Dielectric breakdown properties of Al-air mixtures
2021-05-22XiyuanCAI蔡喜元XiaoZHANG张晓JunyongLU鲁军勇SaiTAN谭赛YongshengZHANG张永胜andGuanxiangZHANG张冠祥
Xiyuan CAI (蔡喜元), Xiao ZHANG (张晓), Junyong LU (鲁军勇),Sai TAN(谭赛),Yongsheng ZHANG(张永胜)and Guanxiang ZHANG(张冠祥)
National Key Laboratory of Science and Technology on Vessel Integrated Power System,Naval University of Engineering, Wuhan 430033, People’s Republic of China
Abstract In order to investigate the influence of aluminum vapor on the breakdown performance of air,this paper makes a study of the dielectric breakdown characteristics of Al-air mixture in the temperature range of 300–5000 K at atmospheric pressure.A Boltzmann analysis method is used to deal with the electron energy distribution function(EEDF),the reduced ionization coefficients(α/N), the reduced attachment coefficients (η/N) and the critical reduced breakdown strength((E/N)cr) so as to explore the influence of temperature and mixing ratio on the dielectric breakdown properties.In the temperature range of 300–2000 K, the property of the mixture is mainly determined by the mixing proportion of aluminum vapor because the composition of particles remains unchanged.In the temperature range of 2000–2500 K, the decomposition of Al2O2 leads to the increase of aluminum oxides and NO, and a rise in the percentage of highenergy electrons as well as the increment of α/N.Also,the joint action of O2 and NO makes η/N increase first and then decrease, and (E/N)cr goes down to a smaller temperature range.An increase in the proportion of aluminum vapor causes(E/N)cr to decrease in the low-temperature region and to increase in the high-temperature region, which will reduce the transition between these two temperature regions.
Keywords: aluminum vapor, arc discharges, Boltzmann analysis, dielectric properties,electromagnetic rail launch, muzzle arc
1.Introduction
In electromagnetic rail launch (EMRL), the arcing performance of the arc ignition device and the transfer process of the muzzle arc are the key factors of the ablation of the materials in the muzzle end and initial disturbance of the projectile.When moving in the bore at high speed, the aluminum armature has friction with the copper rails in the case of high temperature and current.Aluminum vapor can be produced[1]and the arcing gap in the arc ignition device will be heated from room temperature to thousands of Kelvins.These are certain to affect the dielectric breakdown properties of air medium in the arc ignition device and further the performance of the device as well as the transfer process of the muzzle arc.Previous researches into the characteristics of Alair plasma have focused on the thermodynamic properties,transport coefficients and radiation [2, 3].However, the dielectric breakdown characteristics of mixed gases need to be studied further.
Some literatures have studied the dielectric breakdown properties of mixed gases under different working conditions[4–6],but those methods are not suitable for Al-air mixed gas because it needs to be maintained at a high temperature.The theoretical calculation methods of gas discharge characteristics are as follows.(a)Theoretical model of steamer[7].The calculation is simple, but low in accuracy as it is not able to reflect the synergistic effect of every component that is caused by the interaction between particles in the mixture.(b) Particle-in-Cell Monte-Carlo collision (PIC-MCC) model [8].This method has high accuracy, but it needs more computer resources.Moreover, it needs a long calculation period when the number of particles is large.Therefore, it is only suitable for low-pressure gases.(c)Boltzmann analysis method[9].At present, the two-term approximate calculation is mainly used for a solution.This method can overcome the shortcomings of the steamer theoretical model and PIC-MCC model.When the cross-section of elastic collision of particles is obviously larger than that of inelastic collision and the electric field strength is low, it is possible to obtain accurate solutions[10, 11].In addition, the calculation is relatively simple.
The purpose of this paper is to investigate the dielectric breakdown characteristics of Al-air mixture in the temperature range of 300–5000 K under atmospheric pressure.First,the composition of particles in the mixture is calculated according to different proportions of aluminum vapor.Then,the obtained cross-section data of main particles are approximated to the unknown cross-section data.These two kinds of data are substituted into Boltzmann equations, and the two-term approximate method is used to solve the equation of mixed gases at different temperatures and in the reduced electric field thus to obtain EEDF, α/Nand η/Nof mixed gases.After their treatment, (E/N)cris derived.
2.Model and parameters
2.1.Hypothesis
In the process of solution, assumptions are given below:
• All particles are in a ground state,with their excited states ignored.
• The superelastic inverse process is not considered.
• The energy of two electrons is equal after the ionization collision reaction.
• What is significant in gas breakdown is collision between electron and heavy particle, so photoionization and photodissociation are not taken into account.
• Three-body collisions in gas increase with pressure.However, this paper only considers the working of mixture at atmospheric pressure, but does not deal with the three-body collisions due to their complexity.
2.2.Boltzmann analysis method
According to the theory of plasma,the distribution functionfof electron satisfies the Boltzmann equation [12].The use of the two-term approximation can obtain the continuous equation of convection diffusion off0,or the isotropic part off.The equation is as follows:

among which,


where ε is electron energy;eis the charge;meis the electron mass; γ=(2e/me)1/2;Eis the electric field;Tis electron temperature; σεis the overall elastic collision cross-section;σmis the total momentum transfer cross-section; ε0is the vacuum dielectric constant;kbis the Boltzmann constant;Nis the particle density;Mis particle mass;Sis the energy loss in inelastic collision;xkis the mole fraction of target particle;Ckis the energy loss in thek-th inelastic collision between electrons and heavy particles; andCexc,Catt, andCionare respectively the energy loss in excitation, attachment and ionization collisions.

Here, Δεkis the threshold energy ofk-th inelastic collision
2.3.Equilibrium composition of Al-air mixtures
The chemical composition of mixed gases is one of the things which will be first handled by the Boltzmann analytical method.The calculation of equilibrium composition of Al-air mixture is based on the principle of Gibbs free energy minimization [13].Air is mainly composed of N2, O2, noble gas and CO2.In this paper,all the noble gases are equivalent to a single component Ar.As the content of CO2in the air is only 0.03%or so,it can be ignored[14].For this reason,this paper takes pure air as a mixture of N2, O2, and Ar.
The aluminum vapor content (Mal) in the temperature range of 300–5000 K that is obtained from calculation is respectively 0, 1%, 5%, 10% and 20%, that is, the composition data of particles in Al-air mixture.Figure 1 demonstrates the main neutrals of 10%Al-90%air mixture.It can be seen that the main neutrals are N2,O2,N,O,NO,Ar,Al,AlO,Al2O, AlO2and Al2O2, and that variation is smaller in the composition of mixed-gas particles but N2,O2,Al2O2,and Ar are larger in proportion when the temperature is below 2000 K.
2.4.Electron collision cross-section

Figure 1.Composition of 10%Al-90%air mixture particles (only main neutrals).
In addition to the equilibrium composition, the data of electron impact cross-section of different particles are also needed in the calculation.In this paper,the data of N2,O2,N,O,NO,Ar,Al and AlO are obtained from LxCat website[15–17],the ionization cross-section data of Al2O, AlO2and Al2O2are calculated by equation (9) [18].Based on the equivalence principle of similar dielectric breakdown properties proposed in [19] and [20], the unknown cross-section data of Al2O,AlO2and Al2O2are assumed to be those of AlO except for the ionization cross-section, which is calculated by equation (9) [18].
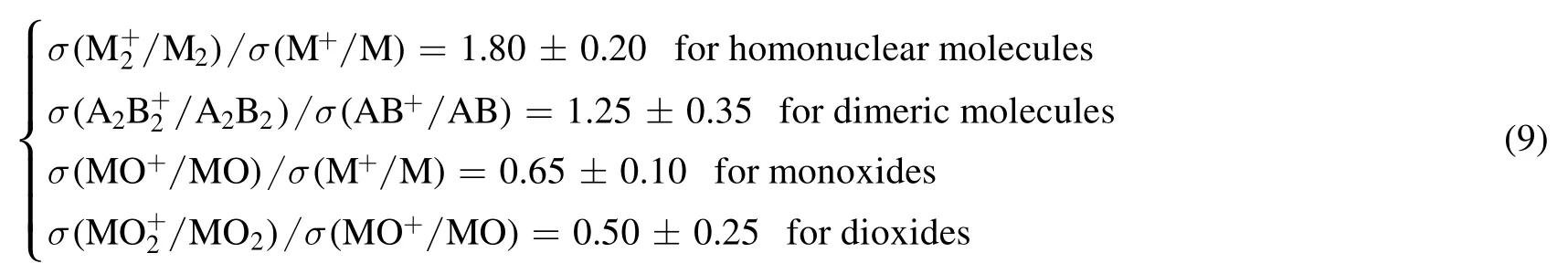
among them, σ is the cross-section, the ionization energy of monoxides is slightly more than that of metal atoms, while that of dioxides is 2 to 3 eV higher.
Using the above calculation methods and parameters,it is possible to obtain EEDF, α/N, η/Nof Al-air mixtures in the temperature range of 300–5000 K by solving the Boltzmann equations with commercial code COMSOL.α/Nand η/Nrepresent coefficients of electron generation and disappearance respectively, and (E/N)crof the mixture is obtained at (α −η)/N=0.
3.Result and analysis
3.1.α/N of air medium

Figure 2.α/N of air medium.
Because there is no experimental data of Al-air dielectric breakdown characteristics, the calculated α/Nof air within 300 Td (1 Td=10−21V·m2) is compared with other experimental data[21–23]to verify the calculation methods and parameters, as shown in figure 2.It can be seen that the calculation result deviates from that of Prasad[21],but it coincides well with the data given by Moruzzi [22] and Stout [23].
3.2.EEDF of Al-air mixtures
Figure 3 shows the EEDF of the mixtures with different proportions of aluminum vapor at 300 K.It is obvious that aluminum vapor will increase the proportion of low-energy electrons and decrease that of high-energy ones,and the effect will be more obvious with an increase in the proportion of aluminum vapor.Aluminum vapor exists mainly in the form of Al2O2.The crosssection of inelastic collision of Al2O2is larger.The inelastic collision electron with Al2O2will absorb the energy of electrons,thereby causing more electrons to be in a low-energy state.
Figure 4 shows the EEDF of 10%Al-90%air mixtures at 100 Td.Its illustration is as follows:
• There is little difference in the EEDF of mixtures at 300–2000 K,which is mainly due to a very small change in the composition of particles at that temperature range,as shown in figure 1.

Figure 3.EEDF of the mixtures with different proportions of aluminum vapor at 300 K.

Figure 4.EEDF of 10%Al-90%air mixtures at 100 Td.
• When the temperature is higher than 2000 K, the energy range can be divided into three regions: low-energy region (0–2 eV), medium-energy region (2–5 eV) and high-energy region (above 5 eV).The proportion of electrons in the medium-energy region increases with temperature, but those in the low- and high-energy regions do not.Temperature is the main factor affecting the electrons in the low-energy region, because its rise leads to the acceleration of electrons and a decrease in the percentage of electrons of the low-energy region.But for changes in the high-energy region, the composition of particles and the cross-section of collision can be used for explanation: on one hand, Al2O2and O2decompose and the proportion of aluminum oxides increases;on the other hand,the most part of the inelastic collision cross-section of aluminum oxides is above 5 eV.As a result, the probability of inelastic collision between electrons and aluminum oxides increases, while the proportion of electrons in the high-energy region decreases.Because of a decrease in the proportion of electrons in the lowand high-energy regions, there is certainly an increase in that in the medium-energy region.
• Variations in the proportion of aluminum oxides have an influence on differences in EEDF at different temperatures.According to the changing rate of aluminum oxides(|ΔMAl,O(T1,T2)|,T1andT2represent different temperatures,the same below),as shown in table 1.It can be seen that the changing rate of EEDF (ΔEEDF(T1,T2)) is consistent with that of aliminium oxides, that is, ΔEEDF(2000, 3000)>ΔEEDF(3000, 4000)>ΔEEDF(4000, 5000)>ΔEEDF(1000, 2000)≈ΔEEDF(300, 1000)= 0, and |ΔMAl,O(2000, 3000)|>|ΔMAl,O(3000, 4000)|>|ΔMAl,O(4000,5000)|>|ΔMAl,O(1000, 2000)|≈|ΔMAl,O(300, 1000)|=0.From the above analysis,it is known that the phenomenon is caused by variation in the composition of aluminum oxides and the concentrative distribution of their inelastic collision cross-sections in the high-energy region.
Figure 5 shows the EEDF of 10%Al-90%air mixture at 300 K.The electric field plays a role in the acceleration of electrons.Therefore,in the energy range above 2.5 eV,EEDF increases withE/N, while it is quite contrary in the energy range below 2.5 eV.
3.3.α/N, η/N of Al-air mixtures
Figure 6 illustrates α/Nof mixture in 10%Al-90%air.It increases withE/Nwithin 500 Td.A rise inE/Nhelps raise the proportion of electrons in the high-energy region, where the collision cross-sections for ionization become larger,which makes an ionization reaction more likely to happen.Within 2000 K,there is hardly any change in α/Nat differentE/N, which is because almost no change appears in the composition of fixed particles.Being above 2000 K, α/Nincreases with the temperature,which is similar to EEDF,and its difference between 3000 K and 4000 K is larger,due to the dissociation of Al2O2and O2.
Figure 7 illustrates η/Nof mixture in 10%Al-90%air.Unlike α/Nwithin 500 Td, η/Nquickly increases at the beginning with the increase ofE/N, but it begins to decline after reaching a certain numerical value.From the curve of EEDF and the electron attachment collision cross-section,it is found that whenE/Nincreases,the percentage of electrons in the energy range of 0–2.5 eV decreases and that in the energy range above 2.5 eV rises.On one hand,the energy range with a larger increase in the percentage is 3–10 eV whenE/Nincreases from 50 Td to 150 Td.On the other hand, only O2and NO among all the components have electron attachment collision cross-sections.As shown in figure 8, these crosssections are just concentrated in the energy range of 4–13 eV.Because of the above two factors, the consequence is that whenE/Nrises from 50 Td to 150 Td, the percentage of electrons in the energy range of 3–10 eV increases more than those in other energy ranges, thereby raising the rate of electron attachment collision reactions of O2and NO and making η/Nincrease rapidly.The coincidence between the EEDF curve and the attachment collision cross-section also shows that η/Nincreases more and more slowly when the temperature is above 3000 K.The concrete explanations are as follows.(a) Beginning from 2000 K, O2decreases significantly.(b) NO comes into being at 1200 K and increases with temperature and then declines after it reaches the climax at 3400 K.(c) The attachment cross-section of NO is smallerthan that of O2in the energy range of 4–10 eV.Therefore,the whole process of the temperature increasing from 300 K to 5000 K can be described as: (a) η/Nchanges slightly at temperature below 2000 K due to little change in the composition of particles; (b) from 2000 K to 3000 K, the percentage of O2declines thus to suppress the attachment collision, and the increase of NO promotes the attachment collision.Between these two factors, there exists a competitive relationship.As both the mole fraction and the attachment collision of O2are larger than those of NO, so O2has the advantage over NO, with the result that the growth of η/Nis not as significant as that at 300–2000 K.(c) When the temperature is above 3000 K, both O2and NO tend to decrease, which causes the reaction rate of attachment collision to slow down.
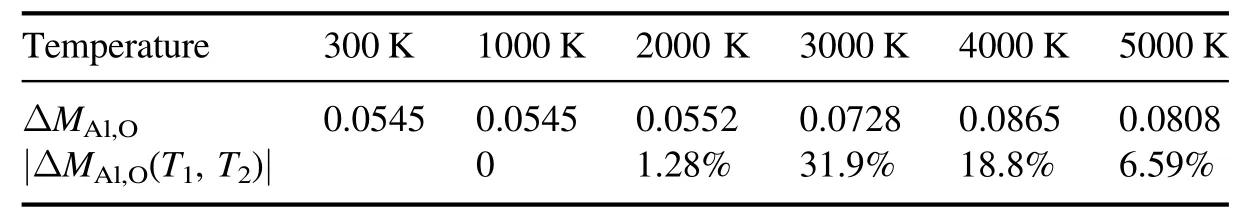
Table 1.Variation of the proportion of aluminum oxides at different temperatures.

Figure 5.EEDF of 10%Al-90%air mixture at 300 K.

Figure 6.α/N of 10%Al-90%air mixture.

Figure 7.η/N of 10%Al-90%air mixture.

Figure 8.Attachment cross-sections of particles in Al-air mixture.
After being up to 150 Td, η/Ngradually decreases asE/Ncontinues to increase.According to the EEDF curve,the range of electron energy is found to expand with the increase ofE/Nafter reaching 150 Td,and electrons tend to spread in the high-energy range,while there is no significant increase in the number of electrons in the energy range of 3–10 eV.The increase of high-energy electrons weakens the effects of O2and NO and suppresses the reaction rate of attachment collision, thereby resulting in the inconsistency of η/Nbefore and after 150 Td.
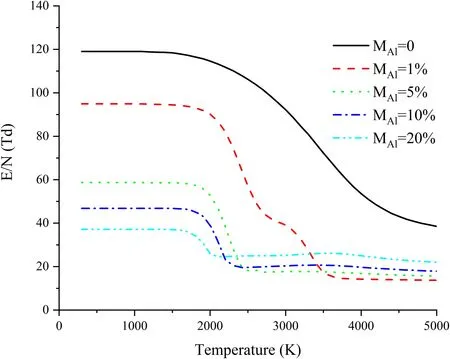
Figure 9.(E/N)cr of Al-air mixtures.

Table 2.Variation of the proportion of aluminum oxides at different temperatures.
3.4.(E/N)cr of Al-air mixtures
Figure 9 shows medium(E/N)crwith different proportions of aluminum vapor.With a rise in the temperature, (E/N)crof pure air experiences three stages: first, keeping basically constant; then, dropping rapidly; finally, declining slowly after reaching a certain temperature.Based on the variation of(E/N)crwith temperature, the temperature range can be divided into three regions: low-temperature region (300–2000 K),medium-temperature region (2000–2500 K; 2000–3500 K especially at a time whenMal=1%), and high-temperature region (2500–5000 K; 3500–5000 K especially at a time whenMal=1%.
In the low-temperature region, (E/N)crbarely changes with temperature.This is because there is little variation in the composition of particles in this region.Aluminum vapor can significantly reduce (E/N)crand will decrease greatly as the vapor content increases.Different proportions of aluminum vapor have a weakening effect on the breakdown strength of mixture in the low-temperature region, as shown in table 2.Obviously a small amount of aluminum vapor can significantly reduce(E/N)crof the medium.Even one percent of aluminum vapor can decrease (E/N)crby 20.3%, and five percent has already been able to decrease (E/N)crby 50.7%.With a continuous increase inMal, the decrease of (E/N)crwill slow down.(E/N)crhas only a 10% change whenMalincreases from 5% to 10%, and its change rate is less than 10%whenMalincreases from 10%to 20%.This means a highproportion of aluminum vapor has a limited effect on the reduction of (E/N)cr.

Table 3.(E/N)cr of Al-air mixtures in high temperature region.
(E/N)crchanges greatly in the medium-temperature region.The increase ofMalwill narrow the range in which(E/N)crchanges.The medium-temperature region will be narrowed from 2000–3500 K to 2000–2500 K whenMalincreases from 1% to 5%.For the other three mixed gases with higherMal, each of their medium-temperature regions is roughly determined as 2000–2500 K, but there is a smaller difference in (E/N)crchanging in the region, that is to say,with the increase ofMal,(E/N)crwill gradually fulfill its rapid decline, and its characteristics of change begin to emerge in the third stage.
In the high-temperature region, (E/N)crchanges in the same way as pure air whenMal=1%.Both of them decrease at relatively low speed as the temperature rises.AfterMalis increased, (E/N)crwill slightly rise in the range of 2000–3500 K and then fall at the same small slope.Furthermore, (E/N)crincreases gradually withMal, which is completely contrary to that in the low-temperature region.(E/N)crof Al-air mixtures in the high-temperature region is shown in table 3, from which it can be seen that (E/N)crof Al-air mixtures in a hot state is far lower than(E/N)crof pure air in a cold state.
4.Conclusion
This work has calculated the equilibrium composition of Alair mixtures with a different proportion of aluminum in the temperature range of 300–5000 K under atmospheric pressure, obtained the electron collision cross-sections of main particles, and solved Boltzmann equations for EEDF, α/N,η/Nand further(E/N)cr.The main conclusion is given below:
• There was no significant difference in EEDF,α/Nand η/Nin the temperature range of 300–2000 K because the composition of particles almost remains constant in this temperature range.After the temperature is above 2000 K,Al2O2and O2are decomposed,while there is an increase in the proportion of aluminum oxides and NO.The proportion of electrons proportion in the 2–5 eV energy range increases with the temperature,but it decreases in the other temperature range, in which α/Ngradually increases and η/Ntends to change.
• AsE/Nincreases,the proportion of high-energy electrons and α/Nkeeps a rising trend.η/Nincreases rapidly and then begins to decline after reaching a certain numerical value.WhenE/Nrises from 50 Td to 150 Td,the energy range with a larger increase in the proportion of electrons coincides with the energy range in which the attachment collision cross-section lies.In this stage, η/Nincreases rapidly.AfterE/Nexceeds 150 Td, these two energy ranges will deviate from each other,so that the attachment collision will be suppressed.
• Aluminum vapor can significantly weaken the breakdown strength of the mixture.In the low-temperature region,(E/N)crcan be reduced more significantly as the content of aluminum vapor increases, but the effect of a high proportion of aluminum vapor on the reduction of(E/N)cris limited.When pure air is at 300 K,(E/N)cris 119.0 Td.WhenMalis 1%, 5%, 10% and 20%, (E/N)crin the lowtemperature region is respectively 94.9 Td, 58.7 Td,46.8 Td and 37.1 Td.Furthermore, the increase of aluminum vapor can make (E/N)crgo into the hightemperature region, where (E/N)crgradually increases with a rise in the proportion of aluminum vapor,which is contrary to the case in the low-temperature region.(E/N)crin the high-temperature region is lower.The data of the aforementioned four kinds of mixed gases are respectively about 14 Td, 17 Td, 20 Td and 25 Td.
Acknowledgments
This work was supported in part by National Natural Science Foundation of China (Nos.51522706, 51877214, and 51607187) and in part by the National Basic Research Program of China (973 Program) (No.613262).
猜你喜欢
杂志排行
Plasma Science and Technology的其它文章
- Programmable electron density patterns induced by the interaction of an array laser and underdense plasma
- Study of the tungsten sputtering source suppression by wall conditionings in the EAST tokamak
- Explicit structure-preserving geometric particle-in-cell algorithm in curvilinear orthogonal coordinate systems and its applications to whole-device 6D kinetic simulations of tokamak physics
- Comparison between fluctuation of floating potential gradient and velocity of blob structure on HL-2A tokamak
- Magnetic diagnostics for magnetohydrodynamic instability research and the detection of locked modes in J-TEXT
- Experimental investigation of the electromagnetic effect and improvement of the plasma radial uniformity in a large-area,very-high frequency capacitive argondischarge
