Manipulating metal-support interactions of metal catalysts for Fischer-Tropsch synthesis
2021-10-12QingpengChengYunhaoLiuShuaishuaiLyuYeTianQingxiangMaXingangLi
Qingpeng Cheng,Yunhao Liu,Shuaishuai Lyu,Ye Tian,Qingxiang Ma,Xingang Li,4,*
1 Collaborative Innovation Center of Chemical Science and Engineering (Tianjin),State Key Laboratory of Chemical Engineering,Tianjin Key Laboratory of Applied Catalysis Science and Technology,School of Chemical Engineering and Technology,Tianjin University,Tianjin 300072,China
2 Advanced Membranes and Porous Materials Center,Physical Sciences and Engineering Division,King Abdullah University of Science and Technology (KAUST),Thuwal 23955-6900,Saudi Arabia
3 State Key Laboratory of High-efficiency Coal Utilization and Green Chemical Engineering,Ningxia University,Yinchuan 750021,China
4 School of Chemical and Biological Engineering,Lanzhou Jiaotong University,Lanzhou 730070,China
Keywords:Metal-support interactions Fischer-Tropsch synthesis Modification strategies Activity Selectivity
ABSTRACT For supported metal catalyst systems,the impact on catalysis originates from the interaction between metal nanoparticles and their support.Metal-support interactions(MSI)can change electronic properties,geometric morphologies,or chemical compositions of metal nanoparticles to make active sites have specific properties and catalytic activities.Fischer-Tropsch synthesis (FTS) is one of the most effective ways to convert cheap non-petroleum-based carbon sources into high value-added chemicals or ultraclean liquid fuels.In this review,we summarize and classify the impact of MSI on the catalytic activity,selectivity and stability of FTS catalysts.The strategies to tune MSI are introduced in detail,and the recent development of high-efficiency FTS catalysts through the manipulation of SMI strategies has been highlighted.It is emphasized that the active metal sites,which are endowed with special functions by MSI,can change the strength of adsorption bond of adsorbates,consequently controlling the product distribution.
1.Introduction
Concerns about global oil depletion and increasingly stringent environmental regulations raise urgent demands to find alternative feedstocks to produce important petrochemicals and fuels.Fischer-Tropsch synthesis (FTS),a key step of technologies of gas to liquid (GTL),coal to liquid (CTL),and biomass to liquid (BTL),can synthesize clean liquid fuels such as diesel fuel,jet fuel and gasoline (Fig.1)viacatalytic polymerization of syngas (CO and H2) predominantly derived from the above mentioned low-value non-petroleum-based carbon sources [1–4].In terms of FTS in industry,the development and application of cobalt-based and iron-based catalysts have achieved great success.Besides South Africa (Sasol) and Netherlands (Shell),where FTS has been long industrialized,many countries currently have FTS plants or completed technology packages,such as Australia,Bolivia,Chile,China,Egypt,Germany,Indonesia,Iran,Italy,Nigeria,Russia and USA by different companies including Bioliq,BP,EniTechnologie,Exxon-Mobil,Shenhua,Synfuels China,Synthroleum and Yankuang group[5].The interest of FTS in the academic community has also increased significantly in recent years.The number of FTS related publications increases from 893 in 2011 to 2016 in 2020 based on Web of Science (Fig.2).
FTS,which can produce hundreds of products due to the diversity of reaction paths,is one of the extremely complex reaction systems [6–8].The main reactions in FTS can be expressed with following Equations:
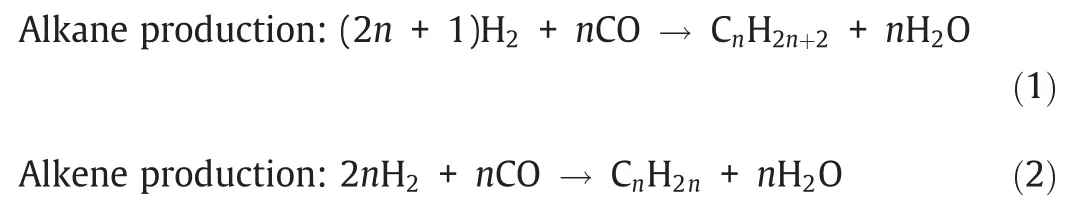
Side reactions in FTS including water-gas shift,alcohol production,and Boudouard reactions:


Fig.1.Catalytic transformation of non-petroleum carbon resources into liquid fuels and chemicals via syngas using the FTS technology.

Fig.2.Numbers of FTS related publications in the past 10 years.Data achieved from Web of Science using the keyword ‘‘Fischer-Tropsch synthesis or CO hydrogenation”.

FTS is a polymerization process,involving chain initiation,chain propagation and chain termination.In the chain initiation step,CO dissociates into active C* or non-dissociates adsorption of CO at metal or carbide active sites,and CH2* intermediate is formed via the hydrogenation reaction;in the chain propagation step,chain growth occursviaC-jianchang coupling over active sites;finally,in the chain termination step,the resulting alkyl chain desorbs from metal or carbide sites after hydrogenation or β-scission,forming paraffin or olefins.Generally,the resulting products of FTS follow a statistical distribution identified as the Anderson-Schulz-Flory (ASF) law [9–13].Eq.(6) shows that the chain growth probability (α) describes the function using molar fraction (Mn) of the product with a carbon number ofnand depends on the rates of propagation (rp) and termination (rt).

As shown in Fig.3,the FTS product selectivity is limited by the ASF distribution.For example,the maximum selectivity of gasoline fractions(C5-C11)is limited to 45%,and the maximum selectivity of diesel fractions (C10-C20) is limited to 39%.Therefore,the biggest challenge in the FTS field is how to design efficient catalysts with a high target product selectivity.

Fig.3.Product distribution in FTS as a function of the chain growth probability(α).
Both supported and unsupported catalysts are widely applied in FTS,especially for cobalt-,iron-,and ruthenium-based supported catalysts [5,6,8].The supports can provide an effective surface and a suitable pore structure,resist the aggregation of active metal components,and enhance the mechanical strength of catalysts.The supports can also provide additional active centers (such as bifunctional catalysts [1,3,5–7]).More important,there is usually some kind of interaction between the metal and supports,which changes the electronic structure and geometric structure of the active component,inducing different activities.For supported metal catalysts,metal-support interactions (MSI) generally determine charge transfer,nanoparticle morphology,and chemical composition,etc.For example,Qinet al.[14]fabricate a stable optimal cobalt-facet (Co(10–11)) catalyst Co@C-SiO2using the intermediate carbon and the silica as a protective shell.The moderate MSI endowed by carbon,combining the confined effect of the core–shell structure,protects and maintains the single-crystal structure,resulting in a long-term stable operation with a low methane selectivity,high activity andselectivity.Such MSI have a profound impact on the resulting performance of the catalysts.Therefore,it is one of the effective strategies to optimize FTS performance by manipulating MSI during the catalyst design.Recently,this topic has attracted widespread attention,as judged from an increasing number of such reports.
Some reviews on MSI are available [15–17],but few focus on the tuning MSI in FTS.This review starts with a brief introduction of the relevant concepts of the MSI phenomenon,and the next section will discuss the latest reports on the effect of MSI adjustment strategies on the activity,selectivity,and stability of the FTS catalysts.
2.Metal-support Interactions
Metal nanoparticles stabilized on support can catalyze lots of major chemical reactions.In the past,metal-support interactions were often overlooked,and only naive metal active sites were considered to be decisive for catalytic reactions.In fact,for supported metal catalysts,metal sites interacted with supports can exhibit a special electron cloud density,geometry structure/particle morphology,chemical composition,or strong metal-support interaction (SMSI)etc.,which is the essential factor to determine catalytic performance (Fig.4a) [17].In addition,the changed geometric structure and/or electronic structure caused by MSI and/or SMSI are also determine the reactivity on active center.This phenomenon may be very complicated,when several factors are entangled together.
2.1.Electronic interactions
The change of the local electron cloud density over the active sites induces the difference of the adsorption and desorption performance of the reactants,intermediate species,and products,and ultimately influences catalytic performance,including activity and selectivity.In general,the interface between the metal nanoparticles and support is likely to occur charge transfer[18–20],which is governed by the fundamental principles,such as energy minimization and continuity of potential in solids.And the difference in the Fermi level between them plays a dominant role in the magnitude and direction of the charge transfer via balancing the electronic chemical potential.Based on the degree of the interaction,it can be classified as weak metal-support interaction(WMSI)or SMSI[21–24].For WMSI,the electron cloud density of metal sites has little change.However,SMSI can cause major changes in electronic properties.Typically,although metal nanoparticles can easily migrate electrons,the charge transfer of metal with support is affected by the physicochemical properties of the support,such as its electrical conductivity,reducibility,exposed crystal plane,morphology,incorporation of heteroatoms,and occurrence of defects.In addition,the particle size of the active metal also reflects the interaction between the metal and support to a certain extent.Generally,stronger MSI is likely to induce the formation of smaller-sized metal particles.And the small-sized metal nanoparticles always exhibit different reactivity of supported metal catalysts in comparison to the larger sized one.In terms of geometric structure,as the size of metal particles decreases,low-coordination atoms are gradually exposed and their proportions gradually increase,which significantly changes the structure and proportion of active centers.From the point of view of electronic structure,the electronic energy level of metal particles also changes significantly due to the size effect,which greatly affects the orbital hybridization and charge transfer between the active metal sites and reactants.Indeed,de Jonget al.[25] studied the origin of the cobalt particle size effects in Fischer-Tropsch catalysis by the steady-state isotopic transient kinetic analysis.It is found that the smaller cobalt exposes more surface atoms with a lower coordination number,leading to an increased localization of the valence electrons.This localization causes an upward shift of the center of d-band,which will strengthen the bond between cobalt and adsorbate.As such,it is a matter of concern to rationally modify the metal-adsorbate bond and further enhance the catalytic performance by tuning electron transfer.

Fig.4.(a) The main phenomena of metal-support interactions.Adapted from Ref.[17],Copyright © 2019,Elsevier.(b) Illustration of one-step hydrogen reduction of Pt-Co catalyst.Adapted from Ref.[29],Copyright © 2013,American Chemical Society.(c) Illustration of irreducible Co2SiO4 as a promoter to modify catalytic performance in FTS.Adapted from Ref.[32],Copyright©2020,Elsevier.(d)STEM-EDX,atomic Co-Ni distribution,and schematic diagram of mechanism of the niobia-supported catalyst.Adapted from Ref.[39],Copyright©2020,American Chemical Society.(e)A schematic illustration of the structural evolution of Ru/TiO2-450H catalysts as Ru loadings vary from 0.5%to 4%(mass),where the gradually brighter color suggests the improvement of reduction degree for Ru NPs.Adapted from Ref.[45],Copyright © 2020,American Chemical Society.
2.2.Geometry structures
Metal nanoparticles,even nanoclusters,have different shapes and proportions of exposed crystal planes.The shape of metal nanoparticles can produce different proportions of crystal planes that may be beneficial or unfavorable to the adsorption and dissociation of reactants to affect the catalytic performance.It is often controlled by adhesion energy at metal-support interfaces [15].For example,a support with stronger adhesion will induce metal nanoparticles to expose more facets [26,27].Of course,this kind of support with strong adhesion energy is conducive to stabilizing and fixing metal nanoparticles during activation or reaction.
Meanwhile,for unsupported metal catalysts,metal nanoparticles with large surface free energy often led to its diverse morphology.It is conceivable that the support can determine the shape of metal nanoparticles due to the reduction of its surface free energy.For example,Labichet al.[28]reported the formation of an encapsulated catalyst driven by minimizing the surface energy,which is to encapsulate the metal (Rh) with relatively high surface energy using the oxide support (TiO2) with low surface energy.Besides,the mismatch of crystal lattice between supports and metal nanoparticles will produce strains and defects,thereby changing the morphology of metal nanoparticles.For example,Maet al.[29] synthesized Pt-Co bimetallic catalysts using a new one-step hydrogenation-reduction method,as shown in Fig.4b.The obtained catalyst exhibits an exceptional high aqueous-phase FTS activity at a low reaction temperature (160 °C).The authors believed that the lattice mismatch of the supported Co layers induced the enhanced energetics and kinetics from the change of the transition states,resulting in dramatically enhanced FTS performance.
2.3.Chemical compositions
During the calcination and reduction process,the interaction between the metal and the support induces the formation of new chemical components.This new phase may be beneficial or unfavorable to the catalytic reaction.Generally,conventional oxide supports (SiO2,Al2O3,etc.) can hardly avoid solid-phase reaction with cobalt species to form silicate or aluminate species that are difficultly reduced,leading to a decrease in catalytic activity [30–34].Of course,irreducible species are a double-edged sword,depending on how scientists choose it.Liet al.[32]used irreducible Co2SiO4as FTS catalyst promoters to effectively increase the dispersion of metallic Co0and inhibit the formation of more Co2SiO4.This modified catalyst can enhance CO adsorption and increase the local CO/H2ratio of active sites,which ultimately stimulates the occurrence of carbon–carbon coupling reactions (Fig.4c).
Alloy is a substance with metallic characteristics synthesized by fusing two or more metal elements,which is formed by the process of one component entering the structure of a basic component(metal or metal compound) [35–41].Generally,alloys reconstruct the electronic structure of pure metals.It means that valence electrons are rearranged in the new potential fields formed by different metal element nucleus and related electrons,which can significantly improve their catalytic performance,including activity,selectivity and stability [42,43].As with monometallic sites,the binding ability of the alloy with the reactants is affected by the support.Recently,Mejiaet al.[39] reported that the combination of Co-Ni alloy and reducible oxides supports (TiO2and Nb2O5)(Fig.4d) offers unique and complex interactions that can be exploited for improved catalyst stability,activity,and selectivity for long-chain hydrocarbons at 0.1MPa.Contrastingly,catalysts supported on α-Al2O3independently of the metal composition showed lower activities,high methane selectivity,and severe deactivation.The advanced characterization results prove that the surface composition of nanoparticles is affected by MSI,and CO is weakly adsorbed on Co-Ni alloy supported on TiO2and Nb2O5,rather than α-Al2O3,modifying the rate-determining step and reactivity in FTS.
2.4.Strong metal-support interaction
The term strong metal-support interaction first proposed by Tausteret al.in 1978[44],which refers to metal nanoparticle coverage by reducible oxide supports(TiO2[45,46],Nb2O5[47,48],and CeO2[49,50]) under the high-temperature reduction condition.Current thinking on the basic concept of SMSI additionally focuses on interface and transport phenomena,as well as the redistribution of charges during the formation of the metal-support interface.The metal nanoparticles covered by reducible oxide support with a few atomic layers may change the original metal-support interaction,thereby causing changes in catalytic performance.Huanget al.[45] prepared different-sized Ru catalysts supported on TiO2via varying the Ru loading (Fig.4e).They found that the coating of TiOxon Ru greatly depends on the size of Ru.This size-dependent SMSI on Ru/TiOxleads to a varied reduction degree of Ru to alter CHxtransformation therein.In their work,large-sized Ru nanoparticles with less TiOxcoating possess an improved reduction degree,which is preferable for methanation.While small-sized Ru suffers an intense TiOxcoating,the lower reduction degree of Ru can modulate the bonding capability toward CHxand shows advantages on chain growth in FTS by suppressing the competitive methanation.As such,the changed structures caused by SMSI determine the reactivity on active sites by modulating the bonding capability between active sites and adsorbent.
However,the metal sites covered by reducible oxide supports may be blocked by the amorphous layer and cause degradation of catalytic performance.For example,Liet al.[46] found that the active cobalt species covered by the TiO2islands over the spent Co/TiO2,resulting in the catalyst inactivation.
3.Strategies of Tuning MSI to Enhance FTS Performance
The choice of supports is a vital factor in designing of efficient catalysts because it can improve the metal dispersion,reducibility,stability,mechanical strength and facilitate heat and mass transfer[51–57].More importantly,the resulting MSI can induce changes in adsorption energy of active metal sites to adsorbates,which can significantly affect the kinetics of a reaction.
3.1.Oxide supports
Oxides such as SiO2,Al2O3,TiO2,etc.are ubiquitous as support materials for metal nanoparticles in FTS,and have even been successfully developed in industrial applications.Moderate-strength metal-support interactions are often favored.It is usually undesirable that this interaction is too strong to make the metal precursor to be readily reduced.Alternatively,if this interaction is too weak,it will cause catalyst aggregation and deactivation [54,58,59].Therefore,a lot of detailed research work has been carried out on balancing MSI in FTS.Jacobset al.[59]compared the catalytic performances of Co catalysts supported on the oxide supports of SiO2,Al2O3,and TiO2.They found that MSI determined the reduction degree of Co species via temperature-programmed reduction(TPR) and hydrogen chemisorption combined with reoxidation measurements,and the strength of interactions decreased in the order of Al2O3>TiO2>SiO2.The addition of noble metal promoter Ru or Pt to Al2O3-and TiO2-supported Co catalysts can significantly accelerate the reduction of Co species and increase the number of active sites,leading to a significant increase in the initial activity of the catalysts.In addition,MSI can induce significant changes in adsorbate binding energies,and consequently influence reaction kinetics by predicting the influence of various oxide supports on elementary association/dissociation reactions in terms of electronic (charge transfer) and geometric (relaxation) effects.As shown in Fig.5a,Jennesset al.[60] computationally studied the role of MSI over oxides (SiO2and TiO2) supported on Rh catalysts to modulate the activity and selectivity of FTS to ethanol by isolating their electronic and geometric aspects.They found that compared with unsupported Rh37nanoparticles,the SiO2-supported Rh catalyst had little effect on the binding energies of adsorbates to a representative Rh nanoparticle,while the TiO2-supported Rh catalyst yielded considerable changes by populating or depopulating states of antibonding character around the Fermi level.The enhanced MSI increases the activation energy for hydrogenation,resulting in the methane production coupled with the decrease in activation energy for CH3insertion in CO to produce ethanol.
Since the type of oxides supports could induce specific MSI,then this feature may be utilized by modifying an oxide with other oxides to tune MSI.As reported by Jacobset al.[59],compared with the SiO2support,the Al2O3support has a stronger interaction with metal.Based on this point,Wanget al.[61] prepared cobalt catalysts supported on SiO2-modified high thermal conductivity Al2-O3@Al composites.They believed that the reduced metal-support interaction via modification of SiO2on the surface of Al2O3@Al increased the CO conversion andselectivity,and decreased the CH4selectivity.Using the similar catalyst design concept,Liuet al.[62] synthesized Al2O3or SiO2nanoshell-modified TiO2samples (Al-TiO2and Si-TiO2) to support cobalt catalysts by a conventional incipient wetness impregnation method.The Al2O3nanoshell-modified TiO2enhances the Co dispersion,while the SiO2nanoshell-modified TiO2improves the reducibility of Co species (Fig.5b),both of which can increase cobalt-time-yield of the catalysts from 1.1 × 10-5mol · g-1·s-1(Co/TiO2) to 1.8 × 10-5mol · g-1·s-1.Guoet al.[63] reported that the ZrO2modification of the Al2O3nanosheet support could improve the reduction degree of Co catalysts to enhance FTS catalytic performance.
Even metal catalysts on the same oxide support may have different metal-support interactions,resulting in differences in catalytic performance.Broget al.[64] prepared a total of 26 catalysts by one-step incipient wetness impregnation of γ-Al2O3or α-Al2O3with different cobalt nitrate solutions.Interestingly,they found that theselectivity of the α-Al2O3based catalysts is higher than that of the γ-Al2O3based catalysts at all particle sizes,which is related to the different MSI caused by the chemical properties of the Al2O3surfaces.Especially,controlling the morphology of the oxide support will induce different MSI,which can improve metal utilization,and sintering resistance,and ultimately improve catalytic performance.Liuet al.[65] prepared the cobalt catalyst supported on γ-Al2O3nanofibers(Al2O3-f)with controllable crystallite size distribution by an ultrasonication assisted mixing method.Compared with the Co catalyst supported commercial Al2O3,the obtained Co/Al2O3-f catalyst exhibits the higher catalytic activity andselectivity due to its improved reducibility,and stabilizes the catalyst by inhibiting aggregation of Co nanoparticles.Fuet al.[66] synthesized the Co3O4-Al2O3mesoporous hollow spheres (MHS) composed of thermally stable Co3O4nanoparticles partially anchored to amorphous interfacial Al2O3using a transient aerosol-assisted self-assembly method(Fig.5c).Compared with the catalytic performance of the Co3O4-Al2O3catalysts with a solid architecture,the Co3O4-Al2O3MHS exhibits the higher catalytic performance and selectivity for the desired gasoline products(52.4%),which is attributed to its hollow spheres structure and moderate interfacial interaction.
Liet al.[67] synthesized a series of Ru/TiO2catalysts with the average diameter of Ru nanoparticles at~ 3 nm through a dopamine sacrificial coating strategy to adjust the ratio of the surfaceexposed metallic Ru sites to the metal-support interface sites on the Ru/TiO2catalyst (Fig.5d).The two kinds of active sites were analyzed in detail by in-situ high-pressure DRIFTS and other characterizations.The results show that the TOF value of metal exposed sites is 5.3 times that of metal-support interface sites(Fig.5e).The as-synthesized Ru/TiO2-500-H catalyst could effectively prevent Ru nanoparticles from sintering,significantly increase the number of surface-exposed Ru active sites and exhibit the high catalytic performance.Through in-situ DRIFTS experiments using CO/syngas as a probe molecule,it is found that the CO species adsorbed on the exposed Ru sites can stably exist,and the bond of the generated CHxintermediate species with the Ru sites can be strengthened(Fig.5f).Thus,it is easy to stimulate the C-C coupling reaction and improve theselectivity.

Fig.5.(a)Graphical representations of the oxide-supported Rh37 nanoparticle.Adapted from Ref.[61],Copyright©2013,American Chemical Society.(b)TEM images and TPR profiles of catalysts.Adapted from Ref.[63],Copyright©2018,American Chemical Society.(c)Schematic illustration on the stepwise formation of Co3O4-Al2O3 MHS.Adapted from Ref.[67],Copyright © 2017,Elsevier.(d) Schematic overview of the pretreatments applied to the Ru/TiO2 catalysts.(e) Activity of Ru/TiO2 catalysts.(f) In situ DRIFTS spectra of CO adsorption at 2.0 MPa and 220 °C.(d-f) Adapted from Ref.[68],Copyright © 2020,Elsevier.
3.2.Carbon supports
Carbon-based materials,one of the vital topics in modern materials science,are encountering its rapid development in many application fields because of their unique characteristics,such as excellent electrical and thermal conductivity,adjustable physicochemical properties,and diverse structures,as well as mechanical strength and lightness [68].Unlike traditional oxide supports,carbon-based materials such as carbon nanotubes (CNTs) [69–71],carbon nanofibers (CNFs) [72–74],carbon nanospheres (CSs)[75–77],ordered mesoporous carbon (OMC) [78–80],graphene[81–83],etc.are difficult to form irreducible species due to its inert surface.However,the synthesis of highly dispersed and stable metal catalysts supported on carbon materials is still confronted with enormous challenges due to the weak MSI.The contradiction between metal reduction and dispersion can be effectively resolved via chemically modifying the surface of the carbon support to modulate MSI by carbon defect engineering design and heteroatom (N,O,B,etc.) doping [58].
3.2.1.Carbon defects
The inevitable defect sites in the framework of carbon network greatly affect the physicochemical properties of carbon nanomaterials,and thus defect engineering has recently become one of the vital research directions of carbon-based catalysts.Generally,the synergistic interaction between intrinsic defect sites of carbon supports and captured metal species can optimize their electronic structure by localizing electron re-distribution and adsorption/desorption behavior of adsorbate [84].Zhanget al.[85]manipulated the Fe-C interaction by modifying the surface chemical property of multiwalled CNTs.They found that the defect-rich high-temperature pretreated CNT (HT-CNT) with electronwithdrawing weakened the reducibility of FeOxand the C3H6adsorption on Fe (Fig.6a).It is worth noting that the defectanchored Fe/HT-CNT promotes the formation of the‘‘Fe-C”coordination complex and phase transformation toward active-Fe2.2C/ε-Fe2C,improving CO adsorption and activation.Similar conclusions had been reported by Tianet al.[86].The authors increased the number of defects on graphene-like layers(a shell)to encapsulate potassium-modified Fe catalysts,which formed a core–shell structure by pyrolysis of an iron-glucose precursor.And they found that the defect-rich graphite layers facilitated the formation of iron carbides during the catalyst preparation.The unique structure of graphene-like carbon layers encapsulating iron carbides inhibited the catalyst sintering to achieve a high stability.And,electronrich surfaces made it hard for hydrogen dissociation to hydrogenate unsaturated intermediates,which led to a high olefin selectivity.
3.2.2.Heteroatom doping

Fig.6.(a)Promotional effects of multiwalled carbon nanotubes on iron catalysts.Adapted from Ref.[86],Copyright©2018,Elsevier.(b)Illustration of the formation process of core–shell FexCy particles.Adapted from Ref.[100],Copyright © 2019,The Royal Society of Chemistry.(c) Schematic illustration on the stepwise formation of the PDA nanospheres.(d) N effect of Co/NCs catalysts in FTS.(c-d) Adapted from Ref.[101],Copyright © 2019,Elsevier.
Heteroatom doping is a process,in which carbon atoms in the carbon skeleton can be replaced by heteroatoms with different electronegativity from that of the carbon atoms to redistribution of the charge on the carbon support [87–93].The charge modulation can be achieved via direct charge transfer with dopants,regardless of whether the electron acceptor (as B,P,S)/ donor (as N),which in turn weakens/enhances interaction with reactants to impact catalytic performance.N-doped carbon-based catalysts have attracted immense attention in FTS because of the characteristics of the electron donor heteroatoms N and the diversity of N species.Huet al.[94] synthesized N-doped carbon nanotubes(NCNTs)with a nitrogen content of 3%-5%by chemical vapor deposition and prepared NCNTs-supported iron catalysts by the incipient wetness impregnation method.The obtained Fe/NCNTs catalyst exhibits high catalytic performance in FTS with the high selectivity toward lower olefins of up to 46.7%,as well as the high activity and good stability.They believed that the introduction of N atoms into CNTs facilitates the immobilization of iron species,promotes the reduction of iron oxides,and accelerates the formation of the active phase of χ-Fe5C2.And it generates basic sites,thereby enhancing the adsorption and dissociation of CO,and inhibiting the secondary hydrogenation of lower olefins.However,after longterm reaction with syngas Fe nanoparticles readily aggregate to form the core–shell structure of FexCyparticles,causing the deterioration of the catalytic performance(Fig.6b).It seems that doping more N atoms into carbon supports can further suppress the formation of iron carbonyls,which effectively regulates the sizes of FexCynanoparticles and alleviates the formation of core–shell structures [89,95–98].Huet al.[99] prepared the durable Febased catalysts supported on the 3D hierarchical nitrogen-doped carbon nanocages with a high N content(12.0%),and the resulting catalyst presents a high-selectivity with a maximum up to 54.1%.Even after the reaction for 200 h,the catalyst only shows a slight increase in sizes of the FexCynanoparticles and retains the high selectivity.Recently,Chernyaket al.[82]expanded N research in FTS.They focused on the effect of localization of nitrogen species over the N-doped graphene nanoflakes(GNFs)and found that the edge localization(the cross-linking of support particles) of N-groups over carbon support hindered the diffusion of reactants and products.
In addition to the research on the N content and position for tuning the MSI in the FTS field,the influence of the types of N dopants on the catalytic performance has also been studied.In general,N-doped carbon materials contain three types of N atoms,including pyridinic-N,pyrrolic-N,and graphite-N with ratios depending on the specific system.It is a challenge to identify the relationship between the N dopant types and catalytic performance because of the uncontrollable contents and types of N dopants in catalysts.Liet al.[100]synthesized a series of homogeneously dispersed and size-controlled N-doped carbon nanospheres (NCS)viaa simple yet green strategy,using biomolecule dopamine as the carbon and nitrogen resources (Fig.6c).Through adjusting the carbonization temperatures,the contents of the N dopants over the NCS-based materials could be precisely controlled.Compared with N-free Co/CS catalyst,doping N into the carbon support is beneficial to improve the dispersion of metallic cobalt,which increases the number of the active sites,leading to the enhanced activity.And the electron-rich N atoms on the support cause the transfer of electrons to the adjacent active cobalt sites,which facilitates dissociation of CO,to further enhance the catalytic activity.For N-doped Co/NCS-T catalysts,after quantitative analysis of the XPS results,it is found that the priority for anchoring cobalt nanoparticles follows the sequence of pyrrolic N >pyridinic N >graphitic N.
For the catalytic performance of the Co/NCS-T catalysts in FTS,as the carbonization temperature increases from 500 to 800 °C,the cobalt-specific activity decreases from 2.5 × 10-5to 1.3 ×10-5mol ·g-1·s-1,the TOF value decreases from 1.0 × 10-2to 7.6 × 10-3s-1,the CH4selectivity slightly increases from 4.4% to 5.2%,and theselectivity decreases from 74.4% to 66.0%(Fig.6d).The results of in situ XPS show that the reduced Co/NCS-500 catalyst with the highest content of pyrrolic N exhibits the strongest interaction between cobalt and support among the catalysts.The strong interaction initiated by pyrrolic N induces the formation of small-sized cobalt,and can effectively resist catalyst sintering in FTS.Via the chemical adsorption,spectroscopy,and microscopy characterizations,Liet al.demonstrated that the pyrrolic N dopants play a leading role in tuning the interaction between cobalt and support.The controllable interaction will change the chemical environment of active cobalt sites and ultimately affect the catalytic performance.Pyrrolic N,which is enriched on the surface of NCS-500,is beneficial to strengthening the interaction between cobalt and support.It improves the dispersion of metallic cobalt,which will expose more active cobalt sites to participate in activating CO and contribute to a higher catalytic activity.Moreover,during CO adsorption the electron-enriched cobalt sites resulting from the modification of pyrrolic N will strengthen the Co-C bond and weaken the C-O bond.Thus,the dissociative adsorption of considerable CO and the subsequent formation of CH2via hydrogenation ultimately stimulate C-C coupling reactions and produce heavy FTS products.It can be concluded that the enhanced activity andselectivity are ascribed to the increased pyrrolic N content in the Co/NCS-T catalysts.
4.Design of Highly Efficient FTS Catalysts by Tuning MSI
As described in the mechanism above,FTS is a complex chemical reaction that grows the carbon chain through C-C coupling.Thus,it is eager to obtain target products with specific carbon numbers such as low-carbon olefins(-),gasoline(C5-C11),diesel (C10-C20),etc.Unfortunately,it is always restricted by the ASF law [1–3].Industrially,CO hydrogenation synthesizes olefins through the methanol route,or the FTS products are subjected to further hydrocracking treatment to improve the selectivity of target fuels.The direct production of target-range gas/liquid fuels will save energy consumption,and be more economical than the multiple-stage process.
Recently,metal-organic frameworks (MOFs) have been regarded as a promising precursor for the synthesis of nanomaterials because of its unique structure,atomic metal dispersion,and textural properties [101–105].Santoset al.[105] synthesized highly dispersed iron carbides (averagedFe=3.6 nm) with a high Fe loading (38%,mass) embedded in a matrix of porous carbon by a MOF-mediated synthesis strategy (Fig.7a).The synthesized Fe@C catalyst offers intimate contact between Fe and C,favoring the formation of the active phase in the FTS process.It exhibits the excellent catalytic performance and 1 order of magnitude higher than that of previously reported Fe catalysts.Furthermore,the authors found that the Fe@C catalyst promoted with 0.6%(mass) of K displayed the optimal selectivity to C2-C5olefins(20.5% carbon selectivity and 44.6% CO2-free selectivity),the increased activity,and the reduced methane selectivity (5%).The catalyst prepared by this strategy has also been applied to other metal catalysts in FTS,such as Co-based catalysts,etc.[106–114].
Combining of zeolites with metal FTS catalysts to directly synthesize liquid fuels from syngas has achieved great success[12,13,115–126].The formed strong MSI between the metal catalyst and the zeolite support severely restrained the reduction of the metal catalyst precursor,which makes it unable to fully utilize metal sites.Liet al.[127] supported highly dispersed metallic Co nanoparticles on H-ZSM5 with narrow size distribution using a hexahedral-barrel sputtering system.The prepared catalyst exhibits WMSI because Co nanoparticles are wedged onto supports by physical forces.WMSI facilitates the reduction of cobalt oxide nanoparticles,and the high cobalt dispersion accelerates nparaffin diffusion to neighboring acidic sites on the H-ZSM5 support for isomerization and hydrocracking (Fig.7b),resulting in the high CO conversion of 75.2%and the gasoline product selectivity as high as 67.3%.
MSI will greatly affect the size of metal particles or clusters during activation and catalysis.The study of metal size effects in FTS has received great attention [25,64,128–131].Generally,the crystallite sizes of cobalt can dramatically affect the catalytic activity and selectivity for FTS.However,FTS is an extremely harsh and exothermic catalytic reaction.How to inhibit metal aggregation is a permanent scientific problem.Liet al.[132] synthesized sizeuniformed cobalt nanocrystals that were embedded into mesoporous SiO2supports.It is likely the structure of watermelon seeds inside pulps(Fig.7c).It was surprising to find that the selectivity of FTS products can be tuned from the C10-C20hydrocarbons with the selectivity of 66.2% to the C5-C11selectivity of 62.4% by only varying the crystallite sizes from 7.2 to 11.4 nm of confined cobalt nanocrystals without any other modification.This phenomenon differs from previous reports in FTS:that is,larger Co nanocrystals generally favor the chain growth and the formation of heavier hydrocarbons [25,129,130].Detailed characterizations indicate that the smaller Co nanocrystals with a narrow size distribution could strongly adsorb and capture the larger amount of active C*species,accelerating the formation of CHxintermediates inside the confined space to stimulate carbon-chain growth.The confined structure may not only avoid the aggregation of Co particles but also inhibit the escape of reaction intermediates,resulting in higher selectivity towards longer chain hydrocarbons.

Fig.7.(a)MOF-mediated synthesis strategy.Adapted from Ref.[106],Copyright©2015,Springer Nature Ltd.(b)Possible reaction routes of the Co/H-ZSM5-S catalyst during the FTS,isomerization,and hydrocracking reactions.Adapted from Ref.[128],Copyright © 2013,Springer Nature Ltd.(c) Synthesis of size-uniformed cobalt catalyst embedded into mesoporous SiO2 supports.Adapted from Ref.[133],Copyright © 2018,Springer Nature Ltd.(d) Evolution of Ru/TiO2 catalysts at different reduction temperatures.Adapted from Ref.[136],Copyright © 2020,Springer Nature Ltd.1Å=0.1nm.
Reducible oxide can migrate to the metal surface by forming a thin overlayer under the reduction condition,which then results in a unique metal-support interface and variegates the catalytic behavior [17,133,134].In very recent work,a significant discovery reported by Huanget al.[135] indicates that the reactivity of Ru/TiO2nanocatalysts can be differentially modulatedviavarying the reduction condition to regulate the TiOxoverlayer on Ru nanocatalysts (Fig.7d).The activity of the obtained catalysts depends on the reduction temperature and shows a volcano-like trend with elevated reduction temperature from 200 to 600 °C.The Ru/TiO2catalyst reduced at 450°C exhibits the highest intrinsic TOF value of 0.039 s-1and excellentselectivity with a value up to 90% under a relatively mild condition (160 °C).It is claimed that the optimized TiOxoverlayer formed on the Ru nanoparticles during reduction at 450°C is evidently able to capture oxygen from the carbonyl group adsorbed on the interface of Ru-TiOx.As such,in turn,it facilitates the cleavage of the C-O bond.And the active C*species is promising to be hydrogenated to CHxspecies and realize the C-C coupling on Ru sites to produce hydrocarbons.This discovery not only provides an understanding of the mechanism of CO activation but also offers an effective approach to tuning reactivity of metal catalysts based on the utilization of MSI.
5.Summary and Outlook
By properly tuning MSI,metal catalysts can be immobilized on a support material to achieve high metal dispersion,shape stability,modifiable electronic properties,etc.to consequently affect catalytic performance.This review focuses on the function of MSI on FTS catalysts.Here,we summarize and classify various types of MSI recently reported in the literatures to promote the catalytic performance of FTS.And we also give some insights and routes into how to develop efficient FTS catalysts by manipulating MSI.It is helpful to clearly understanding of the essential factors on catalytic performance in-depth,and provides the feasible yet new strategies for development of highly efficient FTS catalysts.
Although there have been encouraging advances in optimizing FTS performance by manipulating MSI,there are still some challenges as described below.
(1) Supported metal catalyst systems are usually accompanied by multiple manifestations of MSI,and it is difficult to identify which form plays a dominant role in FTS.The strength of MSI is related to the morphology of support materials,metal size,charge transfer,interfacial platform,and chemical composition,etc.These phenomena are usually entangled.Thus,it is difficult to avoid some controversy regarding functions of MSI in literatures because of ignoring other important factors.
(2) Establishing a descriptor between the modified active site and catalytic performance under real conditions by combining advanced characterization techniques with density functional theory (DFT) methods is essential for deeper understanding of MSI at atomic level.However,the problem lies in the mismatch between theoretical and experimental results.The construction of a reasonable model catalyst system will simplify the research of MSI in reactions.
(3) The control of C-C bond formation from CO and selective dehydrogenation to olefins at chain termination on active metal sites are also very appealing research targets in FTS.The nature of exposed metal sites and associated activity is expected to be controlled by manipulating MSI.Active sites modified by MSI have endowed a specific ability,which changes the active energy of reactants and the original bonding ability with adsorbates.Modification of metal active sites with MSI and their local environments may be a new strategy to achieve selective C-C coupling,as well as selective β-H desorption to produce olefins at chain termination.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors are grateful for financial support from the National Natural Science Foundation of China (21676182 and 21476159),the Program for Introducing Talents of Discipline to Universities of China (BP0618007),State Key Laboratory of High-efficiency Utilization of Coal and Green Chemical Engineering (2020-KF-26)and open foundation of State Key Laboratory of Chemical Engineering (SKL-ChE-20B01).
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Interactions of dynamic supercritical CO2 fluid with different rank moisture-equilibrated coals:Implications for CO2 sequestration in coal seams
- Hydroformylation of formaldehyde to glycolaldehyde:An alternative synthetic route for ethylene glycol
- The latest development on amine functionalized solid adsorbents for post-combustion CO2 capture:Analysis review
- Review on the effect of heat exchanger tubes on flow behavior and heat/mass transfer of the bubble/slurry reactors
- Reviews of clean coal conversion technology in China:Situations &challenges
- Speciation and thermal transformation of sulfur forms in high-sulfurcoal and its utilization in coal-blending coking process:A review
