苯嗪草酮疏果剂对苹果边果营养与激素含量的影响
2021-06-30薛晓敏韩雪平聂佩显王金政
薛晓敏,韩雪平,聂佩显,董 放,王金政
苯嗪草酮疏果剂对苹果边果营养与激素含量的影响
薛晓敏,韩雪平,聂佩显,董 放,王金政
(山东省果树研究所,泰安 271000)
为明确苯嗪草酮疏果剂对苹果边果的疏除作用,以9年生天红2号/SH38/八棱海棠为试材,在最大边果直径6 mm左右时喷300 mg/kg苯嗪草酮2次,清水为对照,生理落果后调查坐果率及坐果比例;喷药后7、9、11、17、29 d采集处理和对照边果,测定氮磷钾矿质营养,淀粉、葡萄糖、果糖、蔗糖和山梨醇碳水化合物,可溶性蛋白质含量以及玉米素(Z)、赤霉素(GA3)、生长素(IAA)和脱落酸(ABA)激素含量。结果显示,苯嗪草酮处理后,花序坐果率和花朵坐果率较对照降低17.95%和27.63%,坐单果比例显著提高,坐三果及三果以上的比例显著降低(<0.01);处理总体上提高了边果无机营养含量,显著降低了可溶性蛋白质含量(<0.05);淀粉含量较对照提高了5.57%~37.20%,但不同程度地降低了蔗糖、葡糖糖、果糖及山梨醇含量,从而使可溶性碳水化合物含量较对照降低了9.55%~52.60%;处理后脱落酸含量显著升高(<0.05),(Z+GA3+IAA)/ABA比值显著降低。说明苯嗪草酮在苹果上具有较好的疏除边果作用,其疏除作用与可溶性蛋白质含量降低,可溶性碳水化合物供应不足,以及ABA含量升高及生长型激素/抑制型激素比值降低有关。
激素;营养;苯嗪草酮;坐果率;苹果
0 引 言
苹果疏花疏果是调整树体负荷、提高果实品质、减轻大小年结果的必要技术,包括人工疏花疏果、化学疏花疏果和机械疏花疏果。人工疏花疏果主要在中国应用,美国、德国的商业化果园开始试验示范机械疏花疏果,化学疏花疏果则是欧美发达国家普遍采取的苹果生产花果调控技术[1-4]。疏果相对于疏花来说,安全系数高,更受果农青睐。研究表明,萘乙酸、西维因、6-BA、ACC、乙烯利等在一定浓度范围内均有疏除幼果、平衡负载量作用[5-8],对其疏除机理也有相关报道,如西维因阻碍营养物质运输养分、萘乙酸干扰激素代谢、乙烯利促进乙烯生成等造成幼果脱落[9-10]。苯嗪草酮是一种光合系统Ⅱ(PSⅡ)抑制剂,主要用作除草剂。2014年安道麦公司发布苯嗪草酮可作为一种低毒、安全的果树疏果剂,主要应用在苹果和梨上,不少学者开展了相关研究[11-12]。本课题组从光合和荧光角度研究了苯嗪草酮对苹果叶片抑制作用的影响[13],但是,对于苯嗪草酮如何影响幼果的生理代谢国内外均未见相关报道。
本文以矮砧苹果树为试材,研究了在最大边果直径6 mm左右时喷施苯嗪草酮对苹果的疏果效应及对边果营养和激素含量的影响,从碳水化合物及激素调控角度初步揭示了苯嗪草酮的疏果机制,为生产提供了参考依据。
1 材料与方法
1.1 试验材料
试验在山东省果树研究所天平湖基地(北纬36°12′55.36″,东经117°01′09.87″,海拔168 m)进行。试材为9年生天红2号/SH38/八棱海棠(‘天红2号’为红富士芽变品种),株行距0.75 m×4.0 m,采用高纺锤树形、行间生草、树盘覆盖、肥水一体化技术,管理水平中等偏上。试验地有机质质量分数0.79%,速效氮86.11 mg/kg,速效磷73.71 mg/kg,速效钾116.32 mg/kg。
1.2 处理方法
选取长势基本一致健康的苹果树30株,设喷药和对照2个处理,各处理15株树,5株树为1个小区,3次重复,处理和对照之间保留2株树作为保护株。每株树随机选3个主枝,统计花序数和花朵数,挂牌标记。
2018年4月21日进行了苯嗪草酮试验浓度筛选试验,设置100、200、300、400、500 mg/kg 5个浓度处理,清水为对照。选用背负式电动喷雾器对全树进行喷布,喷至幼果湿润轻微滴水为止。生理落果后调查有挂牌的主枝坐果情况,统计花序坐果率和花朵坐果率,花序坐果率(%)=坐果花序数/总花序数×100%,花朵坐果率(%)=坐果数/总花朵数×100%。
2019年4月19日在最大边果直径6 mm左右时喷300 mg/kg苯嗪草酮,4月22日喷第二次;对照喷清水。喷施方法同2018年,生理落果后调查坐果率和坐果比例,单果率(%)=坐单果花序数/总花序数×100%,双果率(%)=坐双果花序数/总花序数×100%,三果及以上(%)=100−(单果率+双果率)。
1.3 取样与测定
分别在第一次喷药处理后7、9、11、17及29 d从处理及对照试验树上取边果,前3次每个小区(5株树)共采果90个左右,后2次每个小区(5株树)共采果60个左右,均为3个重复,去除果柄及萼片后,用铝箔纸包好,放入液氮罐中速冻,带回实验室放至−80 ℃冰箱保存,用于测定氮磷钾、碳水化合物、蛋白质及激素。
1.3.1 矿质营养测定
全氮测定用半微量蒸馏法,全磷测定用钼锑抗吸光光度法,全钾测定用火焰光度计法,具体测定步骤参考崔建宇等[14]方法。所有指标均重复3次。
1.3.2 碳水化合物含量测定
单糖和低聚糖提取参考Kang 等[15]方法,葡萄糖、果糖、山梨醇用水提取,称取约0.2 g样品,加入1 mL水,匀浆,过夜浸提。蔗糖用乙腈提取,称取约0.2 g样品,加入1 mL 80%乙腈,匀浆,50 ℃水浴30 min,8 000离心10 min,取上清液,针头式过滤器过滤后待测。用Waters 1525高效液相色谱仪测定,示差检测器为Shodex RI-201H。葡萄糖、果糖、山梨醇用Carbomix Ca-NP 8%色谱柱(300 mm×7.8 mm,10m),柱温80oC,流动相为水,流速0.4 mL/min,进样体积10L;蔗糖用Sepax HP-Amino氨基柱(4.6 mm×250 mm,5m),柱温40 ℃,流动相乙腈∶水=80∶20,流速0.4 mL/min,进样体积10L。根据葡萄糖、果糖、山梨醇、蔗糖标准曲线和样品峰面积计算含量。
将提取可溶性糖的残余物用高氯酸水解成葡萄糖,蒽酮比色法测定葡萄糖含量,测定波长为620 nm,由葡萄糖标准曲线计算淀粉含量。
1.3.3 可溶性蛋白质含量测定
可溶性蛋白含量测定采用考马斯亮蓝染色法[16]。
1.3.4 激素含量测定
激素提取方法参考Yan 等[17],称取约0.2 g样品,加入1 mL预冷的20%甲醇,4 ℃浸提过夜;8 000离心10 min,取上清液,残渣用0.5 mL 20%甲醇水溶液浸提2 h,离心后取上清液,合并2次上清,40 ℃减压蒸发至不含有机相,加入2 mL石油醚60~90 ℃萃取脱色3次,移去石油醚;向下层水相中加入2 mL乙酸乙酯萃取,转移上层有机相至新的EP管,重复萃取3次,合并3次有机相,氮吹吹干,加入0.2 mL流动相溶解,混匀,针头式过滤器过滤后待测。采用RIGOL L3000高效液相色谱仪,Kromasil C18反相色谱柱(250 mm×4.6 mm,5m),波长为254 nm,柱温30 ℃,流动相流速0.8 mL/min,进样体积10L,走样时间35 min。
1.4 数据分析
所有数据均采用SPSS软件进行差异显著性比较,应用GraphPad Prism 5 软件绘图。
2 结果与分析
2.1 苯嗪草酮适宜浓度筛选
不同浓度苯嗪草酮处理对坐果率的调查结果如图1所示。可以看出,与清水对照相比,100~500 mg/kg的苯嗪草酮处理降低了花序坐果率和花朵坐果率,其中对照与200~500 mg/kg处理间的花序坐果率存在显著性差异(<0.05),而对照与所有苯嗪草酮处理的花果坐果率均存在显著性差异(<0.05)。200~400 mg/kg 3个浓度之间差异性不显著,以300 mg/kg的花序坐果率和花朵坐果率最低,分别为72.39%和23.52%,故选300 mg/kg作为后续试验的喷施浓度。
注:不同小写字母表示不同浓度处理0.05水平差异性显著。
Note: Different small letter indicates significant difference at 0.05 level between different concentrations.
图1 不同浓度苯嗪草酮处理对坐果率的影响
Fig.1 Effect of metamitron with different concentrations on fruit setting rate
2.2 苯嗪草酮处理对坐果率和坐果比例的影响
由图2a可见,与对照相比,幼果期苯嗪草酮处理显著降低了坐果率(<0.01),其中花序坐果率降低17.95%,花朵坐果率降低27.63%。同时,苯嗪草酮处理显著提高了花序坐单果比例(<0.01),处理单果率为44.80%,为对照的5.28倍,且保留的单果均为中心果,说明苯嗪草酮仅对边果起作用,对中心果无影响;处理显著降低了花序坐三果及以上的比例(<0.01),处理为对照的47.91%;坐双果比例也有所增加,但未达到显著水平(图2b)。说明苯嗪草酮在富士苹果上有较好的疏果作用,疏除力强,单果率高,分布均匀。
2.3 苯嗪草酮对无机营养的影响
由表1可以看出,苯嗪草酮处理后,所有时期的全氮含量均高于对照,在喷施后7和9 d二者差异不显著,可能与处理时间较短有关;喷施11 d之后,处理的全氮含量均显著高于对照(<0.05)。苯嗪草酮处理对全磷和全钾的影响更为显著,几乎所有处理的全磷和全钾含量均显著高于对照(<0.05),喷后11、29 d处理的全磷含量为对照的2.05和1.67倍,全钾含量为对照的1.93和1.51倍。说明苯嗪草酮的疏果作用不是由于氮磷钾无机养分的缺失造成的。
注:**表示处理与对照间0.01水平差异性显著,下同。
Note: ** indicates significant difference at 0.01 level between treatment and control, the same below.
图2 苯嗪草酮处理对坐果率及坐果比例的影响
Fig.2 Effect of metamitron treatment on fruit setting rate and ratio
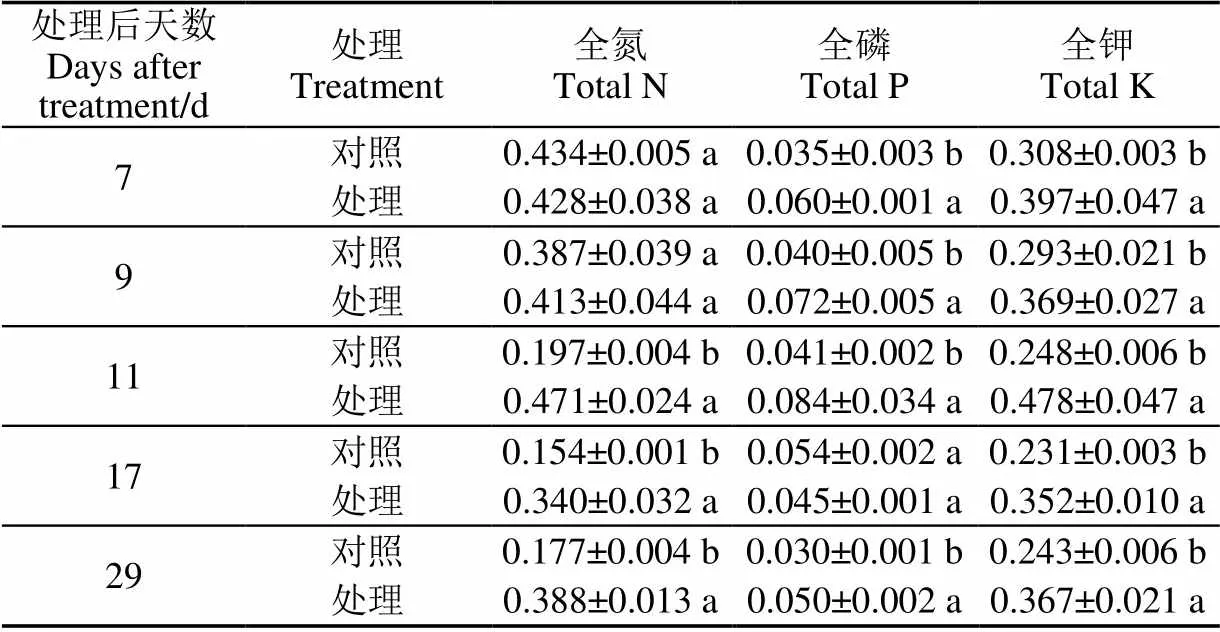
表1 苯嗪草酮处理对氮磷钾营养元素的影响
注:不同小写字母表示处理与对照间0.05水平差异性显著。
Note: Different small letter indicates significant difference at 0.05 level between treatment and control.
2.4 苯嗪草酮处理对有机营养的影响
苹果有机营养成分主要是碳水化合物,包括淀粉、葡萄糖、果糖、蔗糖、山梨醇[18]。由图3a可以看出,尽管苹果幼果中淀粉含量较低,但苯嗪草酮处理的淀粉含量多明显高于对照,增幅为5.57%~37.20%;图3b~图3e为可溶性碳水化合物葡萄糖、果糖、蔗糖及山梨醇含量,可以看出,整体趋势为对照的可溶性碳水化合物含量高于苯嗪草酮处理。为了整体比较处理与对照可溶性碳水化合物含量,对葡萄糖、果糖、蔗糖及山梨醇的总含量进行了分析,如图3f所示,可见苯嗪草酮处理的可溶性碳水化合物均显著低于对照(<0.05),降幅为9.55%~52.57%。而可溶性碳水化合物是幼果发育的直接营养物质,说明苯嗪草酮疏果可能是由于可溶性碳水化合物的供应不足造成的。
注:*表示处理与对照间0.05水平差异性显著,下同。
Note: * indicates significant difference at 0.05 level between treatment and control, the same below.
图3 苯嗪草酮处理对果实碳水化合物含量的影响
Fig.3 Effect of metamitron treatment on fruit carbohydrate content
2.5 苯嗪草酮处理对可溶性蛋白质含量的影响
如图4所示,苯嗪草酮处理后苹果幼果可溶性蛋白质含量明显降低,差异达显著水平(<0.05)。各个时期处理的可溶性蛋白质含量分别为对照的71.42%、72.37%、69.68%、64.20%和86.02%,说明苯嗪草酮处理使苹果幼果的总体代谢能力减弱,进而造成幼果脱落。
2.6 苯嗪草酮处理对激素的影响
苹果幼果脱落受内源激素调控[19-21]。在处理早期(7和9 d),对照的玉米素含量显著高于处理(<0.05);而在处理后期,对照的玉米素含量则明显低于处理(图5a)。苯嗪草酮处理对赤霉素也有影响(图5b)。苯嗪草酮处理显著提升了幼果生长素水平(<0.05),图5c可见,所有处理的IAA含量均明显高于对照,差异性多为显著水平(<0.05)。脱落酸是幼果脱落的主导激素种类,苯嗪草酮处理后脱落酸含量普遍升高,各时期ABA含量均显著高于对照(<0.05),处理为对照的1.37~3.11倍,说明苯嗪草酮提高了幼果的脱落酸含量,从而促进了幼果脱落(图5d)。同时,对(Z+GA3+IAA)/ABA的比值(值)进行了分析,如图5e所示,除喷施早期(7d)外,对照的值均高于处理,其中9、17和29 d 3个时期二者间差异达显著水平(<0.05),降幅最大为51.55%,说明低值与幼果脱落相关。
3 讨 论
苯嗪草酮作为一种苹果疏果剂有近十年的时间,不少学者对不同生态条件下的不同品种进行了喷施时期、喷施浓度、对产量品种调控效应等研究[22-24]。Steven等[25]研究认为,苯嗪草酮适宜喷施浓度为300 mg/kg,适宜喷施时期为盛花后23和38 d,与对照相比,花朵坐果率降低了50.63%,单株产量降低了37.10%,单果质量提高了8.54%,分析为苯嗪草酮影响叶绿素荧光所致,使开放的PSⅡ反应中心的能量捕捉效率值、PSⅡ光合量子产量及相对电子传导率均下降。嘎啦果适宜喷施时期为中心果直径6.0~13.5 mm,浓度为1.65 kg/hm2,喷施2次疏果效应更好,分析认为嘎拉幼果脱落与叶绿素荧光及夜间温度有关[11,26]。富士适宜喷施时期为幼果直径5~10 mm,浓度为350 mg/kg,落果率35.6%~50.9%,且显著提高了成熟期单果质量和商品果比例[8]。本试验中,幼果直径6 mm左右喷施300 mg/kg苯嗪草酮2次,花朵坐果率24.20%,比对照降低27.63%,与多数学者的疏除效应一致。然而,也有学者认为苯嗪草酮适用的幼果直径较大,可作为一种补救型疏果剂[27-28]。
不少学者认为,矿质养分及碳水化合物不足是导致幼果脱落的主要因素之一[29-31]。杨波等[32]研究发现,扁桃在生理脱落期,正常幼果的N、P、K、B、Zn 5种矿质元素的浓度均高于落果,认为扁桃幼果脱落与矿质元素浓度降低有关;而本试验结果则表明,处理的氮磷钾含量多高于对照,分析认为,疏果剂处理后果实变小直至萎蔫脱落,氮磷钾含量在其中相对“浓缩”,从而高于正常发育的果实,这与关军锋等[33]的研究结果一致。徐昌杰等[31]研究认为,柑橘幼果脱落与淀粉含量降低有关,本试验结果则显示,苯嗪草酮处理后幼果的淀粉含量没有降低,降低的是可溶性碳水化合物含量,而可溶性碳水化合物含量对幼果发育的作用更为直接。
众多研究表明,不少果树幼果脱落与激素含量及激素比例有关。易落果的柑橘品种具有较高的ABA含量与较低的GA3含量[34];同样扁桃幼果脱落与ABA含量升高及GA3和IAA含量降低有关[35];苹果幼果脱落与ZT、IAA、GA的减少及ABA的增加有关[36]。本试验中,苯嗪草酮处理后ABA含量显著升高,与前人的研究相一致;另外果实脱落往往不是由某一激素单独控制的,而是由多种激素协同作用的结果,因此本研究对(Z+GA3+IAA)/ABA进行了分析,显示苯嗪草酮处理后(Z+GA3+IAA)/ABA比值降低,说明苯嗪草酮疏果作用与低的(Z+GA3+IAA)生长素类激素/ABA比值有关。
4 结 论
1)在边果直径 6 mm时喷施2次300 mg/kg苯嗪草酮,具有疏除边果的作用,花序坐果率和花朵坐果率较对照降低17.95%和27.63%,差异达极显著水平(<0.01);处理的单果率44.80%,为对照的5.28倍,差异达极显著水平(<0.01)。
2)苯嗪草酮处理后,淀粉含量不降反升,涨幅为5.57%~37.20%;可溶性碳水化合物总量(葡萄糖、果糖、蔗糖、山梨醇)显著降低(<0.05),降幅最大的为52.57%;可溶性蛋白质含量显著降低(<0.01),降幅最大的为35.80%。
3)激素测定结果显示,苯嗪草酮处理后脱落酸含量显著升高(<0.05),处理为对照的1.37~3.11倍;生长型与抑制型激素比值[(Z+GA3+IAA)/ABA]降低,降幅最大的为51.55%。
[1] 王学府,孟玉平,曹秋芬,等. 苹果化学疏花疏果研究进展[J]. 果树学报,2006,23(3):437-441.
Wang Xuefu, Meng Yuping, Cao Qiufen,et alAdvances in research on chemical thinning for apple trees[J]. Journal of Fruit Science, 2006, 23(3): 437-441. (in Chinese with English abstract)
[2] Lordan J, Alins G, Àvila G, et al. Screening of eco-friendly thinning agents and adjusting mechanical thinning on ‘Gala’, ‘Golden Delicious’ and ‘Fuji’ apple trees[J]. Scientia Horticulturae, 2018, 239: 141-155.
[3] Goulart C, Braga de AndradeS, Bender A, et al. Metamitron and different plant growth regulators combinations in the chemical thinning of ‘Eva’ apple trees[J]. Journal of Experimental Agriculture International, 2017, 18(2): 1-6.
[4] Gabardo G C, Petri J L, Kretzchmar A A, et al. Different sources and concentrations of 6-BA in chemical thinning of post-flowering in apple trees[J]. Journal of Experimental Agriculture International, 2019, 32(6): 1-9.
[5] Basak A. Use of benzyladenine endothall and ammonium thiosulfate for fruitlet thinning in some cultivars[J]. Acta Horticulturae, 2000, 517: 217-226.
[6] Eccher G, Begheldo M, Boschetti A, et al. Roles of ethylene production and ethylene receptor expression in regulating apple fruitlet abscission[J]. Plant Physiology, 2015, 169(1): 125-137.
[7] Fruk M, Vuković M, Jatoi M, et alTiming and rates of application of NAA as blossom and fruitlet chemical thinner on apple cv. Braeburn[J]. Emirates Journal of Food and Agriculture, 2017, 29(2): 156-162.
[8] Gabardo G C, Kretzchmar A A, Petri J L, et alInfluence of post-flowering chemical thinning on development and fruit quality of ‘Fuji Suprema’ and ‘Maxigala’ apple trees[J]. Journal of Experimental Agriculture International, 2019, 32(4): 1-13.
[9] Milić B, Tarlanović J, Keserović Z, et alThe growth of apple central fruits as affected by thinning with NAA, BA and Naphthenic Acids[J]. Erwerbs-Obstbau, 2017, 59(3): 185-193.
[10] Gonzalez Luis, Torres E, Carbó J, et alEffect of different application rates of metamitron as fruitlet chemical thinner on thinning efficacy and fluorescence inhibition in Gala and Fuji apple[J]. Plant Growth Regulation, 2019, 89(3): 259-271.
[11] Raphael A S. The photosynthesis inhibitor metamitron is an effective fruitlet thinner for ‘Gala’ apple in the warm climate of Israel[J]. Scientia Horticulturae, 2014, 178: 163-167.
[12] Stevanovic M, Dolovac N, Marisavljevic D, et al. Efficacy of metamitron in apple thinning in Serbia[J]. Communications in Agricultural and Applied Biological Sciences, 2015, 80(2): 261-267.
[13] 薛晓敏,韩雪平,王来平,等. 苯嗪草酮对苹果坐果和光合生物学特征的影响[J]. 应用生态学报,2021,32(2):557-563.
Xue Xiaomin, Han Xueping, Wang Laiping, et alEffects of metamitron on fruit set and photosynthetic biological characteristics of apples[J]. Chinese Journal of Applied Ecology, 2021, 32(2): 557-563. (in Chinese with English abstract)
[14] 崔建宇,陈范骏,朱洪群. 土壤、植物与环境分析实验[M]. 北京:中国农业大学出版社,2007.
[15] Kang J, Yu H P, Tian C H, et alSuppression of photosynthetic gene expression in roots is required for sustained root growth under phosphate deficiency[J]. Plant Physiology, 2014, 165: 1156-1170.
[16] 李合生. 植物生理生化实验原理和技术[M]. 北京:高等教育出版社,2000.
[17] Yan H Y, Wang F, Han D D, et al. Simultaneous determination of four plant hormones in bananas by molecularly imprinted solid-phase extraction coupled with high performance liquid chromatography[J]. Analyst, 2012, 137: 2884-2890.
[18] 齐秀东,魏建梅,李永红. 苹果果实质地软化过程中碳水化合物代谢及其关键酶基因表达的变化[J]. 园艺学报,2015,42(3):409-417.
Qi Xiudong, Wei Jianmei, Li Yonghong. Carbohydrate metabolism and the key gene expression in apple during fruit texture softening[J]. Acta Horticulturae Sinica, 2015, 42(3): 409-417. (in Chinese with English abstract)
[19] Luckwill L C. The hormone content of the seed in relation to endosperm development and fruit drop in the apple[J]. The Journal of Horticultural Science and Biotechnology, 2015, 24(1): 32-44.
[20] Wani A W, Hassan G I, Dar S Q, et alInfluence of different phytohormones and nutrients on fruit set and chemometric attributes of apple[J]. An International Journal of Plant Research. 2017, 30: 492-498.
[21] Luckwill L C. Studies of fruit development in relation to plant hormones: II. the effect of naphthalene acetic acid on fruit set and sruit development in apples[J]. The Journal of Horticultural Science and Biotechnology, 2015, 28(1): 25-40.
[22] Rosa N, Verjans W, Oliveira C, et al. Comparison between 6-benzyladenine and metamitron as thinning agents in ‘Royal Gala’, ‘Cripps Pink’ and ‘Red Delicious’ apple cultivars[J]. Acta Horticulturae, 2018, 1221: 51-58.
[23] Lafer G. Effects of chemical thinning with metamitron on fruit set, yield and fruit quality of ‘Elstar’[J]. Acta Horticulturae, 2010, 884: 531-536.
[24] Greene D W. Use of metamitron alone and in combination with 6-benzyladenine for thinning apples[J]. Acta Horticulturae, 2014, 1042: 167-172.
[25] Steven J M, John D O. Comparison of the effects of metamitron on chlorophyll fluorescence and fruit set in apple and peach[J]. Hort Science, 2012, 47(4): 509-514.
[26] Gonzalez L, Torres E, Àvila G, et alEvaluation of chemical fruit thinning efficiency using Brevis® (Metamitron) on apple trees (‘Gala’) under Spanish conditions[J]. Scientia Horticulturae, 2020, 261: 1-8.
[27] Greene D, Costa G. Fruit thinning in pome and stone fruit: State of the Art[J]. Acta Horticulturae, 2013, 998: 93-102.
[28] Gabardo G C, Petri J L, Hawerroth F J, et alUse of metamitron as an apple thinner[J] Revista Brasileira de Fruticultura, 2017, 39(3): 1-9.
[29] 吕秉鼎,胡佳佳,汤丽云,等. 阳春砂落果规律及其生理机制[J]. 植物生理学报,2021,57(2):429-438.
Lü Bingding, Hu Jiajia, Tang Liyun, et al. The study on the fruit dropping law and physiological mechanism of[J]. Plant Physiology Journal, 2021, 57(2): 429-438. (in Chinese with English abstract)
[30] Ruiz R, Guardiola J L. Carbohydrate and mineral nutrition of orange fruitlets in relation to growth and abscission[J]. Physiology Plant, 1994, 90: 27-36.
[31] 徐昌杰,张上隆. 柑橘幼果发育期碳水化合物代谢及其与生长发育的关系[J]. 果树学报,2001,8(1):20-23.
Xu Changjie, Zhang Shanglong. Carbohydrate metabolism of citrus fruitlets in relation to growth and abscission[J]. Journal of Fruit Science. 2001, 18(1): 20-23. (in Chinese with English abstract)
[32] 杨波,车玉红,郭春苗. 扁桃幼果生理脱落与矿质元素浓度的关系[J]. 新疆农业科学,2015,52(5):852-857.
Yang Bo, Che Yuhong, Guo Chunmiao. Relationship between young almond fruits dropping and the concentration of mineral elements[J]. Xinjiang Agricultural Sciences, 2015, 52(5): 852-857. (in Chinese with English abstract)
[33] 关军锋,马智宏,张华,等. 授粉受精期苹果幼果发育与Ca,Mg,K含量的变化[J]. 河北农业科学,1999,3(3):1-5.
Guan Junfeng, Ma Zhihong, Zhang Hua, et alThe apple fruit development and changes in contents of Ca, Mg, K during the pollination and fertilization stage[J]. Journal of Hebei Agricultural Sciences, 1999, 3(3): 1-5. (in Chinese with English abstract)
[34] 董倩倩,龚桂芝,彭祝春,等. 柑橘采前落果与果实不同部位内源激素含量关系分析[J]. 植物生理学报,2018,54(10):1569-1575.
Dong Qianqian, Gong Guizhi, Peng Zhuchun, et alAnalysis on the relationship between pre-harvest fruit drops and content of endogenous hormone in different parts of fruit in citrus[J]. Plant Physiology Journal. 2018, 54(10): 1569-1575. (in Chinese with English abstract)
[35] 杨波,车玉红,郭春苗,等. 扁桃生理落果期不同组织激素浓度的动态变化及其对落果的影响[J]. 西北植物学报,2015,35(1):118-124.
Yang Bo, Che Yuhong, Guo Chunmiao, et alDynamic change of hormones in the different tissue of almond during the physiological fruit drop and its effect on fruit drop[J]. Acta Bot. Boresl.-Occident. Sin, 2015, 35(1): 118-124. (in Chinese with English abstract)
[36] 张晓明,黄卫东,韩振海. 苹果坐果的激素调控[J]. 植物生理学报,1998,24(4):361-366.
Zhang Xiaoming, Huang Weidong, Han Zhenhai. Study on the hormonal regulation of fruit setting in apple tree[J]. Acta Phytophysiologica Sinica, 1998, 24(4): 361-366. (in Chinese with English abstract)
Effects of fruit thinning agent “metamitron” on nutrition and hormone content of apple lateral fruits
Xue Xiaomin, Han Xueping, Nie Peixian, Dong Fang, Wang Jinzheng
(,271000,)
The work aimed to clarify the effects of metamitron as the fruit thinning agent on the apple fruit setting rate as well as the mineral nutrition, carbohydrates, and hormones of lateral fruitlets, thus providing a reference for applying chemical thinning technology of apples. Thirty 9-year-old apple trees (Tianhong 2/SH38/Malus micromalus) were used as test materials, and 300 mg/kg metamitron solution was sprayed 2 times when the diameter of the biggest lateral fruits were around 6 mm. Spraying water was used as the control. The setting rate of inflorescence and flower were investigated after physiological fruit drop. The mineral nutrition, carbohydrate, soluble protein, and hormone content of lateral fruits were measured after spraying 7, 9, 11, 17, and 29 d. The total nitrogen, phosphorus, and potassium were determined by semi-micro distillation, Mo-Sb-Vc colorimetry, and flame photometer, respectively. Glucose, fructose, sorbitol, sucrose, and hormone contents were determined by HPLC, while the soluble protein content was determined by Coomassie brilliant blue staining. The results showed that the fruit setting rate of inflorescence and flowers decreased by 17.95 and 27.63% compared with the control, respectively. The proportion of inflorescence with single fruit increased by 5.28 times in the treatment, while that of sitting three fruits and more decreased significantly, which was 47.91% of the control. The results of fruit setting rate and fruit setting ratio showed that metamitron could significantly reduce the fruit setting rate, and the single fruit rate was high, with the setting fruits distributed evenly. The total nitrogen content was higher than that of the control at all stages, and the difference between the control and the treatment was significant except 7 and 9 d. The total phosphorus and potassium contents of almost all treatments were significantly higher than those of the control. The total phosphorus contents of 11 and 29 d after spraying was 2.05 and 1.67 times of the control, and the total potassium content was 1.93 and 1.51 times of the control, respectively. The results of mineral nutrition showed that the fruit thinning effect of metamitron was not caused by the deficiency of inorganic nutrients. The content of the soluble protein decreased significantly compared with the control, and the content of each treatment period was 71.42%, 72.37%, 69.68%, 64.20%, and 86.02% of the control, respectively. The starch content of metamitron treatment was significantly higher than that of the control, with an increase of 5.57%-37.20%, yet the content of sucrose, glucose, fructose, and sorbitol decreased with different degrees. Therefore, the soluble carbohydrate content decreased significantly to 9.55%-52.57%. Soluble carbohydrates are the direct nutrients for the development of young fruits, so fruit thinning of metamitron may be caused by insufficient supply of soluble carbohydrates. The abscisic acid content generally increased after the treatment, and the ABA content in each period was 1.37-3.11 times that of the control. The ratio of (Z+GA3+IAA)/ABA decreased significantly, which could cause falling off of young fruits. As a result, metamitron has a good fruit thinning effect on apples, related to the decreased soluble protein content, an insufficient supply of soluble carbohydrate, increased ABA content, and decreased (Z+GA3+IAA)/ABA ratio.
hormone; nutrition; metamitron; fruit setting; apple
2020-09-22
2021-02-07
现代农业苹果产业技术体系(CARS-27)
薛晓敏,研究方向为水果遗传育种与栽培。Email:xuexiaomin79@126.com
王金政,研究员,研究方向为水果遗传育种与栽培。Email:wjz992001@163.com
10.11975/j.issn.1002-6819.2021.07.025
S661.1
A
1002-6819(2021)-07-0206-06
薛晓敏,韩雪平,聂佩显,等. 苯嗪草酮疏果剂对苹果边果营养与激素含量的影响[J]. 农业工程学报,2021,37(7):206-211. doi:10.11975/j.issn.1002-6819.2021.07.025 http://www.tcsae.org
Xue Xiaomin, Han Xueping, Nie Peixian, et al. Effects of fruit thinning agent “metamitron” on nutrition and hormone content of apple lateral fruits[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2021, 37(7): 206-211. (in Chinese with English abstract) doi:10.11975/j.issn.1002-6819.2021.07.025 http://www.tcsae.org
