Approaches to evaluate nutrition of minerals in food
2021-06-05XunWngYifnHeQinGoDongYngJinfenLing
Xun Wng, Yifn He, Qin Go, Dong Yng,b, Jinfen Ling,b,*
a College of Food Science and Nutritional Engineering, China Agricultural University, Beijing 100083, China
b Beijing Key Laboratory of Functional Food from Plant Resources Beijing 100083, China
ABSTRACT
Numerous techniques have been developed to determine the bioavailability of minerals. Each method has specific detection objects with certain procedures to assure the results. This review focuses on a comprehensive comparison of the applications, advantages, and critical control points of these techniques. The commonly used approaches for assessing mineral bioavailability can be divided into three categories-chemical methods,in vitro models and in vivo tests. Chemical methods are first developed, and mainly simulating the digestion environment to give a rough prediction of mineral bioavailability. In vitro models mainly used different cells to simulate the process and environment of food digestion to assess the availability of minerals. In vivo tests are employing complex models to observe the bioavailability of minerals after complicated digestive process in animal models or human volunteers. This review summarizes the critical points of establishment of these relevant models, compares the advantages and limitations among three categories. Although no single bioavailability method is ideal for all micronutrients, certain methods under proper operation can indeed be employed to minimize the differences between simulated results and reality for effective evaluation of the bioavailability of minerals.
Keywords:
Mineral
Bioavailability
In vivo
In vitro
1. Introduction
Micronutrients deficiency, mainly minerals, affects more than 50% of the world’s population, particularly in low-middle income countries [1]. Minerals have diverse functionalities and potentials in the body’s metabolism and homeostasis, such as building strong bones, making different hormones, transmitting nerve impulses and being a structural part in many enzymes [2]. Iron (Fe) deficiency is the most prevalent dietary micronutrient insufficiency worldwide, due to insufficient Fe intake, poor Fe absorption, and/or additional dietary Fe requirements, such as pregnancy need. [3]. Dietary Zn deficiency is affecting approximately 17% of the global population, which is the most common micronutrient deficiency primarily on cereal based diets and low consumption of animal products, leading to poor Zn bioavailability [ 4]. Osteoporosis is second only to cardiovascular disease as a global healthcare problem and medical studies, which is mainly related to long term lack of dietary calcium [5,6].
Many physiological and dietary variables could influence the bioavailability of minerals. Ph ysical factors, such as stage of development, individual mineral status. Iron deficiency is one of the most common micronutrient deficiencies in the world, affecting women, children, and infants severely, especially for the women of reproductive age because of menstrual losses and the high physiologic requirement for iron [7]. The other host factor are grouped as internal factors, while diet-related factors are considered external factors.Food is the only source of mineral intake, however, not all minerals in food can be abosorbed and consequently used for usual physiological functions via body cells. Minerals strongly interact with their chemical environment in food and other components during passage through the digestive system. For iron and zinc, the bioavailability especially in staple foods is low because of the highly complex chemical interactions with other compounds, including enhancers and inhibitors.The major inhibitor was phytate, which chelates iron or zinc and forms insoluble complexes. Other minor antinutrients could includ polyphenols and fibers [8]. Hart et al. [9] used the Caco-2 cell model to characterize the effects of 43 additional polyphenols on iron bioavailability, and identified them as being promoters or inhibitors.Conversely, ascorbic acid was a good enhancer of iron because it reduced iron from the diet to ferrous iron, a form absorbable by the intestinal cells. Other compounds, such as sulfur amino acids or organic acids, could also promote zinc absorption [8].
Mineral nutrition can be improved by 1) increasing the concentration of the nutrients while maintaining their bioavailability;2) maintaining the concentrations while improving bioavailability;or 3) increasing both the concentration and bioavailability of the minerals. Though bioavailability of minerals can’t be assessed easily and expressed intuitively, its effects have been explored by a variety of research models and methods. According to their principles, major materials involved, and operation means, these mineral bioavailability assessment models and methods are classified into three groups:chemical methods,in vitromodels, andin vivotest procedures.This review discusses the development of each methodology,summarizes the critical points during establishment of the relevant models, compares the advantages and disadvantages among the three categories, and finally focuses on respective applications of each.
2. Chemical methods
The chemical method uses HCl-extractability as the indicator for available minerals. To date, it is the first developed, the simplest and most economical method for studying mineral nutrition. HClextractability (HCl-E) refers to the solubility of the mineral in 0.03 N HCl after incubation at 37 °C for 3 h with constant stirring. At the end of the incubation time, the mixtures would be filtered through an ashless filter paper, and then the HCl-E is calculated as Equ (1)[10]:

WhereSMrepresents mineral content (mg) soluble in 0.03 N HCl at 37 °C for 3 h,TMrepresents total mineral content.
In this model, the process of incubation at 37 °C for 3 h in 0.03 N HCl simulates the human gastric system and the corresponding stomach environment food passing through. This method has also been used to study the effects of food processing (e.g., germination,fermentation, soaking, dehulling, and cooking), as well as the influence of components (e.g., phytic acid and polyphenols) on the mineral nutrition of grains and legumes[11-13].
This model was used to determine the food mineral dissolution rate in the body, but the action of enzymes in the digestive tract and the minerals actually utilized were not properly taken into consideration. Hence some researchers introduced pepsin and pancreatin to mimic the digestive enzymes [14]. Overall, the accuracy and application of this model was limited. It was rapidly evolved into thein vitromodel as discussed below, which took the influence of the human body’s microenvironment into account.
3. In vitro models
Thein vitromodel dated back to the 1980s, when most studies simulated only the pH of the gastric environment, thein vitromodel simulated the process and environment of food digestion in the human body [14-22]. It is a rapid and relatively accurate model for assessing the availability of minerals, and is currently the most often used model for food nutrition research. Solubility, dialyzability, and absorbability are the most often used parameters.
3.1 Solubility
Solubility, i.e.in vitrosolubility, is the proportion of ionizable minerals in the digestive system after digestion steps (soluble minerals in the solution) out of total minerals in certain foods. In the initial configuration of the model, food samples are incubated/digested with pepsin-HCl (0.5% pepsin in 0.1 N HCl) for 90 min,then the pH is adjusted to 7.5 followed by centrifugation and filtration; ionizable mineral (iron) in the digested solutions at pH 7.5 is then defined as bioavailable [23]. Because the principal site of iron absorption is in duodenum, the model has been further modified with an additional step that mimics intestinal digestion(incubation with pancreatin-bile extracts at pH 6.8-7.0) before centrifugation[14].
In vitromodel is a simple, effective, and reproducible method for estimating mineral bioavailability. It has been successfully applied in estimating the bioavailability of iron and zinc in several different foods (Table 1).

Table 1Studies using mineral solubility as indicators of bioavailability.
3.2 Dialyzability
Dialyzability is the proportion of minerals that can cross a 6 000-8 000 Da molecular weight cutoff semipermeable membrane after thein vitrodigestion procedure [14]. Compared to solubility,the property of dialyzability is more stable and reproducible.
On the basis of the application of pepsin and other digestive enzymes, Miller et al. [15] adjusted the pH from gastric to intestinal levels in a mixture of food samples and digestive enzymes by adding NaHCO3to the dialysis tube. In order to increase the efficiency of assessing dialyzability for a more diverse samples, Argyri et al. [18] modified the setup of gastro-intestinal digestion by carrying out tests on six-well plates and fractionating the digests with a dialysis membrane. Dialyzability has been applied to food solutions in both liquid-state and solid-state form.Table 2 summarized the studies on mineral bioavalability using the dialyzability as a indicator.

Table 2Studies using mineral dialyzability as indicators of bioavailability.
In vitrosolubility and dialyzability represent the first stage of biological utilization and are well-correlated with human studies.However, both of them are only suitable for the prediction of nonheme iron bioavailability, as gastro-intestinal conditions have practically no influence on heme iron [33]. Solubility and dialyzability do not involve the subsequent mineral absorption step, and not all soluble minerals can be recognized as bioavailable, thus their configuration do not accurately reflect the entire virtue conditions though they do provide an index of available minerals in food.
3.3 Absorbability
Absorbability is the proportion of a mineral that can be absorbed by certain cells from the total content in food.Absorbability is normally determined by tissue culture models that are used to simulate the second stage of biological utilization of minerals (i.e., absorption) by the human body. The human colon adenocarcinoma (Caco-2) cell line is the most popular one used to examine transport and uptake of minerals. Under appropriate conditions, Caco-2 cells differentiate spontaneously into polarized,enterocyte-like monolayers. They exhibit many characteristics such as the formation of brush border microvilli and the presence of brush border-associated enzymes, similar to small intestinal cells[14-18,21,22]. Other cells, such as human colon adenocarcinoma(HT-29) [34] or rat small intestine (IEC-6)[35] cells, have also been used for this purpose.
Normally, the gastro-intestinal digestion model and Caco-2 cell monolayer uptake model are combined for studying mineral bioavailability.Caco-2 cells are cultured in six-well plates, and an upper chamber is created over the cells by inserting a 12 000-14 000 Da molecular weight cut-off dialysis membrane, which allows low molecular weight mineral complexes to diffuse into the lower compartment where its uptake takes place. Many studies on the enhancers and inhibitors of iron uptake have used the combined models [14-18,21,22]. Caco-2 cell ferritin is one of the sensitive and clear markers of cell iron uptake since cells produce ferritin proportionately in response to increases in intracellular iron. Glahn et al.[15] first introduced ferritin formation into the prediction of nonradiolabeled food iron availability.
A combination ofin vitrodigestion and Caco-2 cell line modeling appears to be the most widely appliedin vitromodel in the field of iron availability, as it has demonstrated the capability to examine a broad range of foods and meal conditions, and produces results that consistently agree with human studies, and also can investigate interactions and factors related to rion bioavailability in foods.Although it is a useful and relatively accurate tool for estimating the mineral bioavailability, it is of high cost and stringent technical requirements compared to the simple gastro-intestinal digestion model. Additionally, Caco-2 cells are derived from the colon carcinoma, and the extent to which normal metabolic processes are maintained in these cells remains debatable.
Researchers have also applied different cells in combined models. For example, intestinal iron absorption is regulated by body iron storage and the cell iron storage affects iron uptake into the cells. To partially alleviate the efflux pathway blockage, Bering et al. [34] extended thein vitrodigestion/Caco-2 cell culture model to a three-tier system. In this system, Caco-2 cells are grown on a semipermeable membrane so that iron can pass across the cell into the basolateral chamber. Thus mimicking the status of the basolateral side becomes possible with this system. Because of the tight junctions of Caco-2 cell monolayers and the lower permeability in comparison to the human intestine, recent works have added goblet cells to the co-culture. Maares et al. [37], for example, utilized co-cultures of Caco-2 and HT29-MTX cells in a zinc resorption assay to obtain highly accurate zinc bioavailability predictions. As for other minerals,there has been limited utilization of Caco-2 cell culture to study the mechanisms of absorption or factors affecting absorption.
Studies on mineral bioavailability usingin vitromodels are summarized in Table 3.
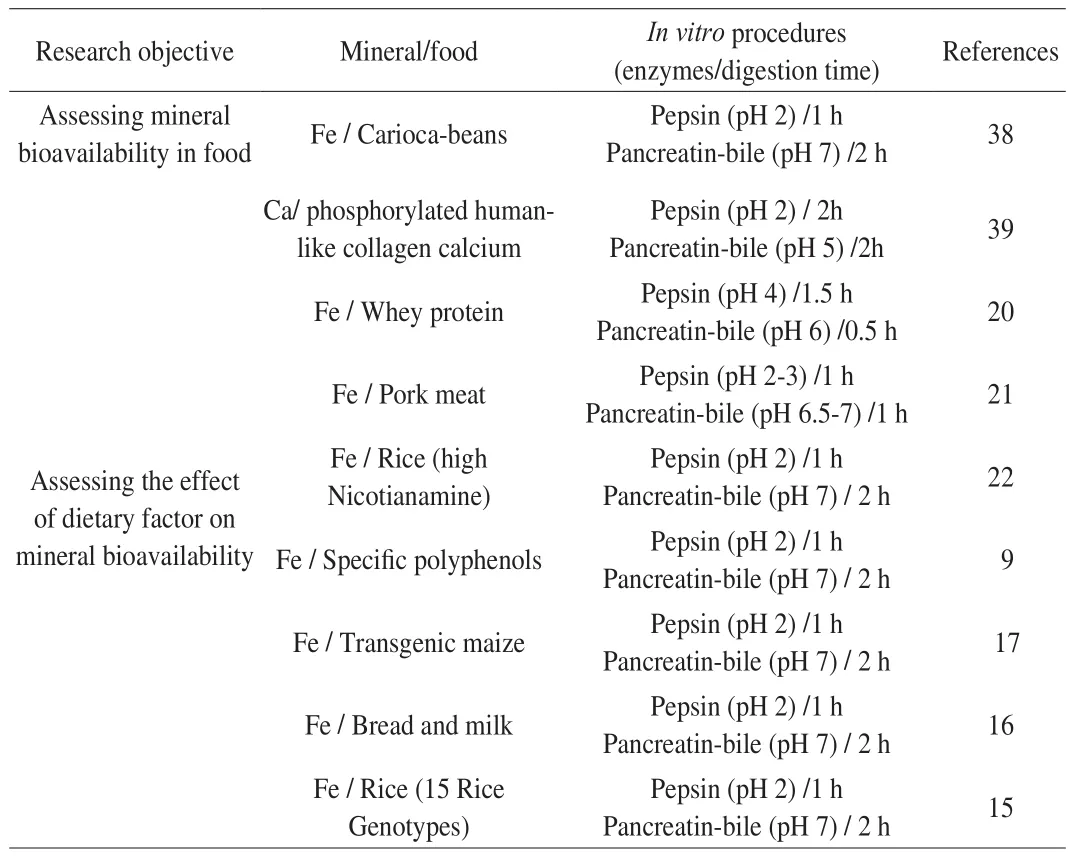
Table 3Studies using in vitro digestion/Caco-2 cell culture models.
4. In vivo test
Currently,in vivotests (based on humans or animals) for mineral bioavailability mainly include balance study, mineral concentration in serum and urine assay, tissue indicators development, and expressions of relative genes or protein study. In vivo tests are mainly employed by human nutritionists, not popular in food sciences research.
4.1 Balance study
“Balance study” is defined as the difference between mineral intake and excretion as it represents the apparent absorption of minerals. During balance study, the total minerals provided and remaining in the diet, feces, and urine are collected and used to calculate the absorption [40]. Balance study is the most traditional in vivo test for mineral absorption.
Balance study is conceptually simple, easily operated, and commonly applied in the mineral nutrition field. It can be conducted in both animals or humans and is applicable to all types of food,supplements, and even drugs. However, there are significant drawbacks of this method: 1) it is a time consuming and expensive process, and 2) its accuracy is relatively poor, as the mineral intake is often overestimated (incomplete consumption) while the excretion is underestimated (incomplete fecal and urine collection). Thus the absorption of minerals derived via balance study is normally higher than actual absorption due to the existence of endogenous minerals.
Radioisotope techniques, mainly radioisotope and stable isotope techniques, were introduced for trace element studies in the late 1940s and have been widely used since [41]. The use of isotopes in minerals absorption is feasible, and a combination of isotope and chemical balance techniques can yield significantly more information than either technique used alone [42].
For most minerals, the tracer methods would be optimal. They are highly sensitive and reproducible, and depending upon the tracer used,can be very quick and inexpensive [43]. The high sensitivity of the method is supported by very low levels of the tracer (particularly if radioactive) in the normal background. The method requires the mineral source be homogenously labeled, i.e., that every atom or molecule of the test sample in the ingesta has the same probability of containing the isotopic tracer as every other atom or molecule, and labeling isotopes equably is very challenging (especially in a mixed diet). Accordingly, this method is mainly used for studies on single minerals.
4.2 Minerals in serum and/or urinary increment
The increment of mineral concentration in serum after 24 h ingestion is defined as “mineral bioavailability”. Its principle relates to pharmacokinetics: after a one-time oral dose of medicine, drugs are absorbed and gradually enter the blood circulation, causing the concentration in serum to increase, and minerals work similarly [44].
The absorptive increment usually tends to be only a small fraction of what is already present, and homeostatic forces actively damp the absorptive rise [45]. Studies on calcium have indicated that serum calcium begins to rise at 1 h and reaches its peak at 5 h, but only increases 5% overall from the baseline [44]. The in serum method thus requires that dose levels generally be considered pharmacological rather than physiological and can obtain a significant response.Simplicity, speed, and easy operation are the principal advantages of this method. It is also inexpensive and suitable for multiple tests, but it tends to have a low signal-to-noise ratio.
The urinary increment technique works similarly to that in serum technique. When the concentration of nutrients in the blood increases,the amount excreted in the urine also increases. Hence, an increase in urinary mineral excretion within 24 h after oral administration is an indicator of bioavailability. This method was initially developed for the identification of patients with renal stones presenting with intestinal hyper-absorption of calcium.
Urinary increment is much less expensive than the classical serum method. For many trace elements such as iron, zinc, and copper, however, urinary excretion is often deemed insignificant or entirely negligible, rendering the test inapplicable. Similar to the serum method, it is suitable for the comparison of two or more kinds of supplements. Mortensen and Charlesused [46] compared the absorption of calcium, calcium carbonate, and calcium carbonate plus vitamin D in milk to find that a calcium carbonate regimen is at least a good calcium supplement as milk, and that an addition of 600 IU vitamin D promptly results in an increase in urinary calcium excretion after increase in calcium absorption, even in healthy women.
4.3 Tissue indicator
Body tissues can also be used as a mineral absorption indicator based on their specific position relevant to mineral deposition.Depending on the trace element studied, minerals in tissues, whole body weight, bone gain, and/or liver concentrations can be used as indicators. For example, Veum et al. [47] used weight gain and bone zinc as indicators for zinc availability. Heart, liver, and bile are suitable tissues for copper bioavailability study [48]. In vivo tests used in different studies are summarized in Table 4.

Table 4In vivo tests for various foods.
4.4 Expression of gene or protein
In 1995, the divalent metal transporter 1 (DMT1) (also called“Nramp2-nature resistance-associated macrophage protein 2”, or“DCT1-divalent cation transporter 1”, or SLC11A2-solute carrier family 11, member 2) was discovered. It was considered as one of the most significant breakthrough in the field of mammalian mineral metabolism [69]. Till now, many different proteins such as ferritin,ferroportin and duodenal cytochrome b (Dcytb) for iron transport;CaT1, calbindin-D9k, and claudins for calcium; and zip4, ZnT1 and MT4 for zinc have been proven related to metal transfer [7,68-73].The up-regulation of mineral metabolism-related gene expression indicates the possible enhancement of the intestinal development,overall digestive capacity or the mineral uptake and celllular storage,so measurement on expression of proteins or related genes is suitable for evaluating mineral nutrition.
Van Cromphaut et al. [73] assessed serum45Ca accumulation rate within 10 min of oral gavage in two strains of VDR-knockout (KO)mice and determined the expression of intestinal candidate genes involved in transcellular calcium transport. Calcium transport protein1(CaT1) was shown to more abundantly expressed at mRNA level than the epithelial calcium channel (ECaC) in the duodenum, but both expression were considerably reduced (CaT1 > 90%, ECaC > 60%) in the two VDR-KO strains on a normal calcium diet.
The activity of enzymes and expression of proteins and genes do not precisely reflect the absolute absorption of minerals, but they are helpful for investigating mechanisms such as the mineral absorption pathway and the regulation of mineral transport.
5. Summary
All methods mentioned above are applicable for both animal models and human models, although there are, of course, some differences between the two. Extrapolating certain results from the animal model to human beings can be challenging or restrictive.The mouse model, the most commonly used (and economical) one,represents larger differences from the human body than other animal models such as pigs. Rats absorb minerals much more efficiently from plant foods than humans, and can be used to give rough estimates of bioavailable minerals in certain foods. The human model is the most realistic, but because of the individual differences and difficulty in enlisting volunteers, animal models are used much more often. Meanwhile, the combination of cell culture and in vitro digestion models presents an alternative for studies that are frequently challenging to conduct in humans and animals because of ethical concerns.
In studies on mineral bioavailability, the techniques chosen(in vivo or in vitro) are often predetermined by the resources available to the investigator. No single bioavailability method is ideal for all micronutrients, and all bioavailability methods present advantages and limitations, and they all have the potential to provide useful information if properly conducted and carefully interpreted. The equipment required, costs of labor and animals, and funds available all play important roles in selecting the method of choice. Given the need for mineral nutrition research (and economic restrictions under which the research conducted), it is necessary to utilize in vitro and animal models first before application the information obtained to human clinical trials.
Conflict of Interest
The authors declare no conflict of interests.
Acknowledgement
This manusciprt is supported by China Agriculture Research System - Green Manure.
杂志排行
食品科学与人类健康(英文)的其它文章
- Bioactive compounds and probiotics-a ray of hope in COVID-19 management
- The role of glutamine in supporting gut health and neuropsychiatric factors
- Anti-hyperglycemic effects of dihydromyricetin in streptozotocin-induced diabetic rats
- Aroma profile of two commercial truffle species from Yunnan and Sichuan, China:inter- and intraspecific variability and shared key compounds
- Stability of phenolic compounds and drying characteristics of apple peel as affected by three drying treatments
- Development of water-soluble zein colloid particles and in situ antibacterial evaluation by multiple headspace extraction gas chromatography
