CO2 High Temperature Corrosion and Its Prevention of Chromia Forming Fe-base Alloys
2021-05-08JianqiangZHANG
Jian-qiang ZHANG
腐蚀与防护
CO2High Temperature Corrosion and Its Prevention of Chromia Forming Fe-base Alloys
(School of Materials Science & Engineering, University of New South Wales, Sydney NSW2052, Australia)

high temperature corrosion; CO2; chromia-forming alloys; carburisation; alloying
Introduction
High temperature CO2corrosion problem can be traced back to early 1950’s when CO2was used as a coolant in gas-cooled nuclear reactors[1-4]. Recently, interest in this topic has been regained because of its relevance to oxyfuel combustion[5-6]. In this process, coal is burnt in pure oxygen rather than air, so the flue gas contains mainly CO2and water vapour, which makes CO2collection and sequestration feasible but raises corrosion problem. The CO2corrosion could also be a problem in the use of supercritical CO2as a heat transfer medium and turbine working fluid[7-9]in solar thermal energy production.
Heat-resisting alloys resist corrosion at high temperatures by form a slow growing oxide scale, for example, Cr2O3, to protect the underlying metal. Conventional methods of alloy design for this purpose based on Wagner’s diffusion theory[10-11]are reasonably successfulwhen the service environment is oxygen or air. However, in the atmosphere of CO2, many chromia-forming alloys which are protective in oxygen or air undergo a rapid “breakaway” corrosion. CO2-rich gas not only changes oxidation kinetics and oxide morphologies, but also leads to internal carburisation of the alloy, forming chromium carbides which make the repassivation even worse[12-13]. Such carbide formation has been demonstrated to be feasible at the scale-alloy interface, where a low oxygen potential leads to an elevated carbon activity[12-14]. Our recent atom probe tomography results[15-17]have revealed that carbon indeed penetrates chromia scales via grain boundaries. The water vapour in the CO2produces very fine-grained chromia microstructures and accelerates whisker formation, which affects the reaction kinetics and the stability of otherwise protective chromia scale[18-19]. As a result, significant increases in critical alloy levels for chromia scale formation for chromia- forming alloys[12-14,17-19]have been observed in CO2.
Based on this understanding, strategies to resist alloy corrosion in CO2-rich gas by creating additional diffusion barriers and modifying oxide grain boundary to promote chromia-scale protection have been proposed and investigated[17,20-23]. This paper reviews the work carried out at UNSW on chromia-forming Fe-base alloys in CO2-rich gases, the effect of Si and Mn alloying, and the modification of grain boundaries by sulphur[19,24-25], one of the surface-active elements.
1 Experiments
Model Fe-9Cr, Fe-20Cr, and Fe-20Ni-20Cr (all in wt%) alloys and these alloys with small amount of alloying additions of Ce (0.1wt%), Mn (2wt%) and Si (0.1,0.2,0.5wt%) were prepared by arc melting of high- purity metals under a protective Ar-5%H2gas atmosphere, using a non-consumable tungsten electrode. All alloys underwent annealing at 1100 ℃ for 50 h under a flowing Ar-5%H2gas to ensure homogenization, and then slowly cooled in furnace. The alloys were cut into slices in a dimension of (10~14) mm×(6~8) mm×(0.8~ 1.3) mm, where a hole of 1.5 mm in diameter was drilled near an edge for hanging. The specimens were ground with SiC paper to 1200 grit surface finish and cleaned ultrasonically in ethanol prior to reaction.
The reaction gases were Ar-20%CO2(dry CO2) or Ar-20%CO2-20%H2O (wet CO2) atmosphere at 650 and/or818 ℃. In some experiments, a small amount of SO2gas (0.1, 0.5, 1.0vol%) was added to investigate the sulphur effect. Water vapor was generated by passing a mixture of Ar and CO2through a thermostatted water saturator at a fixed temperature. Individual gas flows of CO2, Ar and SO2were controlled by mass flow controllers (brooks 5850E). Reactions were conducted in a vertical furnace equipped with a silica reactor. More details about the experimental procedures can be found in Refs[12,13,17-25].
The weight of each sample was measured before and after corrosion tests using a microbalance (Mettler Toledo XP 205) with an accuracy of 0.01 mg. Surface morphologies and cross-sections of oxidized samples were analysed using optical microscopy and scanning electron microscopy (SEM, Hitachi S3400) equipped with energy dispersive X-ray spectroscopy (EDS, Bruker). Some corroded samples were characterized by transmission electron microscopy (TEM; Philips CM200) and atom probe tomography (APT) with a picosecond-pulse ultraviolet laser (Cameca LEAP 4000X SiTM).
2 Results and discussion
2.1 Corrosion behaviour of Fe-base chromia forming alloys in CO2
CO2corrosion problem can be demonstrated in Figure 1 by comparing model Fe-9Cr-(0.1Ce, 2Mn) alloys (in weight %) exposed to air and Ar-20CO2(in vol%), respectively, at 818 ℃ for 120 h. In dry air, all alloys formed thin and protective Cr2O3oxide scales, while in CO2, thick, multi-layered iron-rich oxide scales were grown. Cr concentration increased to 20% formed a partly protective oxide scale, together with non-protective iron-rich oxide scale on the surface in CO2after 380 h reaction (Figure 2). Detailed reaction product identification can be referred to[12]. Investigation of corrosion evolution of this alloy revealed that a protective chromia scale was formed initially but then damaged by the nucleation and growth of iron-rich oxide nodules, which spread and merged to form a continuous, rapidly growing layer. As seen in Figure 2, carbide formation can be identified beneath both the chromia layer in the protective range and iron-rich oxide nodules in the non-protective zone. Once an iron-rich nodule forms, the rate of carburisation beneath it becomes more rapid, forming both intergranular and intragranular carbides (Fig.2b). Under the protective chromia scale region, carburisation is much slower with only intergranular carbides identified along the grain boundaries (Fig.2a).

Fig.1 Metallographic cross-section of Fe-9Cr-(Ce,Mn) alloys reacted in (a) dry air and (b) Ar-20%CO2 at 818 ℃ for 120 h

Fig.2 Carbide formation in (a) protective region and (b) non-protective region of Fe-20Cr in dry CO2 at 650 ℃ for 380 h
The similar nonprotective behaviour is found for the austenitic steel Fe-20Cr-20Ni, forming iron-rich oxide nodules with a partly protective thin chromia scale[26]. However, the growth of these nodules is rather slow, because of the presence of an inner layer of spinels FeCr2O4and NiFe2O4, both being in the slow diffusing phases.
2.2 Carburisation in CO2 gas
Carburisation of the alloy is, at first sight, surprising as the carbon activity aCin CO2gas is too low to carburise any alloy component (C=1.6×10−15in Ar-20CO2at 650 ℃ calculated using FactSage 7.1). According to the following reactions:

2CO=CO2+C (2)
Carbon activity can be expressed as:






Fig.3 Schematic illustration of and aC in growing oxide scale
Although above discussion shows that carbide formation is thermodynamically possible in CO2gas atmosphere, it is not clear how carbon transfers from the gas phase, through the scale to the scale-alloy interface. Carbon solubility in Cr2O3, FeO and Fe3O4is low[27]and therefore the diffusion of carbon through the oxide lattice is negligible. The ideal passage of carbon through scales of these oxides should be the defects, e.g. porosity and grain boundaries of the oxides. Iron-rich oxide scales are normally formed in accompanying with the occurrence of non-protective oxidation. This iron oxide scale, in particular thick manganite scale, is very porous[3,28], which could provide mass transfer channel for both carbon and oxygen. It seems possible[12-13,15,17-25]that CO2migrates through the inner layer via a combination of gas phase transport in pores, and grain boundary diffusion.
Carbide formation underneath the chromia scale indicated that chromia scales was also permeable by carbon. Analysis by atom probe tomography[15-16]of chromia scales grown in CO2had shown that carbon was segregated to oxide grain boundaries and the scale- alloy interface, for the case of Fe-20Cr reacted at 650 ℃. Pre-oxidation of this alloy in oxygen did not prevent subsequent penetration by carbon when exposed to CO2, which further carbon penetration via chromia grain boundaries (Figure 4).

Fig.4 Atom probe tomography of chromia grown at 650 ℃ on Fe-20Cr in oxygen and subsequently exposed to CO2[16]
This mechanism understanding in atomic scale provides the strategies for preventing CO2corrosion by either adding additional diffusion barriers to strengthen chromia protection, e.g. Si and Mn alloying, or oxide grain boundary modification to reduce carbon diffusion via the grain boundary, e.g. sulphur addition in the gas.
2.3 Additional diffusion barriers by alloying


Fig.5 Si effect on (a) weight gain kinetics of Fe-9Cr alloy in Ar-20%CO2 at 818 ℃, and (b) cross-section of Fe-9Cr-0.2Si (showing a thin SiO2 sublayer underneath the chromia scale)[20]
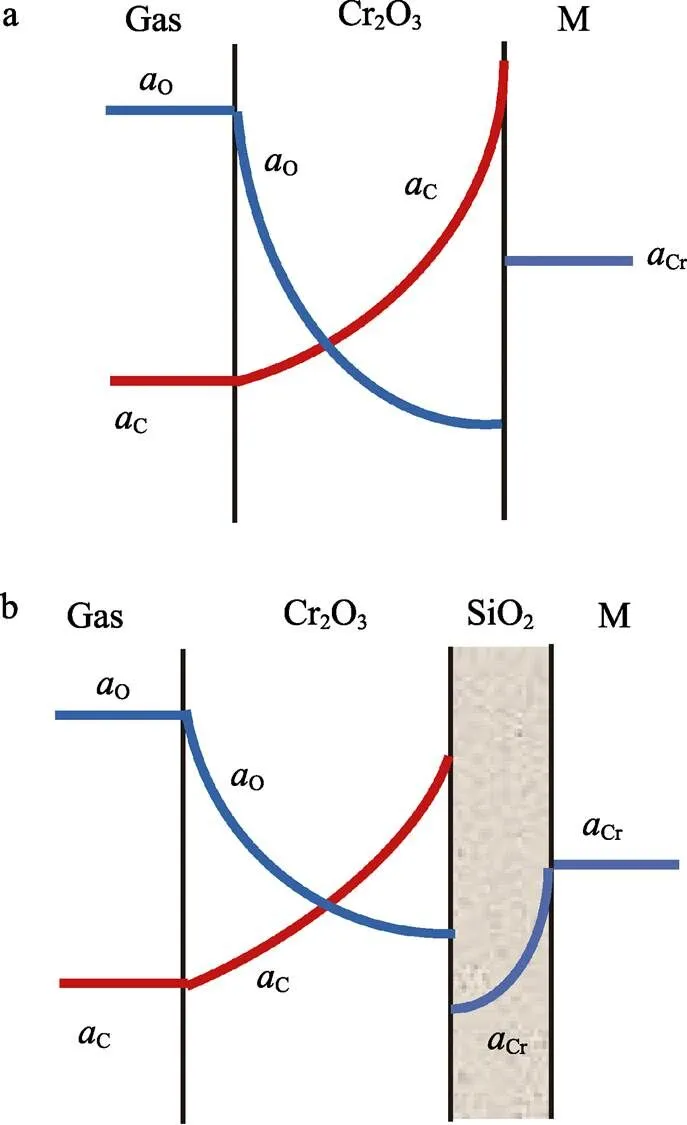
Fig.6 Schematic diagram showing the profile of oxygen, carbon and chromium activities in the growing oxide scale in the case of (a) only Cr2O3 formation and (b) Cr2O3 together with sublayer SiO2
However, in CO2-H2O gas mixtures, silicon effect is limited only for ferritic alloys with more than 20% Cr but demonstrates insignificant effect for austenitic alloy e.g. Fe-20Cr-20Ni. The failure of the latter has been attributed to the combination of thermal stress arising from the coefficient of thermal expansion difference between austenitic metal and scale with larger growth stresses generated when water vapour is present[21,23].
Prevention of carbon entry into chromia scale grain boundaries can also be achieved by alloying with Mn, so as to form an additional, outermost scale layer of Mn-rich oxide. These have been shown to be resistant to carbon entry in both CO2and CO2-H2O gases[17,22,26,30]for Fe-20Cr alloy as shown in Figure 7.

Fig.7 Cross-section of Fe-20Cr-2Mn alloy reacted in Ar-20CO2 at 650 ℃ for 1000 h (a) TEM bright field image and (b) EDS mapping[22]
2.4 Surface/grain boundary modification by sulphur
It is well known that sulphur is a strong surface adsorption element which has a significant effect on retarding carburisation. It is expected that sulphur in the gas will be preferentially adsorbed on the surface/grain boundary which will reduce the occupancy of carbon on the grain boundary. As a result, carbon penetration via oxide grain boundary will be retarded, and so will the carburisation. It was found that adding only 0.1%SO2converted Fe-20Cr from partly breakaway oxidation to fully protective oxidation after 500 h reaction (see Figure 8). Addition of sulphur also reduced the carburisation kinetics significantly as compared in Figure 8b and 8c. Sulphur effect was also very significant for austenitic Fe-20Cr-20Ni alloy.
2.5 Water vapour effect
Water vapour is unavoidable in the combustion gas. A typical oxyfuel combustion gas composition wouldbe 61.2%CO2, 30.3%H2O, 4.5%N2, 3.3%O2and 0.7%SO2[5]. A small amount of oxygen is beneficial for chromia formation and appears to suppress carburisation in many cases[31]. The presence of water vapour leads to the formation of finer grained chromia scale than that in dry gas. It also initiates the formation of whisker chromia on the surface. Water vapour decreases carburisation to a low extent when added to CO2, but is harmful in promoting breakaway by iron-rich nodule formation on ferrous alloys[32]. All of these effects have been rationalised in terms of “competitive adsorption”, the ability of one contaminant species to occupy sites in oxide grain boundaries (and their external surfaces), thereby excluding other, more weakly adsorbing species[13,19,24,25,31,33].
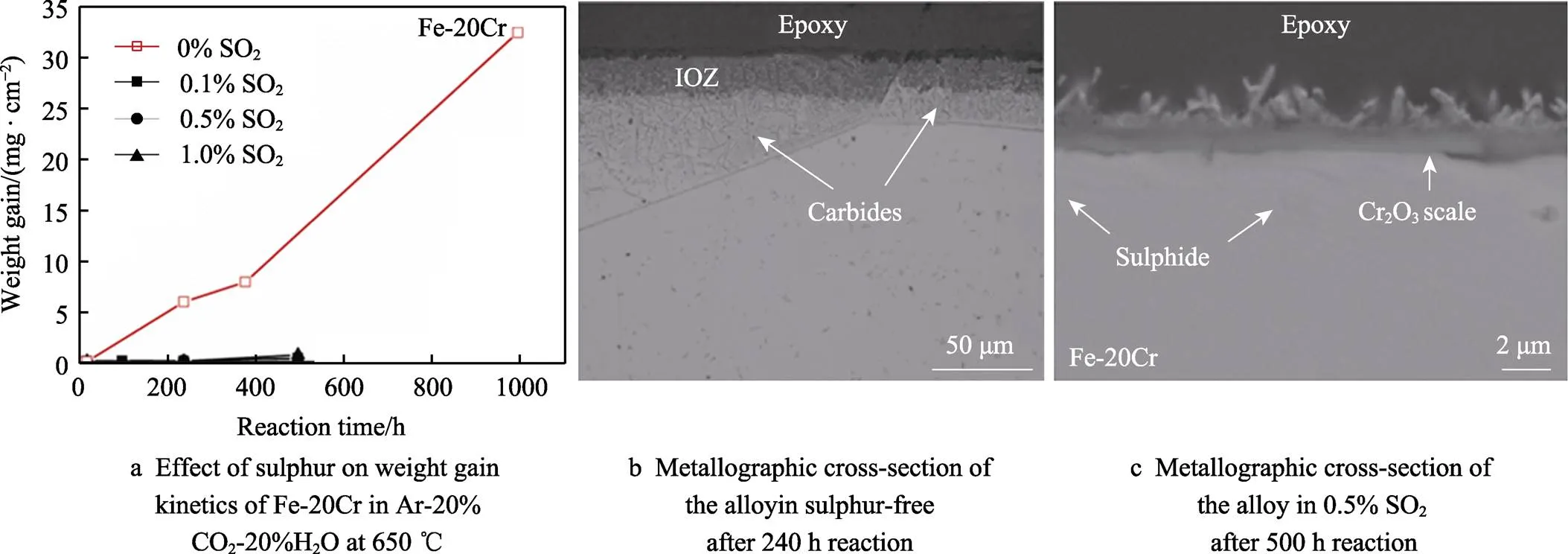
Fig.8 Effect of SO2 on wet CO2 corrosion[25]
3 Conclusions
In CO2rich gas, oxygen partial pressure of the gas is high enough to oxidise all alloy components of the chromia-forming Fe-base alloys investigated. For these alloys, breakaway oxidation is found to occur in relatively higher chromium containing alloys, indicating an accelerated corrosion in CO2-rich gas. In other words, heat resisting steels require higher chromium levels to resist CO2than those needed for service in air. The reason is partly related to chromium depletion by internal precipitation of chromium-rich carbides.
Although carbon activity of the gas is low enough for carburising any alloy components of the alloy, high carbon activities are produced beneath Fe-rich oxide scales by local equilibrium with the low oxygen activity at the alloy-scale interface. The thermodynamic analysis of carbon activity distribution explains well the formation of carbide in low carbon activity CO2gas.
Carbon penetration through chromia scales as CO2via oxide grain boundaries is revealed by APT. This observation suggests two possible strategies to resist CO2corrosion. The first one is alloying to form an additional diffusional barrier to assist chromia protection. The typical examples are adding Si, forming an amorphous silica sublayer under chromia, and/or Mn, forming Mn-rich oxides on the top of chromia surface and also at the interface of oxide/metal. The other way is adding sulphur (SO2) in the gas atmosphere which attributes to competitive adsorption and reduces carbon penetration and therefore carburisation and corrosion. These methods have been demonstrated to be effective in enhancing corrosion resistance of chromia-forming alloys in CO2gases.
[1] MCCOY HE. Type 304 stainless steel vs flowing CO2at atmospheric pressure and 1100-1800F[J]. Corrosion, 1965, 21: 84-94.
[2] FUJII CT, MEUSSNER RA. Carburization of Fe-Cr alloys during oxidation in dry carbon dioxide[J]. J Electrochem Soc, 1967, 114: 435-442.
[3] ANTILL JE, PEAKALL A, WARBURTON JB. Oxidation of mild and low-alloy steels in CO2based atmospheres[J]. Corros sci, 1968, 8: 689-701.
[4] GIBBS GB, WOOTTON MR, PRICE WR, et al. Scale stresses during protective and breakaway corrosion of iron and rimming steel in CO2[J]. Oxid met, 1973, 7: 185-200.
[5] VITALIS B. Overview of oxy-combustion technology for utility coal-fired boilers[C]// 5th International Conference on Advance in Materials Technology for Fossil Power Plants. Florida:[s. n.], 2007.
[6] ROBERTSON A, AGARWAL H, GAGLIANO M, et al. Oxy-combustion boiler material development[C]// 35th International Technical Conference on Clean Coal & Fuel Systems. Florida:[s. n.], 2010.
[7] FIROUZDOR V, SRIDHARAN K, CAO G, et al. Corrosion of a stainless steel and nickel-based alloys in high temperature supercritical carbon dioxide environment[J]. Corros sci, 2013, 69: 281-291.
[8] OLIVARES RI, MARVIG P, YOUNG DJ, et al. Alloys SS316 and hastelloy-C276 in supercritical CO2at high temperature[J]. Oxid met, 2015, 84: 585-606.
[9] CAO G, FIROUZDOR V, SRIDHARAN K, et al. Corrosion of austenitic alloys in high temperature supercritical carbon dioxide[J]. Corros sci, 2012, 60: 246-255.
[10] WAGNER C. Reaktionstypen bei der oxydation von legierungen[J]. Zeit elektrochem, 1959, 63: 772-782.
[11] WAGNER C. Theoretical analysis of the diffusion processes determining the oxidation rate of alloys[J]. J Electrochem Soc, 1952, 99: 369-380.
[12] NGUYEN TD, ZHANG J Q, YOUNG DJ. Effects of cerium and manganese on corrosion of Fe-Cr and Fe-Cr-Nialloys in Ar-20CO2gas at 818℃[J]. Corros sci, 2013, 76: 231-242.
[13] GHENO T, MONCEAU D, ZHANG J, et al. Carburisation of ferritic Fe-Cr alloys by low carbon activity gases[J]. Corrosion science, 2011, 53: 2767-2777.
[14] ABELLAN JP, OLSZEWSKI T, PENKALLA HJ, et al. Scale formation mechanisms of martensitic steels in high CO2/H2O-containing gases simulating oxyfuel environments[J].Mater high temp, 2009, 26: 63-72.
[15] YOUNG DJ, NGUYEN TD, FELFER P, et al. Penetration of protective chromia scales by carbon[J]. Scripta mater, 2014, 77: 29-32.
[16] NGUYEN TD, LA-FONTAINE A, YANG L, et al.Atom probe study of impurity segregation at grain boundaries in chromia scales grown in CO2gas[J]. Corros sci, 2018, 132: 125-135.
[17] NGUYEN TD, LA-FONTAINE A, CAIRNEY J M, et al.Effects of Si, Mn, and water vapour on the microstructure of protective scales grown on Fe-20Cr in CO2gas[J].Mater high temp, 2018, 35:22-29.
[18] NGUYEN TD, ZHANG J Q, YOUNG DJ.Growth of Cr2O3blades during alloy scaling in wet CO2gas[J].Corrosion science, 2018, 133: 432-442.
[19] YU C, ZHANG J Q, YOUNG DJ. Corrosion behaviour of Fe-Cr-(Mn,Si) ferritic alloys in wet and dry CO2-SO2atmospheres at 650 ℃[J]. Oxid met, 2018, 90: 97-118.
[20] NGUYEN TD, ZHANG J Q, YOUNG DJ. Effects of silicon on high temperature corrosion of Fe-Cr and Fe-Cr-Ni alloys in carbon dioxide[J]. Oxidation of metals, 2014, 81: 549-574.
[21] NGUYEN TD, ZHANG J Q, YOUNG DJ. Effects of silicon and water vapour on corrosion of Fe-20Cr and Fe-20Cr-20Ni alloys in CO2at 650℃[J].Oxidation of metals, 2017, 87: 541-573.
[22] NGUYEN TD, ZHANG J Q, YOUNG DJ. Effect of Mn on oxide formation by Fe-Cr and Fe-Cr-Ni alloys in dry and wet CO2gases at 650 ℃[J]. Corrosion science, 2016, 112: 110-127.
[23] NGUYEN TD, ZHANG J Q, YOUNG DJ. Water vapour effects on corrosion of Fe-Cr and Fe-Cr-Ni alloys containing silicon in CO2gas at 818℃[J].Oxidmet, 2015, 83: 575-594.
[24] YU C, ZHANG J Q, YOUNG DJ. High temperature corrosion of Fe-Cr-(Mn/Si) alloys in CO2-H2O-SO2gases[J]. Corrosion science, 2016, 112: 214-225.
[25] YU C, NGUYEN TD, ZHANG J Q, et al. Sulphur effect on corrosion behaviour of Fe-20Cr-(Mn,Si) and Fe-20Ni-20Cr-(Mn,Si) in CO2-H2O at 650℃[J].J Electrochem Soc, 2016, 163: 106-115.
[26] NGUYEN TD, ZHANG J Q, YOUNG DJ. Effects of cerium and manganese on corrosion of Fe-Cr and Fe-Cr-Ni alloys in Ar-20CO2and Ar-20CO2-20H2O gases at 650℃[J]. Corrosion science, 2015, 100: 448-465.
[27] WOLF I, GRABKE HJ. A study on the solubility and distribution of carbon in oxides[J]. Solid state commun, 1985, 54: 5-10.
[28] ROUILLARD F, MOLNE G, MARTINELLI L, et al.Corrosion of 9Cr steel in CO2at intermediate temperature I: Mechanism of void-induced duplex oxide formation[J]. Oxid met, 2012, 77: 27-55.
[29] HUNTZ AM, BAGUE V, BEAUPLÉ G, et al. Effect of silicon on the oxidation resistance of 9% Cr steels[J]. Applied surface science, 2003, 207:255-275.
[30] NGUYEN T, ZHANG J Q, YOUNG DJ. Microstructures of chromia scales grown in CO2mater[J]. High temp, 2015, 32: 16-21.
[31] MEIER GH, JUNG K, MU N, et al. Effect of alloy composition and exposure conditions on the selective oxidation behavior of ferritic Fe-Cr and Fe-Cr-X alloys[J]. Oxid met, 2010, 74: 319-340.
[32] GHENO T, MONCEAU D, YOUNG DJ. Kinetics of breakaway oxidation of Fe-Cr and Fe-Cr-Ni alloys in dry and wet carbon dioxide[J]. Corros sci, 2013, 77: 246-256.
[33] YU C, NGUYEN T D, ZHANG J Q, et al. Corrosion of Fe-9Cr-(Mn,Si) alloys in CO2-H2O-SO2gases[J].Corrosion science, 2015, 98: 516-529.
Jian-qiang ZHANG. CO2high temperature corrosion and its prevention of chromia forming Fe-base alloys[J]. Surface technology, 2021, 50(4): 260-266.
TG172
A
1001-3660(2021)04-0260-07
2019-10-30;
2021-03-18
张建强(1964—),男,博士,教授,主要研究方向为金属材料在混合气体环境中的高温腐蚀,包括氧化、渗碳、硫化和氯化。邮箱:j.q.zhang@unsw.edu.au
2019-10-30;
2021-03-18
Jian-qiang ZHANG (1964—), Male, Ph. D., Professor, Research focus: high temperature corrosion of metal materials in mixed gas atmospheres, including oxidation, carburization, sulphidation and chlorination. E-mail: j.q.zhang@unsw.edu.au
10.16490/j.cnki.issn.1001-3660.2021.04.026
