-19, 28]. In previous reports, plant extracts were potential resources for production of anti-agents. For example, garlic extract was demonstrated to significantly decrease the hatching rate ofeggs and oncomiracidia longevity[29]. Trasviña-Moreno, et al.[6]evaluated the efficacy of six water-ethanol plant extracts againstsp. and found that the adult parasite survival rate was significantly reduced when treated with dilutions 1:100 and 1:50 oforextract. In the present study,antiparasitic efficacy of five medicinal plants (,,,,and) extracts againstadults, eggs, or oncomiracidia was evaluated. Among these four medicinal plants,ethanol extract showed best anthelmintic effect against.is a traditional Chinese medicinal plant with the rhizome and roots, known as‘Qianghuo’ in Chinese. It has been used over the years to disperse cold, prevent painful obstructions from wind, damp and warm pain[21]. The extract ofshowed remarkable antibacterial activity against. Bioactivity-guided fractionation led to the isolation of two active compounds, falcarindiol and phenethyl ferulate[30]. In this study, we speculate that falcarindiol or phenethyl ferulate were the active compounds inethanol extract that contributed to the most effective against. However, the exact compound for anthelminticefficacy is unknown and further research is needed.

Table 5 Lethal duration of N. girellaeoncomiracidia in different concentrations of plant extracts during 5 h exposure
Values with different lowercase letters in the same row are significantly different (< 0.05). ND: not detected. +: plant extracts at the listed concentrations did not kill alle oncomiracidiawithin 5 h exposure.
Monogenean eggs have been reported to have high resistance to physical and chemical treatments due to the protection of a proteinaceous shell[6]. Numerous chemical agents have been investigated for their efficiency at preventing hatching of monogenean eggs with little success[15]. However, in this study, it was found thathatching did not occur and larval development failed in 128 mg·L-1extract or 256 mg·L-1ofextract for 10 days of continuous immersion. Similarly, previous reports have demonstratedeggs are highly sensitive to the water-soluble extract of red seaweed[15]. Additionally, oncomiracidia live in water, and are sensitive to parasiticides or chemicals. Reducing the intensity of infective theronts can interrupt infection ofto fish[31]. Hence, it is crucial to kill all oncomiracidia in order to controlinfection. In this study, lethal duration of oncomiracidia after 5 h exposure was used for evaluating antiparasitic efficacy of plant extracts against. The results showed that the ethanol extract ofhad the strongest effect againstoncomiracidia. The lowest lethal concentration ofethanol extract was 8 mg·L-1. Therefore, compared with the other four plant extracts,extract could be a promising alternative parasiticide to controleffectively.
Crude plant extracts are natural and easy to get compared to phytochemicals, so they can be readily used for controlling fish diseases in aquaculture[32]. Thus, crude extracts ofcould be used as the feasible treatments to control. Compared with the other four medicinal plants,has a lower price and better antiparasitic efficacy against. Therefore, crude extract ofis more likely to be applied to the prevention and control ofinfection.anthelminticexperiments were applied to determine potential drugs against adultin this study. However, in order to evaluate the drug comprehensively,anthelmintic and toxicity experiments should be carried out in future studies.
4 Conclusion
In this study, the activity power ofadults and oncomiracidia were evaluated. And the potential use of plant extracts for the treatment ofinfections was determined. Among the five plant extracts,extract proved to be most effective against adults, eggs, and oncomiracidia of. It showed potential for alternative methods to manage parasitic disease in aquaculture. Further studies should be conducted to evaluateefficacy of these plant extracts against, as well as the toxicity of these medicinal plants against various hosts.
[1] OGAWA K, BONDAD-REANTASO M G, FUKUDOME M, et al.(Hargis, 1955) Yamaguti, 1963 (Monogenea: Capsalidae) from cultured marine fishes of Japan[J]. Journal of Parasitology, 1995, 81: 223-227.
[2] MILITZ T A, SOUTHGATE P C, CARTON A G, et al. Dietary supplementation of garlic () to prevent monogenean infection in aquaculture[J]. Aquaculture, 2013, 408: 95-99.
[3] OHASHI H, UMEDA N, HIRAZAWA N, et al. Antiparasitic effect of calcium and magnesium ion-free buffer treatments against a common monogenean[J]. Parasitology, 2007, 134: 229-236.
[4] HARGIS W J. A new species of(Trematoda: Monogenea) from, the Opaleye[J]. Journal of Parasitology, 1955, 41: 48-50.
[5] YANG Wenchuan, LI Liwei, WANG Yanhai. Study on Benedeniasis on maricultured fishes in Fujian[J]. Marine Science, 2004, 28: 39-43.
[6] TRASVIñA-MORENO A G, ASCENCIO F, ANGULO C, et al. Plant extracts as a natural treatment against the fish ectoparasitesp. (Monogenea: Capsalidae)[J]. Journal of Helminthology, 2017, 93: 57-65.
[7] OGAWA K. Diseases of cultured marine fishes caused by Platyhelminthes (Monogenea, Digenea, Cestoda)[J]. Parasitology, 2015, 142: 178-195.
[8] HIRAZAWA N, HAGIWARA H, TSUBONE S, et al. Investigation of the toxicological and histopathological effects of hydrogen peroxide bath treatments at different concentrations onspecies and the effectiveness of these treatments on(Monogenea) infestations[J]. Aquaculture, 2017, 479: 217-224.
[9] THONEY D A, HARGIS J R W J. Monogenea (Platyhelminthes) as hazards for fish in confinement[J]. Annual Review of Fish Diseases, 1991, 1: 133-153.
[10] MORALES-SERNA F N, CHAPA-LóPEZ M, MARTíNEZ- BROWN J M, et al. Efficacy of praziquantel and a combination anthelmintic (Adecto®) in bath treatments againstand(Monogenea), parasites of bullseye puffer fish[J]. Aquaculture, 2018, 492: 361-368.
[11] WUNDERLICH A, GUIMARAES A, TAKEARA R. Plant-derived compounds as an alternative treatment against parasites in fish farming: a review[M]. KHATER H, GOVINDARAJAN M, BENELLI G. Natural Remedies in the Fight Against Parasites. London; UK: InTechOpen. 2017: 246.
[12] SENG L T. Control of parasites in cultured marine finfishes in Southeast Asia--an overview[J]. International Journal for Parasitology, 1997, 27: 1177-1184.
[13] HIRAZAWA N, AKIYAMA K, UMEDA N. Differences in sensitivity to the anthelmintic praziquantel by the skin-parasitic monogeneansand[J]. Aquaculture, 2013, 404-405: 59-64.
[14] HIRAZAWA N, TSUBONE S, TAKANO R. Anthelmintic effects of 75 ppm hydrogen peroxide treatment on the monogeneans,, andinfecting the skin and gills of greater amberjack[J]. Aquaculture, 2016, 450: 244-249.
[15] HUTSON K S, MATA L, PAUL N A, et al. Seaweed extracts as a natural control against the monogenean ectoparasite,sp., infecting farmed barramundi ()[J]. International Journal for Parasitology, 2012, 42: 1135-1141.
[16] BULFON C, VOLPATTI D, GALEOTTI M. Current research on the use of plant-derived products in farmed fish[J]. Aquaculture Research, 2015, 46: 513-551.
[17] ACHARYA K P, ACHARYA M. Traditional knowledge on medicinal plants used for the treatment of livestock diseases in Sardikhola VDC, Kaski, Nepal[J]. Journal of Medicinal Plants Research, 2010, 4: 235-239.
[18] RAHUMAN A A, GOPALAKRISHNAN G, VENKATESAN P, et al. Larvicidal activity of some Euphorbiaceae plant extracts againstand(Diptera: Culicidae)[J]. Parasitology Research, 2008, 102: 867-873.
[19] VALLADAO G M, GALLANI S U, PILARSKI F. Phytotherapy as an alternative for treating fish disease[J]. Journal of Veterinary Pharmacology and Therapeutics, 2015, 38: 417-428.
[20] WANG Tianyuan, ZHANG Feifei, REN Yueyin, et al. Research progress on chemical constituents and pharmacological actions of[J]. Shanghai Journal of Traditional Chinese Medicine, 2017, 51: 109-112.
[21] AZIETAKU J T, MA H, YU X A, et al. A review of the ethnopharmacology, phytochemistry and pharmacology of[J]. Journal of Ethnopharmacology, 2017, 202: 241-255.
[22] XUE Yueqin, SONG Jie, YE Suping, et al. Separation,identification and its antibacterial activity of glycosylflavones inBrongn[J]. West China Journal of Pharmaceutical Sciences, 2009, 24: 218-220.
[23] FU Ruoqiu, MENG Desheng, HU Daqiang, et al. Antibacterial effect of aqueous extract and ethanol extract of[J]. China Pharmaceuticals, 2010, 19: 29.
[24] LI Cuifang, WANG Fang, MA Hao, et al. Study on antibacterial activity of(Royle) Johnst hairy roots extract[J]. Journal of Agricultural University of Hebei, 2010, 33: 92-96.
[25] BRAZENOR A K, HUTSON K S. Effects of temperature and salinity on the life cycle ofsp. (Monogenea: Capsalidae) infecting farmed barramundi ()[J]. Parasitology Research, 2015, 114: 1875-1886.
[26] LIU Yanmeng, ZHANG Qizhong, XU Dehai, et al. Antiparasitic efficacy of commercial curcumin againstin grass carp ()[J]. Aquaculture, 2017, 480: 65-70.
[27] LANKVELD D P K, LOVEREN H V, BAKEN K A, et al.testing for direct immunotoxicity: state of the art[J]. Methods in Molecular Biology, 2010, 598: 401-423.
[28] CHENG Sensung, HUANG Chingi, CHEN Weijune, et al. Larvicidal activity of tectoquinone isolated from red heartwood-typeagainst two mosquito species[J]. Bioresource Technology, 2008, 99: 3617-3622.
[29] MILITZ T A, SOUTHGATE P C, CARTON A G, et al. Efficacy of garlic () extract applied as a therapeutic immersion treatment forsp. management in aquaculture[J]. Journal of Fish Diseases, 2014, 37: 451-461.
[30] MATSUDA H, SATO N, TOKUNAGA M, et al. Bioactive constituent of, falcarindiol having antibacterial activity againstisolated from patients with atopic dermatitis[J]. Natural Medicines, 2002, 56: 113-116.
[31] LIU Yanmeng, ZHANG Qizhong, XU Dehai, et al. Antiparasitic efficacy of curcumin fromagainstin grass carp[J]. Veterinary Parasitology, 2017, 236: 128-136.
[32] FU Yaowu, ZHANG Qizhong, XU Dehai, et al. Parasiticidal effects ofroot bark extracts againstinfecting grass carp[J]. Diseases of Aquatic Organisms, 2014, 108: 129-136.
efficacy of crude plant extracts against
LIU Yanmeng1, a, HOU Tinglong1, a, FU Yaowu1, FENG Juan2, ZHANG Qizhong1, *
1. Engineering Research Center of Tropical and Subtropical Aquatic Ecological Engineering, Ministry of Education, Key Laboratory of Aquatic Eutrophication and Red Tide Prevention of Guangdong Higher Education Institutes, Institute of Hydrobiology, Jinan University, West 601 Huangpu Avenue, Tianhe District, Guangzhou 510632, China 2. Key Laboratory of South China Sea Fishery Resources Exploitation and Utilization, Ministry of Agriculture, South China Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Guangzhou 510300, China
is an ectoparasite of marine fishes, which causes major epidemics in marine aquacultureIn order to investigate the effect of salinity on the activity of the adults and oncomiracidia of, the mortalities ofadults and oncomiracidia were evaluated at different salinities (0, 10, 20, and 30 ppt). Additionally, theefficacy of ethanol extracts of,,,,andagainstwas investigated. Results showed the adults and oncomiracidia ofshowed 0.0% mortality at 30 ppt of salinity after an 8 h and 5 h exposure at (22 ± 1) °C, respectively. They can maintain good activity power. The result provided time basis for theexperiment of.results indicated thatethanol extract resulted in 100% mortality ofadults at a concentration of 16 mg·L-1within 158.7 min and killed all oncomiracidia at 8 mg·L-1within 135.7 min.,,andextracts at ≥ 64 mg·L-1killed alloncomiracidia.extract reduced adults and oncomiracidia survival.extract at ≥ 128 mg·L-1caused 0% hatching rate with no egg development. However, when exposed to 256 mg·L-1of,, andethanol extract for 10 days, there was no significant efficacy on eggs hatching rate. These findings demonstrate that these plant extracts are potential sources of botanical drugs for controllinginfection in aquaculture.
; plant extract; antiparasitic efficacy; activity power
10.14108/j.cnki.1008-8873.2021.05.006
S943S
A
1008-8873(2021)05-040-09
2021-07-01;
2021-07-23
广东省省级科技计划项目(2016B090918112, 2016B090918029); 广东省渔业生态环境重点实验室开放基金(LFE-2016-2); 广州市科技计划项目(201604020040)
刘彦甍(1993—), 男, 湖南衡阳人, 博士研究生, 主要从事水产病害研究, E-mail: liuyanmeng93@163.com
a. 刘彦甍和侯廷龙贡献相同
通信作者:张其中, 男, 博士, 教授, 主要从事水产病害与分子免疫研究, E-mail: zhangqzdr@126.com
刘彦甍, 侯廷龙, 付耀武, 等. 中草药粗提物杀灭离体鱾新本尼登虫效果[J]. 生态科学, 2021, 40(5): 40-48.
LIU Yanmeng, HOU Tinglong, FU Yaowu, et al.efficacy of crude plant extracts against[J]. Ecological Science, 2021, 40(5): 40-48.
