Effects of chronic decaffeinated green tea extract supplementation on lipolysis and substrate utilization during upper body exercise
2021-03-19SofieBlicherEricBartholomaeJochenKressler
Sofie Blicher,Eric Bartholomae,Jochen Kressler*
Department of Exercise and Nutritional Sciences,San Diego State University,San Diego,CA 92113,USA
Abstract
Keywords: Arm exercise;Cardiometabolic disease;Fat oxidation;Nutraceutical
1. Introduction
Green tea or green tea extract(GTE)is made from the leaves of the green tea plant Camellia sinensis,which is high in polyphenols, particularly epigallochatechin-3-gallate (EGCG).1-3Green tea catechins, including EGCG, have good average bioavailability when including colonic metabolites (~39%); however, the variability is large (standard deviation = 19%).4Reported benefits of GTE include increased fat oxidation during endurance exercise with doses of 570-890 mg/day,2,5,6as well as during post-periods of high-intensity exercise.7Others have reported no effect on fat oxidation during exercise, but doses were relatively low (160-270 mg/day8,9) or supplementation was short(2 days of 1000 mg10).The main mechanism underlying the effects of green tea on fat oxidation is believed to stem from the inhibition of the enzyme catechol-O-methyltransferase by EGCG, which results in increased levels of catecholamines and lipolytic activity.3,11There is also some evidence that longterm EGCG supplementation can induce regulatory enzymes involved in mitochondrial biogenesis such as peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1α)and peroxisome proliferator-activated receptors (PPARs), but evidence is inconclusive,and there is a lack of studies involving humans.3An additional issue is that GTE contains caffeine,which may have some individual or interaction effects with EGCG or other compounds in GTE. Dulloo et al.12reported that GTE effects on fat oxidation go beyond its caffeine content.Studies investigating decaffeinated GTE (dGTE) specifically report increased fat oxidation during exercise after 1-4 weeks of 570-890 mg/day.2,5One study reported no effect on fat oxidation during exercise with 1120 mg dGTE,potentially because of the fact that well-trained subjects were investigated.13
One limitation of all of these studies is that they only investigated leg exercise, which is not applicable to all modes of exercise (e.g., swimming) or populations (e.g., people with lower limb injuries or disabilities). The latter is of particular importance because lower levels of fat oxidation are negatively associated with body mass, and these populations are particularly vulnerable to low fat oxidation rates,weight gain,and associated health issues.14-16Another limitation is that,to the authors’knowledge,no study to date has included women in investigations of fat oxidation during exercise. Therefore,the purpose of this study was to investigate the effect of longterm supplementation(4 weeks)of dGTE(650 mg,containing 611 mg EGCG) on lipolysis and fat utilization during 1 h of moderate-intensity arm cycle exercise in men and women.Supplementation length and doses are within ranges previously reported to be effective in humans.2We hypothesized that after long-term dGTE supplementation, lipolysis and fat oxidation during exercise would increase compared with placebo(PLA).This is significant because many individuals cannot or choose not to perform lower body exercise yet still need or want to burn as much fat as possible.
2. Materials and methods
2.1. Subjects
The institutional review board of San Diego State University approved the study(No.2255099).Subjects were 8 healthy adults (4 females, 4 males) 23-37 years of age who volunteered to participate in the study. The subjects did not have specific exercise training, and they were generally active as part of a normal, healthy lifestyle. Measures of body weight and height were taken and body mass index(BMI)was calculated. Age was 28 ± 6 years, BMI was 23 ± 2 kg/m2, and peak oxygen uptake (VO2peak)was 1.98±0.69 L/min. Exclusion criteria were any heart or respiratory disease,any arm or hand injuries that rendered the subject incapable of performing arm cycling exercise, pregnancy, and prohibitive anxiety over blood draws or wearing a rubber one-way breathing mask over the nose and mouth.All subjects completed a written consent form.
2.2. Protocol
In this randomized PLA-controlled, triple-blind, crossover design study, subjects came to the laboratory for a total of 5 visits (Fig. 1). At the first visit, the subjects performed an incremental arm cycle exercise test to exhaustion (ITE) to determine upper limb-specific VO2peak. The next 4 visits consisted of the same protocol.At least 48 h after the ITE,the subject performed a 1-h arm cycle test at 50% of VO2peakas described previously.2Between the 2nd and 3rd visits,subjects were provided with supplements, either PLA or dGTE, for 4 weeks. This was followed by a 4-week washout period and subsequent crossover supplementation in which those who received PLA first now received the dGTE supplement and those who received the dGTE supplement first now received PLA.A 4-week washout period was selected to control for the menstrual cycle phase among female subjects.
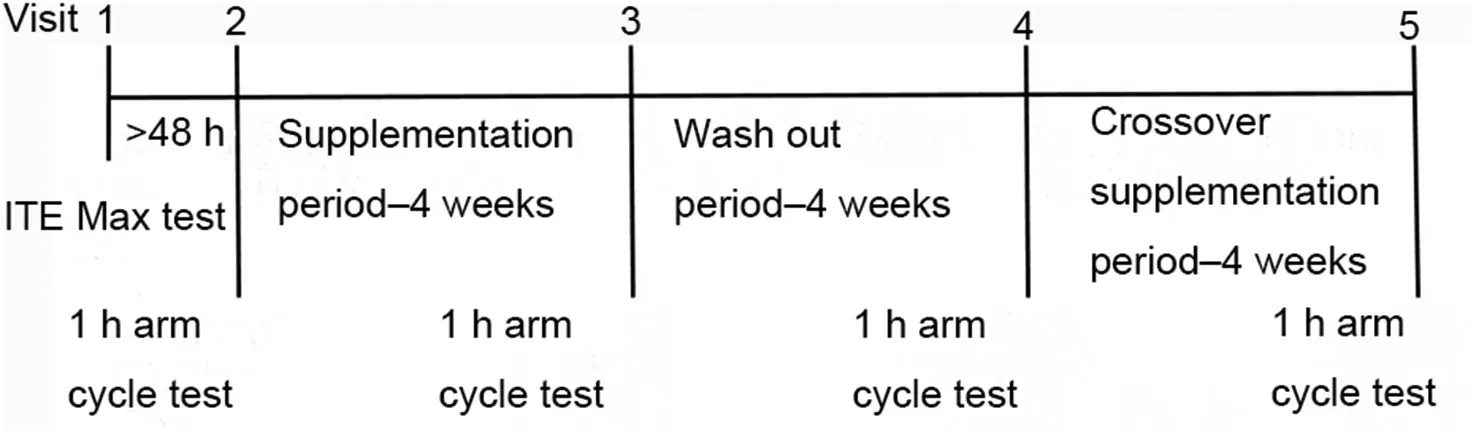
Fig. 1. Schematic overview of the study protocol. ITE = incremental arm cycle exercise test to exhaustion.
2.3. Exercise testing
All exercise testing was performed on an arm cycle ergometer (Monark Rehab Trainer 881E; Varberg, Halland, Sweden).During all exercise tests,subjects wore a Hans-Rudolf 1-way valve mask connected to a metabolic cart (Parvo Medics TrueOne 2400; Parvo Medics, East Sandy, UT, USA), and respiratory gases were measured continuously.The ITE started at an initial workload of 0 Watt(W)for 3 min.Resistance was increased 15 W per 3 min for women and 20 W for men as described elsewhere.17Subjects were instructed to maintain a cadence of 50 revolutions per min(rpm)at all times.When the subject could not maintain the required rpm, the test was terminated. VO2peakwas determined as the maximal value for a 15-breath running average.18
Target intensity for the 1-h exercise test was 50% of VO2peak. The initial target workload for the 1-h tests was calculated as 50%of peak power(Wpeak)using the formula from Jeukendrup et al.:19Wpeak=Wlast+((t/180)·increment W),in which Wlastis the workload of the last completed 3-min ramp stage, t is the time in seconds completed in the last attempted stage, and increment W is the increment in Watts increased per ramp.
After a ≥8 h overnight fast,subjects returned to the laboratory ≥48 h after their first visit to perform a 1-h arm cycle exercise test at 50%VO2peak.Before starting the exercise,subjects sat still for 5 min to get resting respiratory measures.For the exercise,subjects were instructed to maintain a cadence of 50 rpm. To ensure that the subject could perform the full 60 min of arm exercise and that the target%VO2peakwas met,the workload was adjusted within the first 20 min of the test if the respiratory exchange ratio (RER) was >1.0, the rating of perceived exertion was >5, or the VO2was >50% VO2peak.Exact time point and magnitude of the power adjustment were recorded and repeated for all trials within a given subject.All calculations for the exercise trials were based on the final 40 min of steady-state exercise only.
2.4. Supplementation
Subjects were randomly assigned to either 650 mg dGTE(Teavigo; Taiyo International Inc., Minneapolis, MN, USA)containing 611 mg EGCG, or 650 mg PLA supplements containing plant-based protein powder (Optimal Protein; Seeking Health, Bellingham, WA, USA). The remainder of the dGTE supplement was caffeine <0.10%, loss on drying <5.0%,heavy metals(as Pb)<10.0 μg/g,arsenic(as As)<1.0 μg/g,lead(Pb)<1.0 μg/g,cadmium(Cd)<0.5 μg/g,and mercury(Hg)<0.1 μg/g.The PLA was a proprietary blend of pea protein isolate, rice protein concentrate, L-glutamine, L-glycine,and taurine, with 90% protein content. The supplements were taken every day twice a day(once in morning and once at night with or without a meal as desired by participants) for 4 weeks.The supplements were in powder form and packed in single doses into standard, clear polypropylene vials, 325 mg in each vial.Participants were encouraged to mix the powder in juice or other flavored drink,because our pilot work determined that both supplements had an unpleasant taste. Subjects were provided with the supplements for all 8 weeks(56 tubes)and a calendar to record all supplements taken.They were instructed to return the calendar to the investigator at the following visit (i.e., the post visit for the respective supplementation period).No missed supplementations were reported. Subjects were instructed to maintain any current dietary habits throughout the period of the study.
2.5. Measures
2.5.1. Blood biomarkers
Before and after each 1-h arm exercise trial,5 mL of blood was drawn using venipuncture and collected in ethylenediamine tetra-acetic acid tubes (Fisher Scientific International,Inc., Pittsburgh, PA, USA). Samples were then spun in a centrifuge at 2300 g for 10 min at 4˚C.Plasma was extracted and stored at -80˚C for later analysis. The plasma samples were analyzed using standard,commercially available assay kits for glycerol (Glycerol Colorimetric Assay Kit Item No.10010755;Cayman Chemical,Ann Arbor,MI,USA)and free fatty acid(FFA)(Free Fatty Acid Fluorometric Assay Kit Item No.700310;Cayman Chemical).
2.5.2. Respiratory measures
VO2and carbon dioxide production (VCO2) measurements were used to calculate the total energy expenditure(EE),fat,and carbohydrate(CHO)used during the exercise(assuming negligible contribution of protein oxidation), using a stoichiometric formula based on exercise intensity as described elsewhere:20

The substrate utilization rate (g/min) was multiplied by 60 to estimate the total amount in grams of fat or carbohydrates used for 1 h of exercise. The amounts in kcal were calculated by multiplying total amount of fat or carbohydrates with 9.75 kcal for fat or 3.95 kcal for CHO(40%-50%VO2max)or 4.07 kcal (50%-75% VO2max).19Total EE was estimated by adding the total kcal of fat and carbohydrates oxidized.
2.6. Statistical analysis
The sample size was determined with G*Power3,(Heinrich-Heine Universität, Düsseldorf, Germany) based on the effects for increased fat oxidation reported by Roberts et al.2Specifically,given the reported fat oxidation effect size(ηp2= 0.45), it was determined that with a default correlation for repeated measures of 0.5,a sample size n=4 would yield a power(1-β)>95%at the pre-determined α=0.05.Allowing for an attrition rate of 20%, we enrolled 8 subjects, all of whom completed the full protocol.
Statistical analyses were performed using SPSS Version 22(IBM Corp.,Armonk, NY,USA). For blood glycerol and FFA,a 2×2×2 repeated measures analysis of variance was used to determine interaction of treatment, pre/post exercise, and baseline/4 weeks supplementation periods.For respiratory measures,a 2×2 repeated measures analysis of variance was used to determine interactions between treatment and pre/post supplementation period. Normality assumption was tested with the Shapiro-Wilk test.Only 1 measurement crossed the significance threshold after adjustment for multiple comparisons. This was the glycerol measurement at baseline, pre-exercise. Visual inspection of the data identified a single outlier,which was confirmed by Peirce’s criterion testing. The outlier was eliminated from analysis, which did not change significance determination for any outcome compared with the full sample. Data are presented as mean±SD,significance was ascribed to p ≤0.05.
3. Results
3.1. Oxygen consumption,EE,and fat oxidation
The mean VO2for all 4 exercise trials was 47% ± 13%VO2peak. VO2showed no differences across trials ((0.83-0.89)±(0.19-0.25)L/min,p=0.460)and no interaction of treatments and baseline/1 month supplementation (VO2: F(1, 7)=4.05,p=0.084,η2=0.366)(Fig.2A).
Correspondingly, the mean total EE showed no differences across trials ((264-266) ± (59-77) kcal; p=0.420) and no interaction (F(1,7)=3.11, p=0.121, η2=0.308) (Fig. 2B).There were also no changes for absolute(dGTE=0.11±0.08 to 0.12±0.06 g/min vs.PLA=0.10±0.05 to 0.09±0.04 g/min;p=0.220) or relative (23% ± 12% to 25% ± 11% vs. 23% ±10% to 21% ± 9%; p=0.532) fat utilization between baseline and 1-month supplementation for either treatment and or interaction(absolute fat oxidation:F(1,7)=0.27,p=0.220,η2=0.037;fat utilization relative to total EE: F(1,7)=0.43, p=0.532,η2=0.058))(Fig.3).
3.2. Blood glycerol and FFA
Mean glycerol was similar pre-exercise for all four 1-h arm cycle tests(pre-exercise values:(3.4-5.1)±(0.6-2.6)mg/dL).There was a significant main effect for the change from preexercise to post-exercise,with post-exercise values being about twice as high ((7.9-9.8) ± (2.6-3.7) mg/dL) (p=0.001) but with no significant interaction (F(1,6)=0.126, p=0.867,η2=0.005) (Fig. 4). Similarly, the FFA concentration was not significantly different pre-exercise (pre-exercise values:(3.5-4.8)±(1.4-2.2)mg/dL)and significantly increased postexercise ((7.2-9.1) ± (2.6-4.5) mg/dL) (p=0.001) but with no significant interaction(F(1,7)=0.001,p=0.981,η2=0.000)(Fig.4).
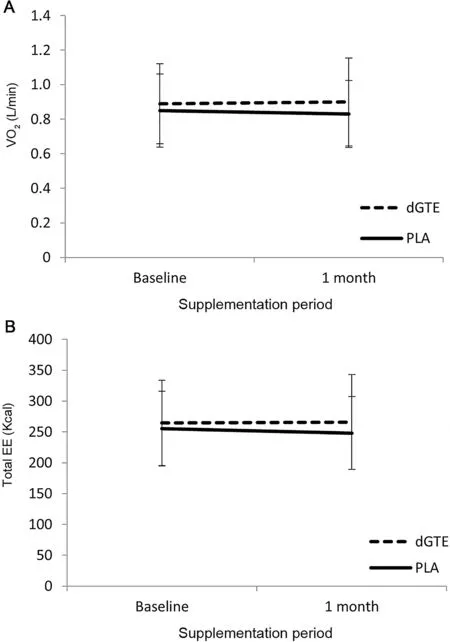
Fig.2. Oxygen consumption(A)and EE(B)before and after supplementation.Data are mean±SD.Baseline,measurement before treatment;1 month,measurement after 1 month of treatment.EE=energy expenditure;dGTE=decaffeinated green tea extract;PLA=placebo;VO2=oxygen uptake.
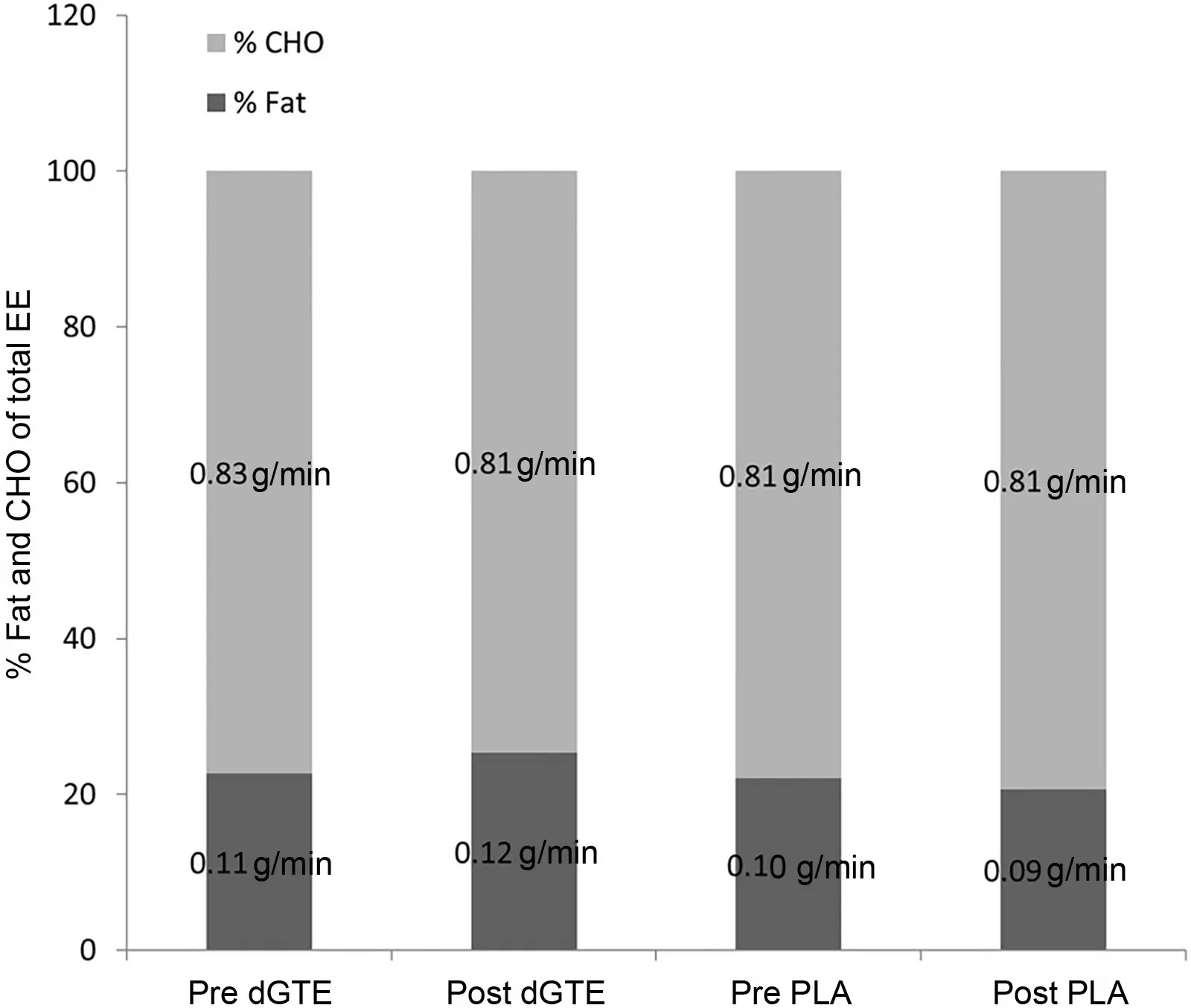
Fig. 3. Substrate utilization parameters during 1-h arm cycling tests before and after supplementation.It shows the percentage of total energy expenditure(EE),as well as the absolute amounts of fat and carbohydrate(CHO)oxidation for all 1-h arm cycling tests in the green tea extract(dGTE)and placebo(PLA)conditions.
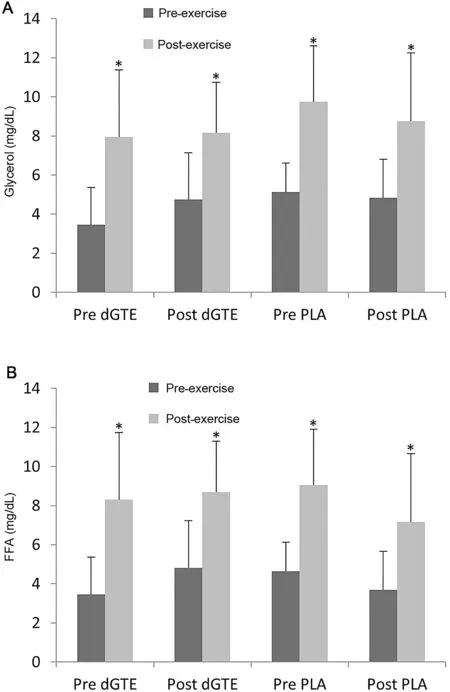
Fig.4. Markers of lipolysis before and after supplementation.It shows the differences in glycerol(A)and free fatty acids(FFA)(B)for the 1-h arm cycling tests for the green tea extract(dGTE)and placebo(PLA)conditions.Glycerol values represent the mean value of 7 subjects because we removed 1 outlier after visual inspection and con-firmation via Peirce’s criterion. * p <0.05,compared with pre values before exercise.
4. Discussion
This was the first study to investigate the effects of dGTE on fat oxidation and lipolysis for upper limb exercise. To the best of our knowledge, it is also the first study that included women in examining GTE and fat oxidation during exercise.It is important to investigate the effect on upper limb exercise because some modes of exercise (e.g., swimming and kayaking) mostly depend on the upper limbs, and several populations (e.g., those with lower limb weakness, instability,paralysis, or amputations) are unable to perform lower limb exercises.We did not find any effects of long-term dGTE supplementation on fat oxidation when performing the arm cycle exercise.There was a tendency for a slightly higher fat oxidation of 0.006 g/min corresponding to about 3% of total EE with dGTE supplementation for 4 weeks when compared with baseline, but these results are unlikely to be meaningful and were not statistically different from PLA. Similarly, when comparing the blood biomarkers pre-exercise and post-exercise, there were significant increases in both glycerol and FFA. This was expected, given that exercise increases lipolysis. There were no differences in the level of glycerol or FFA when comparing pre-exercise and post-supplementation tests.This suggests that neither dGTE nor PLA had any substantial effects on lipolysis.
Our protocol fell well within effective supplementation and exercise ranges reported by others2,5,6but failed to elicit similar results.Specific to dGTE,improvements in fat oxidation of up to 25%have been reported after long-term(4-week)supplementation (400 mg EGCG/day) when performing 1-h cycling exercise at 50%VO2peak.2Others have reported no such effects with 5 weeks of supplementation of 1120 mg.13These differences perhaps result from differences in the training status of subjects between these studies, with dGTE being potentially more effective for less-trained individuals.2,13Recently,green tea in general has been proposed to enhance fat oxidation only in people who are untrained in sports and exercise.21It is difficult to compare these conclusions, which were based on leg exercises, to our study on arm exercise. The subjects in our study were not specifically trained for arm endurance nor did they regularly participate in activities with substantial arm endurance components(e.g.,swimming or pushing the wheels on a wheelchair).This should have ensured plenty of room for improvement with dGTE supplementation because some studies suggest that there is greater effectiveness in untrained individuals compared with trained individuals.2,13,20However,this may be different for upper limb exercise because the arm musculature of the general population is simply not adapted for prolonged endurance activity and may lack the capacity for high levels of fat oxidation irrespective of otherwise effective dGTE supplementation. Supporting this notion is the fact that key components of oxidative capacity, such as mitochondrial and capillary density,are lower in arm musculature than in leg musculature.22However, arm muscle oxidative capacity can be improved with arm-specific endurance training,and fat oxidation during the same type and intensity of exercise is higher in some populations with lower leg disabilities compared with able-bodied individuals.21Therefore, the potential exists that the combination of endurance training and dGTE supplementation may be more pronounced for these populations.
Another possible explanation is that upper limb exercise causes higher rates of lactate production and increased catecholamine responses at the same relative intensity as leg exercise.23The main active ingredient in dGTE is EGCG. The mechanism of EGCG is proposed to involve the inhibition of the breakdown of catecholamines and subsequently higher rates of lipolysis and fat oxidation.1-3,24Lactate is a known inhibitor of lipolysis, and higher levels of lactate production could have inhibited the benefits of dGTE supplementation.We did not measure lactate concentrations,but given the only moderate intensity of exercise(47%±13%VO2peak)that was sustained for 60 min,it is unlikely that substantial lactate accumulation occurred because of its known associations with high intensities and fatigue. It is also possible that higher levels of catecholamines released at the same relative intensity with arm exercise compared with leg exercise25could have resulted in a ceiling effect, blunting augmentation with dGTE. Consequently, a lower intensity might be better suited to determine changes in fat oxidation with dGTE supplementation. However,our pilot data determined that at ~40%VO2peak,60 min of arm ergometry was insufficient to increase lipolysis as measured by glycerol concentration pre-exercise vs. post-exercise(1.3 ± 0.9 mg/dL to 1.4 ± 0.7 mg/dL), leaving no room for the EGCG’s proposed mechanism of action.
Finally, our study is limited by the fact that we did not assess tissue or plasma levels of EGCG or its metabolites.However,it is extremely invasive to measure tissue(e.g.,liver)concentrations.Because of the elimination half-life of <4 h,26plasma or urine levels would be expected to be eliminated with an overnight fast and would only be relevant to an acute effect, which would have confounded our results. In addition,the study is limited by a small sample size. Individuals interested in increasing fat oxidation during upper body exercise are unlikely to benefit from dGTE supplementation.
5. Conclusion
One month of dGTE supplementation does not increase fat oxidation or markers of lipolysis during 1-h moderate-intensity arm cycle exercise in men and women.
Acknowledgments
We thank the following individuals for their assistance with study administration, data collection, and analysis: Zachary Johnson, Alison Meagher, Osiris Orduna, Jonathan Cunha,Traci Roberts, Dr Mark Kern, and Dr Shirin Hooshmand.Assistance with graphical analysis of data was provided by Dr Harsimran Baweja and Hedaya Rizeq. This work was supported by University Grants Program 242545,San Diego State University.
Authors’contributions
SB was the study coordinator, conducted measurements,analyzed data(statistics),and developed the initial draft of the manuscript; EB conducted measurements, analyzed blood samples, and revised the manuscript; JK conceived the study,conducted measurements, supervised all analysis, revised the manuscript,and edited the manuscript(including responses to reviewers). All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
杂志排行
Journal of Sport and Health Science的其它文章
- Factors associated with concussion-symptom knowledge and attitudes toward concussion care seeking in a national survey of parents of middle-school children in the US
- Effects of purposeful soccer heading on circulating small extracellular vesicle concentration and cargo
- The diagnostic and prognostic utility of the dual-task tandem gait test for pediatric concussion
- Impaired eye tracking is associated with symptom severity but not dynamic postural control in adolescents following concussion
- Slowed driving-reaction time following concussion-symptom resolution
- Impaired motor control after sport-related concussion could increase risk for musculoskeletal injury:Implications for clinical management and rehabilitation
