Search for Effective Catalysts for the Development of Engines Based on the Products of Catalytic Decomposition of N2O
2021-03-08Boryaev
A.A.Boryaev
(Saint-Petersburg State University of Architecture and Civil Engineering, 4 Vtoraya Krasnoarmeyskaya St., Saint-Petersburg 190005, Russia)
Abstract:The paper presents requirements for the N2O decomposition catalyst and provides justification for the selection of components to develop its formulation. The methods to study the physical and chemical as well as catalytic properties of catalysts were developed, and the results of experimental studies on the properties of catalysts with different composition in the laboratory conditions were presented. The activity of samples of compact metal catalysts as well as samples of supported metal and oxide catalysts in the nitrous oxide decomposition reaction was studies. The methodology for determining the activity of catalysts in the decomposition reaction of N2O is presented, as well as the design of an experimental reactor for determining the activity of the catalyst by the minimum temperature for the onset of the decomposition reaction of N2O. In the course of the study, the following series of catalyst activity are determined in the N2O decomposition reaction up to a temperature of 600℃: based on platinum group metals applied to the aluminum oxide carrier — Rh>Ru>Ir>Pt>Pd; based on simple oxides applied to the aluminum oxide carrier —CoO>Cr2O3, MnO2>CuO>ZrO2>NiO>Fe2O3. The following activity series for the most promising carriers are also determined: by the onset temperature of decomposition, ZrO2>Al2O3-CaO>Al2O3-ZrO2>Al2O3-AlN>θ-Al2O3>Al2O3-SiO2>SiO2>α-Al2O3; by the rate constants of N2O decomposition, Al2O3-SiO2>Al2O3-AlN>ZrO2>Al2O3>Al2O3-ZrO2>Al2O3-CaO>α - Al2O3>SiO2. The paper presents the results of selecting the active component and carriers for the supported catalyst. The author proposes formulations of the nitrous oxide decomposition catalyst for bench testing in simulated engines.
Keywords:nitrous oxide; engines; catalyst; properties;formulation
Introduction
Due to toughening requirements for the environmental safety of propellants, N2O has recently gained increasing attention as an environmentally-friendly and, at the same time, high-energy monopropellant. The use of N2O, which has a saturated vapor pressure of 5MPa (at 20℃), allows us to implement new design solutions when creating a spacecraft control propulsion system. However, the use of N2O in space equipment is currently constrained by the lack of a highly-efficient decomposition catalyst. The development of such a catalyst is a complex task, which is due to the requirements for the engine operating conditions, on the one hand, the thermodynamic and chemical properties of nitrous oxide itself, on the other hand[1].
The paper presents the developed requirements for the N2O decomposition catalyst, provides experimental justification for the choice of active components and carriers to conduct an experimental assessment of their catalytic properties, and describes the developed methods to study physical and chemical as well as catalytic properties of catalyst samples and conditions of their achievement. Based on the results of experimental studies, the formulation of nitrous oxide decomposition catalysts for engines was selected.
1 Requirements for the catalyst and justification for the choice of components to develop its formulation
1.1 Requirements for the catalyst with regard to conditions of its operation in the engine
High catalytic activity is one of the main requirements for a catalyst. The N2O decomposition catalyst shall initiate the process at the lowest possible temperature. Analysis of the literature data on the activity of various chemicals in the N2O decomposition reaction demonstrates[2]that the best samples of catalysts begin to show their activity at temperatures above 300℃, and decompose nitrous oxide with a noticeable speed only when the temperature increases further. Moreover, its auto-catalytic decomposition is carried out at temperatures much higher than the minimum onset temperature of decomposition. This means that currently it is almost impossible to develop an engine based on the decomposition of such a thermally stable compound as N2O, which could be started without catalyst preheating. Therefore, it is necessary to develop a catalyst that would initiate the decomposition process at the lowest possible temperature (~300℃). A catalyst for nitrous oxide decomposition with high activity can be developed by applying an active component (AC) to a thermally stable carrier of a certain chemical nature with a developed surface (at least 20-30m2/g), which are determined during the formulation elaboration.
When N2O is decomposed, a significant amount of heat is released, and, as a result, depending on the decomposition degree, the temperature in the catalyst layer will vary in a wide range and can reach ~1500℃:
2N2O = 2 N2+O2+163.3kJ
(1)
Therefore, another important requirement for the catalyst is its capability to maintain its constant activity throughout the entire resource, i.e. the catalyst should be thermally stable up to a temperature of 1500℃. One of the products of N2O decomposition is oxygen, which can interact with the AC, thus contributing to changes in the catalyst activity. Therefore, the catalyst should be chemically stable up to the above temperature as well. An important catalyst characteristic is its grain size. Reducing the size of the catalyst grain will help increase the access of the precursor to the catalyst surface. Based on the requirements for the engine, the thermodynamics and chemical properties of the nitrous oxide decomposition process[1-2], it can be assumed that the developed catalyst should:
(1)Be a supported catalyst in the form of rounded pellets (0.8-1.25mm in size), with a developed specific surface area (at least 20-30m2/g), a heterogeneous porous structure, and both micro- (1-10nm) and macro- (more than 100nm) pores and transport pores (up to 100nm);
(2)Ensure the minimum onset temperature of N2O decomposition in terms of activity;
(3)Be thermally and chemically stable up to a temperature of 1200℃ in the atmosphere of N2O and its decomposition products; its properties, as the resource is exhausted, should not undergo significant changes that would lead to a noticeable decrease in its activity and performance.
1.2 Choosing the catalyst type and promising active components
From the analysis of the literature data[2], it is known that metal and supported metal and oxide catalysts can be used for N2O decomposition. Supported catalysts are catalysts where the AC is distributed on the surface of a specially selected carrier. Compact metal catalysts (usually block catalysts-briquettes compressed from a catalytically active metal wire) have some advantages over supported ones. For example, they are characterized by high thermal conductivity, which makes it possible to organize effective redistribution of heat in the reactor, plasticity, significant mechanical and erosive strength increasing the service life of such catalysts.
1.2.1 Metal (compact) catalysts
(1) Choosing the catalyst type
Compact metal block catalysts can be of several types: cellular metal-ceramic catalysts and catalysts in the form of “metal rubber” (compressed metal and catalytically active wire in the form of a briquette). Based on the operating conditions, metal catalysts of the “metal rubber” type are the most promising. These catalysts are made by the special laying of metal wire spirals in a press-die. Their manufacturing technology is simple, and it makes it possible to get blocks with reproducible properties. The total surface of such catalysts can be adjusted by changing the wire diameter, and the porous structure can be adjusted by the size of the briquette pressing.
(2) Active components for compact catalysts
From the above requirements for N2O decomposition catalysts and features of the technology for the manufacturing of metal catalysts of the “metal rubber” type, it follows that the materials taken to manufacture catalysts of this type should meet the following requirements:
Have a melting point above 1500℃;
Be plastic and madein the form of a wire with a diameter of 0.1-0.4mm;
Not interact with oxygen up to a temperature of 1200℃.
The following list shows some metals arranged in ascending order with regard to their melting point (℃), which are of interest for research in terms of their activity[3]:
Ni(1463), Co(1495), Fe(1539), Pd(1550), Pt(1770),Zr(1852), Cr(1890), V(1960), Rh(1966), Ru(2427), Ir(2454), Nb(2539), Mo(2620), Re(3170), W(3410), Os(3227).
Not all of the above metals, despite their high melting point, are stable in an oxidizing environment (especially in an oxygen environment)[4-5].
Zirconium and niobium interact with O2intensively at temperatures above 200℃, and at 700℃, they burn to form ZrO2and NbO2. In a highly dispersed form, they are pyrophoric.
Mo, W and Re metals are stable under normal conditions in the O2atmosphere. However, when heated above the “red heat” temperature -400℃ (and for Re-above 300℃)-even compact metals are oxidized easily. In terms of their oxidation ability (MeO3is mainly formed), they can be arranged as follows: Mo>W>Re.
Re is characterized by the formation of easily volatile Re2O7oxide. It should be noted that at high temperatures (~1000℃), MoO3and WO3oxides sublimate.
When heated in the air up to a temperature of 500℃, Ni and Co are oxidized easily in a compact form (covered with oxide films), and Fe interacts with wet O2at room temperature. It should be noted that these metals are pyrophoric in the air in their highly dispersed state.
Vanadium becomes brittle when heated in the air above 300℃ (O2dissolution in the metal is observed); at 600-700℃, the metal is oxidized intensively to form V2O5, which partially dissociates and partially sublimates above 700℃.
Chromium is one of the most stable metals in the O2atmosphere. When a compact metal is heated, the formation of surface oxide is observed, and only at a temperature of about 2000℃, it starts to burn in O2to form a thermally stable Cr2O3oxide.
All metals of the platinum group (except Os, which is easily oxidized at 120℃ to the very volatile and poisonous oxide OsO4), when heated in the air, form an oxide film that protects the compact metal against further oxidation. However, in a highly dispersed state, when they are calcined in an oxygen atmosphere, the formation of oxides is observed.
For instance, when heated in the air (up to 450℃), the compact Ru is oxidized (a surface oxide film is formed); in a highly dispersed form, oxidation occurs at 100℃ with the formation of volatile RuO4(Tevaporation=60℃) and RuO2, which dissociates at 930-950℃ and sublimates.
Compact palladium is oxidized at 600-700℃, with the formation of PdO; in a highly dispersed form, oxidation occurs at a lower temperature.
Iridium is very stable to oxygen but in a highly dispersed state at a temperature above 600℃, it is also able to form an unstable oxide-Ir2O3, which easily dissociates into Ir and very stable oxide IrO2. At temperatures above 1100℃, its partial dissociation into Ir and O2is observed. Some researchers note the volatility of this oxide. It should be noted that the introduction of Ir in Pt, Pd, Ni and other alloys increases their hardness and corrosion resistance.
When dispersed Rh is heated in the air up to 600-800℃, the formation of Rh2O3oxide is also observed, which, similar to iridium oxide at temperatures above 1100℃, decomposes into Rh and O2.
Pt has the greatest resistance to oxidation; it shows no noticeable oxidation even during melting. However, in a highly dispersed form, when it is heated in an O2atmosphere at 700℃, we observed the formation of a PtO oxide film. Pt-based alloys have high corrosion resistance.
Thus, taking into account the above data on the O2influence on the state of the metal, we can state that, depending on the temperature, the considered metals under the conditions of N2O decomposition will have different degrees of oxidation. In the initial (~300℃) and final states (above 1000℃), some metals will be in the metallic state, and in the intermediate temperature range-in the oxidized form.
Based on the properties of the metals discussed above, metals in the form of a Pt wire and its alloys with Rh andIr, as well as Ni and its alloy with chromium (nichrome) can be taken as precursors for catalysts of the “metal rubber” type for research in the N2O decomposition reaction.
1.2.2 Supported catalysts
It is necessary to select an active component and an appropriate carrier to create a supported catalyst.
(1) Choosing promising carriers
As mentioned above, N2O decomposition catalyst carriers should meet some stringent requirements:
The melting point of the precursors and the carrier itself-above 2000℃;
Inertia in the atmosphere of N2O and its decomposition products-up to 1500℃;
The specific surface area and phase state-stable up to 1200℃;
The initial specific surface area-at least 20-30m2/g;
Total pore volume-at least 0.26cm3/g;
Porous structure-heterogeneous with a set of micro-, macro-, and transport pores;
Mechanically strong and resistant to heat shock and vibration overload;
Pellets-rounded shape, with a size in the range of 0.8-1.25mm.
Taking into account the above requirements, the following high-temperature oxides and compounds could be used as carriers.
Carriersbasedonaluminumoxide.Currently, carriers based on aluminum oxide having several modifications, the stability of which is different at high temperatures, are the most common in catalysis[6].
The most widely used carrier is based on γ-Al2O3. Its manufacturing technology is developed in detail, and its properties are well studied. The carrier can be obtained with a specific surface area of 120-300m2/g and a heterogeneous porous structure with a total pore volume of 0.3-0.7cm3/g. However, the carrier based on γ-Al2O3is of no interest for the N2O decomposition process since in the operating temperature range of 700-1200℃ it will undergo polymorphic transformations into the final product α-Al2O3:
γ-Al2O3→ θ-Al2O3→ α-Al2O3
According to our research, the transformations are accompanied by an increase in the primary particles of γ-Al2O3and, as a result, a decrease in the specific surface area, porous structure and carrier pellet strength is observed.
α-Al2O3is corundum, the most stable form of aluminum oxide (Tmelting~2150℃), which has sufficient mechanical strength and stability in the reaction products. However, the carrier based on it has a small specific surface area (~1m2/g) and a low total pore volume (~0.2cm3/g); therefore, it is impossible to obtain a highly active catalyst supported by such a carrier. In this regard, α-Al2O3is also not considered as a promising carrier for the N2O decomposition catalyst.
In our opinion, the θ-Al2O3form is promising since it is resistant to sintering up to 1000℃. At the final temperature of its production of 750℃, it can have a specific surface area of 80-120m2/g, and a heterogeneous porous structure (a typical pore pattern is shown in Fig. 1) and a total pore volume of 0.30-0.40cm3/g. During heat treatment up to 1200℃, the carrier pellets do not shrink due to the introduction of a certain amount of α-Al2O3into its composition during production. In the finished form, the carrier is a mixture of (γ+θ+α)-Al2O3. In Fig.1 shows the porous structure of three batches of a carrier with the composition(γ+θ+α)-Al2O3, which were manufactured using the same technology (sample 1, sample 2 and sample 3).

Fig. 1 Porous structure of three different samples of the carrier with the composition (γ + θ + α) -Al2O3 of different production batches
Some methods are known to reduce the rate of the phase transition of γ-Al2O3to α-Al2O3. One of them is based on the introduction of alkaline earth metal compounds in the production process, which, by introducing into the octahedral voids of γ-Al2O3, inhibit the process of transition to α-Al2O3. Based on this principle, the aluminum oxide carrier stabilized with calcium oxide retains a high specific surface area (~60m2/g) and a heterogeneous porous structure during heat treatment at 1100℃ for 3 hours. Such a carrier can be recommended for preparing the N2O decomposition catalyst based on it.
Carriersbasedonaluminumnitride. Another promising carrier is a carrier based on aluminum nitride having a high melting point (~2000℃). Its mechanical strength and macroporous structure are based on a framework made of the aluminum nitride sintered at 1700℃, and the specific surface and microporous structure are provided by the aluminum oxide formed during the hydrolysis of the framework surface and subsequent heat treatment at a final temperature of 650℃. The aluminum oxide formed on the framework surface not only contributes to the development of the specific surface but also protects the framework against the chemical interaction with salt solutions during the AC application. Such a carrier can have a specific surface area of 60-120m2/g, a heterogeneous porous structure and a total pore volume of 0.28-0.34cm3/g with the high mechanical strength of pellets.
Carriersbasedonzirconiumoxide. A carrier based on zirconium oxide with a melting point of 2700℃ can be promising for creating a catalyst. Two modifications of ZrO2are known: monoclinic and tetragonal. The monoclinic modification is stable up to 1000℃, and at higher temperatures, it reversibly passes into the tetragonal modification. Therefore, despite the high thermal stability, at temperatures above 1000℃, the carrier based on it may undergo changes in the specific surface, porous structure and mechanical strength. Nevertheless, due to the high activity of ZrO2in the N2O decomposition reaction, this carrier is of considerable interest in research.
In our opinion, a zirconium carrier, where aluminum hydroxide is used as a binding agent, can be more promising. The carrier obtained in this way is a mixture of Al2O3-ZrO2. This high-temperature carrier has a developed specific surface, a porous structure, and a sufficiently high mechanical strength. In addition to the above carriers, carriers based on aluminum oxides with silicon oxide may be of interest.
Based on the above, we selected the following carriers for research:
(γ+θ+α) -Al2O3;CaO-Al2O3;Al2O3/AlN;ZrO2;ZrO2-Al2O3;SiO2-Al2O3
(2) Choosing promising active components
Metals. The analysis of the properties of the most promising metals to create the N2O decomposition catalyst showed[2]that almost all of them will be oxidized during the reaction, depending on the temperature of the catalyst under the action of the released oxygen. The platinum group metals Pt, Rh, and Ir will have the lowest exposure to O2. Their oxidation temperature in a highly dispersed form is above 700℃. It should be noted that, at temperatures above 1000℃, the formed oxides will dissociate into Me and O2. Palladium and ruthenium are oxidized at a lower temperature from 500-600℃. All other metals are oxidized up to a temperature of 400℃. Therefore, during a test of any supported metal catalyst, the active component will turn into an oxide. However, it is of interest to determine the activity of the catalyst, where the metals of the Pt group with different Me content will be used in the initial form as the AC. Such metals are: Rh, Ir, Pt, Ru, and Pd.
Oxides. It is known from the analysis of the literature data[2]that such oxides of transition metals of group VIII as Rh, Ir, Co, Fe, and Ni show the greatest activity in the N2O decomposition reaction; the onset temperature of the reaction based on them is 200-400℃. The oxides of Cu, Mn, W, Mo, and Cr show a slightly lower activity; the minimum reaction temperature on such catalysts is 400-500℃. In terms of their activity as well as thermal and chemical stability in the temperature range of 400-1000℃, the oxides of the above metals are of interest for research to determine the possibility of developing the N2O decomposition catalyst based on them (including their mixtures) for operation in a micro-engine with a thrust of 1 N.
Thus, for the development of the supported catalyst, the requirements for the carrier are formulated and some carriers are selected, based on which the N2O decomposition catalyst can be created. These carriers include high-temperature aluminum oxide carriers containing θ-form and stabilized with calcium oxide, aluminum nitride carriers, zirconium oxide carriers, and aluminum and silicon oxide carriers.
Oxides of the following metals were selected as active components to search for the catalyst composition at the first stage: Rh,Ir, Co, Ni, Fe, Cr, Mn, Cu, Pt, Pd, and Ru.
2 Methods to study the physical and chemical as well as catalytic properties of catalysts
2.1 Methods to determine physical and chemical properties
To study the physical and chemical properties of catalysts, we used the following methods:
(1)Thetotalspecificsurfaceareaof the samples was determined by a chromatographic method based on changes in the volume of argon absorbed by the catalyst surface from the flow of a gas mixture with helium of a given concentration (volume ratio 5%-7%) at the temperature of liquid nitrogen and its subsequent desorption when the temperature increased to 25-30℃.
(2)Thepourdensitywas determined by measuring the volume of the vibro-packed catalyst sample weight and calculated as the sample weight/volume ratio.
(3)Thetotalporevolumewas determined by the volume of distilled water absorbed by a certain weight of the sample during its “titration” and calculated as the ratio of the absorbed liquid volume to the sample weight.
(4)Thespecificsurfaceareaof metals and their dispersion were determined by gas chromatography during oxygen-hydrogen titration of the catalyst surface.
(5)Theamountoftheactivecomponentadded to the catalyst was determined by the calculation method based on the difference between the weight of the taken carrier and the weight of the finished catalyst.
(6)Theporousstructureof carrier samples was studied by mercury porosimetry.
(7)Thephasecompositionwas studied by X-ray diffraction method.
2.2 Method to determine the catalyst activity in the N2O decomposition reaction
To determine the catalyst activity, it was required to develop some new methods.
2.2.1 Determining the activity by the minimum onset temperature of the reaction in a flow-through unit
One of the main tasks was to create a highly active and thermally stable N2O decomposition catalyst that would initiate the decomposition process in the engine with minimum external energy consumption. The minimum onset temperature of N2O decomposition was taken as a measure of the catalytic activity, with chromatographic analysis of its decomposition products. The method for determining this indicator makes it possible to determine the activity of the studied samples and choose the most promising ones for more detailed research and development of the catalyst composition based on them. Fig. 2 shows the layout of a flow-through unit used to determine the activity of catalysts.
The operation of the installation is carried out in three stages:
(1)Stage 1-preparation for testing.
(2)Stage 2-testing.
(3)Stage 3-bringing the installation to its original position (end of tests).
Below is a description of the operation of the installation at stage 2-testing.
The catalyst in the amount of 10cm3(layer height of 25-30mm) with a grain size of 0.8-1.25mm was loaded in reactor 18, which is a quartz tube with a false bottom. The amount of catalyst that was selected when varying the sample weight from 5 to 15cm3makes it possible to determine the onset temperature of the reaction most accurately. In our opinion, the reactor diameter (~18mm) and the volume of the catalyst loaded into the reactor will be close to the parameters of the engine being developed. Pure nitrous oxide was used as the working medium, which made it possible to simulate the operation of the catalyst in the engine, as well as determine the beginning of decomposition more accurately since the use of pure nitrous oxide produces a much greater amount of heat per unit of time than when its mixtures aredecomposed.
To create the conditions for the reaction in a reactor with ideal displacement and heating of the working gas flow (N2O) entering the catalyst, we poured 10cm3of quartz glass pellets with a size of 2-3 mm onto the catalyst layer, having previously found out that the fraction of decomposed N2O on the quartz is insignificant up to a temperature of 600℃.

Fig. 2 Layout of a flow-through unit for determining the activity of the catalysts in N2O decomposition: 1, 2-cylinders; 3, 4-reducing units; 5, 6, 7-gas flow adjusters; 8, 9-manostats; 10, 12, 14-flowmeters; 11-mixer; 13-multi-way valve; 17, 23-valves; 18-reactor with a heater; 21-controller recorder; 24, 25-desiccators; 20, 26-thermostats; 22-control trap
The temperature in the middle of the catalyst layer was measured with a chromel-copel thermocouple installed directly in the layer (when determining the activity up to 600℃) or a chromel-alumel thermocouple (when heating the catalyst to 1200℃).
The reactor, on the outer surface of which achromel-alumel thermocouple was fixed in the catalyst zone for automatic control of its heating rate, was placed in a cylindrical furnace. After installing the reactor, gas lines were connected to it. After checking the unit for tightness, which was determined by changes in the pressure of supplied helium in the system, nitrous oxide was supplied to it. The N2O flow rate through the reactor was 100cm3/min (0.0033g/s), which was selected when changed from 80 to 170cm3/min. The gas from the cylinder, passing through flow meter 10, mixer 11, desiccator 24 for moisture sorption, entered the reactor. From the reactor, the gas was released into the atmosphere after passing desiccator 24 and a chromatograph. After setting the flow rate of nitrous oxide, the reactor heating was switched on. The heating rate was 5deg/min and it was automatically maintained throughout the experiment by voltage thermostat 26. As the reactor was heated, every 20℃ (every 4minutes), the composition of the exhaust gases was determined chromatographically. At the first stage of research, when the most active components were selected for the development of the catalyst composition, the maximum heating temperature of the reactor was 600℃. After reaching 600℃ (the first determination of activity), the reactor heating was automatically switched off, and it was cooled. As the cooling process progressed, the exhaust gases were analyzed every 5minutes to determine the decomposition reaction end temperature. After cooling the reactor to a temperature of 100℃, the heating was switched on and the catalyst activity was re-determined as described above (second determination). The activity of compact metal catalysts was determined at the same facility, where the activity of pellet catalysts was determined, but in that case a model reactor with a diameter of 10 mm was used. The temperature, at which the appearance of oxygen and nitrogen was observed in the composition of the exhaust gases, was taken as the minimum onset temperature of the reaction.
Based on the data on the gas medium composition, we calculated the degree of decomposition depending on the heating temperature of the catalyst and determined the kinetic characteristics of the studied carriers and catalysts to the degree of N2O decomposition equal to 0.3.
To determine the kinetic characteristics, we used first-order equation (2), which was transformed into equation (3) by integration and resolution with respect tokas well as Arrhenius equation (4).
dx/dτ=k(1-x)
(2)
k=1/τ·ln[1/(1-x)]
(3)
k=k0·е-E/RT,
(4)
where:kis the rate constant, cm3/g·s;τis the contact time expressed as the ratio of the catalyst weight to the N2O volume flow rate, reduced to the catalyst temperature, g·s/cm3;k0is the pre-exponential multiplier, cm3/g·s;Eis the activation energy of the catalytic process, J/mol;Ris the universal gas constant equal to 8.315J/mol·K;Т is the catalyst temperature, K;xis the degree of N2O decomposition, calculated based on the experimental data of chromatographic analysis, according to the following equation:
x =CN/(100% -1/2·CN)
(5)
where:CNis the nitrogen concentration, %; 1/2·CNis the oxygen concentration expressed in terms of nitrogen concentration (ratio of the nitrogen concentration to the oxygen concentration in N2O decomposition products is 2), %.
By using experimental valuesτandx, we calculated the rate constant based on equation (3). The pre-exponential multiplier and activation energy were calculated based on the obtained values of the rate constant and catalyst temperature using equation (4) and the least-squares method. To compare the catalytic activity of the studied carriers and catalysts, the rate constants were calculated at a certain temperature.
2.2.2 Determining the activity by the minimum onset temperature of the reaction in a differential reactor
The N2O decomposition reaction is accompanied by the release of a significant amount of heat. However, to initiate this process, the catalyst should be heated to a certain temperature at which the reaction begins. For its determination, we for the first time applied the differential method widely used in the study of solids. The onset temperature of the catalytic reaction in the catalyst layer was determined using a differential thermocouple by comparing the temperatures in the catalyst layer and the inert carrier, through which N2O was passed. The beginning of the reaction is indicated by the deviation of the controller recorder line from the zero value.
As the heating temperature of the catalyst and the carrier increases, the degree of N2O decomposition will increase as well, which is accompanied by an increase in the catalyst layer temperature and, therefore, a large deviation from the zero line. As the temperature rises in the catalyst layer, the conditions, under which N2O is completely converted into reaction products, are reached. As soon as these conditions are reached, the rate of temperature rise in the catalyst layer should become constant and the recorder will show a straight line. The observed temperature differences in the layer of the catalyst and the inert carrier depend on the rate of heat supply to the reactor: at a high rate, the deviation from the zero line is observed more clearly.
The layout of the unit for such studies differs from the layout of the reactor considered above. It is shown in Fig.3. The unit represents a quartz tube with a false bottom, which is divided by an impermeable quartz partition into two cells. An inert carrier (in our case, quartz glass pellets) with a size of 0.8-1.25mm and a weight of 5 g is placed in one cell, and the same amount of the studied catalyst of the same fractional composition is placed in another cell. In the middle of the layer of the inert carrier and the catalyst, a differentialchromel-copel thermocouple is installed, connected to the recording device. The reactor is installed in a furnace, the temperature of which can be increased at a given rate (in our case, 10deg/min) by means of an automatic regulator. Then the reactor is connected to the gas lines. The unit is checked for tightness under the pressure of helium, which is pre-fed into the system. After checking the tightness, the helium is replaced with N2O and the unit is purged with nitrous oxide at a rate of 100cm3/min, which passes after the reactor through the chromatograph and is released into the atmosphere. Then, the reactor heating is switched on; as the temperature rises, the composition of the exhaust gases is determined every 5minutes. The beginning of the reaction is recorded by the deviation of the controller recorder and confirmed by the data of chromatographic analysis. A typical thermogram of the process is shown in Fig. 4.
This method was used to confirm the data on the reaction onset temperature obtained in a flow-through unit since it did not allow for the determination of the decomposition degree (and, therefore, kinetic parameters of the process) due to the passage of a part of N2O through the inert carrier layer.

Fig. 3 Layout of a reactor with a differential thermocouple

Fig. 4 Thermogram for determining the beginning of the decomposition reaction using a differential thermocouple: 1-differential curve, 2-sample heating curve
3 Results of the experimental determination of catalyst properties
3.1 Activity of compactmetal catalysts
3.1.1 Conditions of sample preparation
To obtain compact metal catalysts of the “metal rubber” type, the following precursors were selected in the form of a wire with a diameter of 0.2mm:
(1)Pl-99 platinum;
(2)PlRd-10 platinum-rhodium alloy (10% Rh);
(3)PlI-10 platinum-iridium alloy (10%Ir);
(4)Np-2 nickel;
(5)N80Kh20 nickel-chrome alloy (20% Cr).
Catalyst samples in the form of porous briquettes were made by pressing workpieces from a wire spiral of each material, specially laid in press-dies with a diameter of 10mm. The conditions of workpiece pressing were selected so that the height of the briquette would be 20mm. The length of the wire for the manufacturing of a single sample in all cases was equal to 8m. This technology made it possible to obtain samples with the same surface (determined by the diameter and length of the wire) and the same porosity (determined by the height of the briquette and the pressing force). Before the test, a recess was made in the center of the metal briquettes to a depth of 10mm to install the thermocouple.
3.1.2 Results of the experimental determination of activity. Activity series
All test samples were subjected to heat treatment in the air at a temperature of 600℃ for 3 hours before the test. The platinum-based briquettes did not change by color or by weight. The annealing colors, which indicate the formation of a thin oxide film on the surface of the wire, appeared on the nichrome briquettes. However, the briquette weight did not change. And only the nickel-based briquettes underwent significant changes: their metallic color turned green, and the briquette weight increased by 1.5%. It indicates the wire oxidation.
The activity of the prepared samples was determined by the method described above; the final temperature of sample heating in the N2O flow was 800℃, i.e. below the onset temperature of homogeneous decomposition. It was found that the metal catalysts are arranged in the following series according to the onset temperature of the N2O decomposition process:
Nickel(510℃)>Pt, Pt-Rh, Pt-Ir (~560℃)>nichrome (710℃).
The N2O decomposition degree at 800℃ was as follows: for nickel -0.34; for Pt and its alloys with Rh and Ir -0.26, for nichrome -0.03, i.e. the lowest decomposition rate was observed with nichrome.
An examination of the catalysts after testing showed that the platinum-based samples did not change their color, although there was some weight loss (~0.1%).
The nichrome-based samples turned green; their weight increased, indicating the wire surface oxidation.
The most significant changes were observed in the nickel-based samples: they turned green and their weight increased by 4.5%. It indicates the significant oxidation of the nickel wire. Therefore, the relatively high activity of nickel-metal catalysts can be explained by the formation of nickel oxide, which shows noticeable activity in the N2O decomposition reaction.
Thus, for the output layer catalysts, we can recommend platinum-based catalysts, which are active in the reaction under consideration and almost do not undergo significant changes up to 800℃.
3.2 Experimental studies and selection of carriers for catalysts
It is known that the carriers in the supported catalysts are quite inert themselves. However, they cannot be considered only as a mechanically strong heat-resistant framework with a developed specific surface area and a certain porous structure. According to some studies[2], the nature and properties of the carrier significantly affect the activity of the supported catalyst: they can increase or decrease the activity, or interact with the active component to form a chemical compound. At the first stage of carrier selection for research, the most promising heat-resistant carriers of the following composition were selected:
(1)aluminum oxide stabilized by Ca compounds (Al2O3-CaO,Tcalc—1100℃);
(2)aluminum nitride coated with γ-Al2O3(Al2O3-AlN framework carrier,Tcalc—650℃);
(3)zirconium oxide (ZrO2,Tcalc—1100℃);
(4)zirconium oxide-aluminum oxide (Al2O3-ZrO2,Tcalc—800℃);
(5)aluminum-silicon oxide (Tcalc—1100℃);
(6)aluminum oxide (calcined, α-Al2O3,Tcalc-more than 2000℃):
(7)aluminum oxide (heat-resistant, γ+θ+α-Al2O3,Tcalc—750℃);
(8)quartz (SiO2,Tcalc—1200℃).
The study of the physical and chemical properties of carriers showed (Table 1) that they differ significantly both in their specific surface area (from 110 to 1m2/g), and in their total pore volume (from 0.7 to 0.1cm3/g), and, hence, in their porous structure.

Table 1 Physical and chemical properties of various high-temperature carriers
To assess their possible contribution to the catalyst activity, the activity of these carriers in the N2O decomposition reaction was determined using the method described above. Fig. 5 shows the Arrhenius dependencies for some carriers, calculated by a first-order equation.
Table 2 provides data describing the catalytic activity of carriers, which shows that they have a very long induction period. By the onset temperature of the reaction, the studied carrier samples can be arranged in the following series:
ZrO2> Al2O3-CaO>Al2O3-ZrO2>Al2O3-AlN>θ-Al2O3>Al2O3-SiO2>SiO2>α-Al2O3
As can be seen, the carrier based on zirconium oxide is the most active. The reaction of N2O decomposition on it begins at 280℃. The least activity was shown by the carrier, which is aluminum oxide calcined at a temperature above 2000℃, having a specific surface area of 0.5m2/g. The reaction of N2O decomposition on it begins only at 460℃.
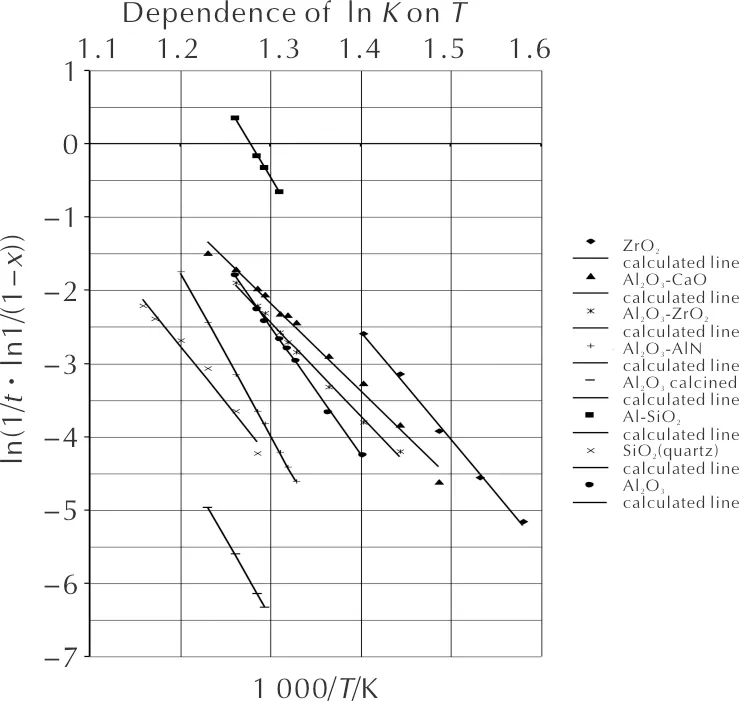
Fig. 5 Dependence of lnk on the temperature for various sample carriers in the N2O decomposition reaction
From Table 2, it is also seen that the activation energy on the carrier samples varies widely: from 100 (Al2O3-CaO) up to 185kJ/mol (Al2O3-AlN). Therefore, when moving to higher temperatures, the activity series should change. It is confirmed by the calculated data for the rate constant at 900℃. It can be seen that at this temperature, the carriers in the N2O decomposition reaction are arranged (in terms of activity) in the following series:
Al2O3-SiO2>Al2O3-AlN>ZrO2>Al2O3>Al2O3-ZrO2>Al2O3-CaO>α-Al2O3>SiO2
It should be noted that since the catalyst weight is taken into account when calculating the rate constant, the carrier with the lowest pour density (Al2O3-SiO2) becomes the most active.
Thus, based on the obtained results, the most promising carriers are the following:
(1)Aluminum oxide carrier, based on which a series of catalyst samples was prepared for the first stage of work;
(2)Carriers based on Al2O3- ZrO2and Al2O3-AlN.
Despite its high activity in the N2O decomposition reaction, the zirconium oxide carrier is not of practical interest due to the destruction of catalysts during preparation and its scarcity.
A test sample of the Al2O3-SiO2composition, which showed high activity in the N2O decomposition reaction, deserves consideration.

Table 2 Properties of carriers in the N2O decomposition reaction
3.3 Activity of supported metal and oxide catalysts
3.3.1 Conditions for the preparation of supported single-component catalysts and their properties
To obtain catalysts using a ready-made carrier, we selected the method of repeated dip ensuring the full absorbency. The size, shape and porous structure of the catalyst are set by the carrier, which is important to ensure the reproducibility of the catalyst properties. In addition, this method makes it possible to use the AC to the maximum extent, whereas, in deposited catalysts, its part is shielded by the carrier and is not available for reagents. When the carrier is dipped, the pore volume filling is carried out due to capillary forces and completed in a very short time. The amount of the AC added to the catalyst depends on the total pore volume of the carrier and the concentration of the dipping solution. To obtain highly concentrated catalysts (more than 5% AC), several dips are carried out. Before each next dip, the dipped carrier is heat-treated to remove the solvent from the carrier pores and convert the AC salt to an insoluble state. The number of dipping / heat treatment cycles is repeated until the required amount of the AC is applied. To reduce the number of dips, heat treatment can be carried out in a hydrogen atmosphere, as a result of which the AC is reduced to metal.
To obtain comparative data on the activity of various metals and oxides, all the studied samples were prepared using a single batch of the high-temperature aluminum oxide carrier, which was a mixture of (γ+θ+α)-Al2O3in terms of phase composition. At the final temperature of heat treatment up to 750℃, it had a specific surface area of 110m2/g, a total pore volume of 0.34cm3/g, a heterogeneous porous structure with a set of micro-, macro-, and transport pores. Its porous structure is shown in Fig. 1 (sample No. 2). For the preparation of samples, a carrier of a rounded shape with a pellet size of 0.8-1.25mm was used.
Solutions of the following compounds were taken as the initial AC salts: H2PtCl6; PdCl3, Pd(NO3)2; RhCl3; RuOHCl3; H2IrCl6; Cu(NO3)2; Co(NO3)2; Ni(NO3)2; H2CrO4; Mn(NO3)2, Fe(NO3)3.
The AC content in the prepared catalysts varied from 3.5 to 36%. The high initial AC concentration in the catalyst is due to the fact that a part of the AC (up to 5%) can interact with the carrier during the preparation of the catalyst (especially during heat treatment). The observed interaction is especially typical for metals of the iron subgroup. It leads to a loss of catalyst activity. The preparation of catalysts in such a wide range of concentrations is due to the fact that the activity with an increase in the AC content for many reactions passes through the maximum, which has its own value for each AC. Therefore, to develop the catalyst composition, it is necessary to determine the optimal AC concentration. Heat treatment of the dipped carriers was carried out at a temperature of 300-350℃. After the application of the set amount of the AC, the platinum group metals were reduced at a temperature of 400-450℃. Table 3 shows the physical and chemical properties of the prepared samples.
To bring all samples to the same initial state, they were subjected to heat treatment in the air at a temperature of 600℃ for 3 hours before testing.
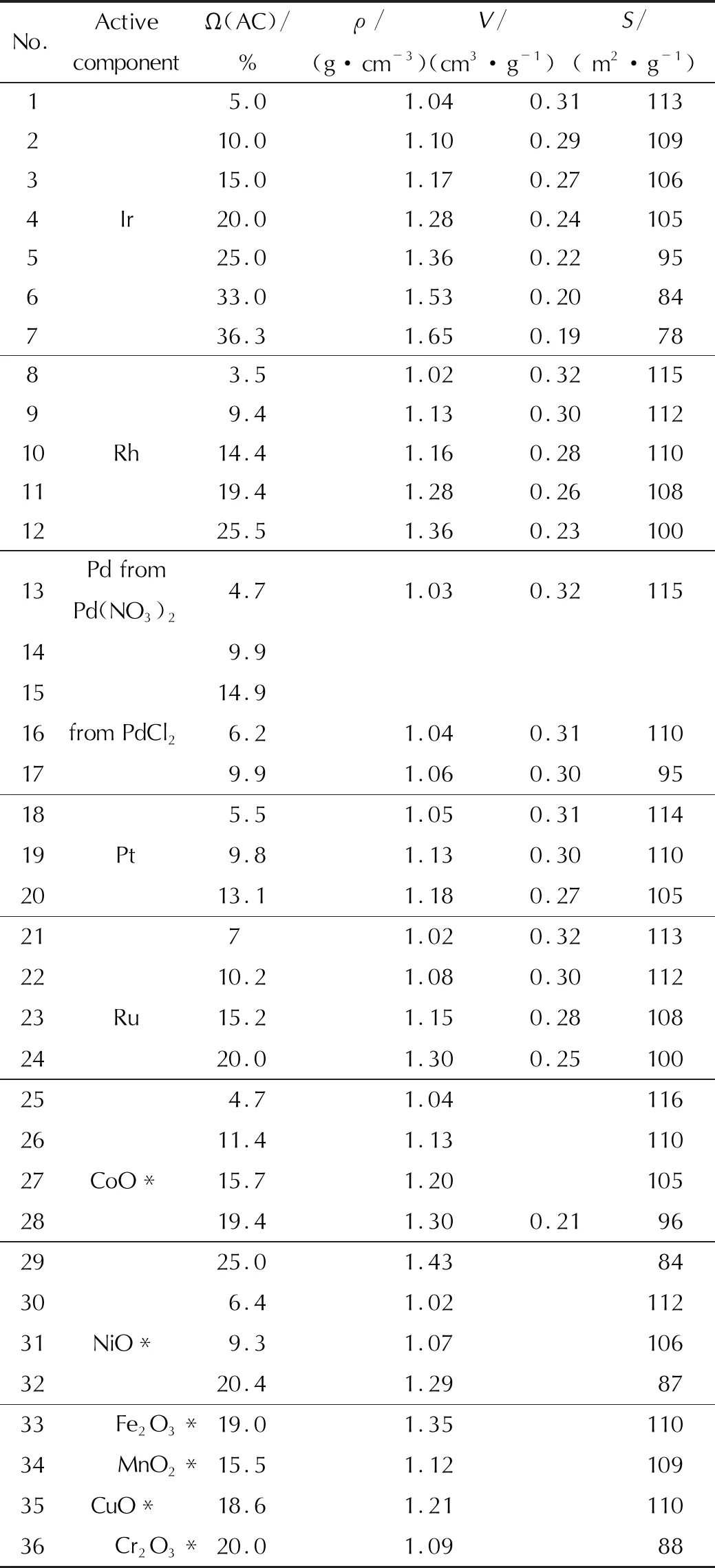
Table 3 Physical and chemical properties of the prepared samples of supported catalysts
3.3.2 Results of the experimental determination of single-component catalyst activity
Following the selected method, each catalyst sample was pre-heated in the air at 600℃ for 3 hours and then it was exposed to N2O for 5 hours during the experiment. During the first two hours, the degree of N2O conversion was determined in the mode of programmable heating of the furnace from 20 to 600℃ (first determination). Then the activity of the sample in the mode of free cooling to temperatures of 100-200℃ (with no reaction) was determined, and then, the programmable furnace heating was switched on again, and the dependence of the N2O decomposition degree on the temperature (second determination) was determined again for the same sample both during heating and cooling.
The study of the activity of the prepared catalysts showed (Table 4 and Fig. 6) that in the continuous heating mode, the curve of the dependence of the N2O decomposition degree on the temperature has a clear S-shape. It means that the induction period of the decomposition process is observed at a relatively low temperature.

Fig. 6 Dependence of the N2O decomposition degree on the temperature for the following samples: Rh2O3/Al2O3; Cr2O3/Al2O3;ZrO2
With a further increase in the temperature, the rate of conversion increases sharply. When the decomposition degree reaches 0.9, it begins to change much more slowly as the temperature increases. For most samples, the induction period was overlong, which made it difficult to determine the onset temperature of the reaction accurately. Therefore, a temperature, at which the decomposition degree reaches the value of 0.05, was adopted for the comparison. The comparison of the activities of the supported catalysts showed that the samples prepared based on Pt-group metals that were subjected (like all the studied ones) to standard pre-treatment (3 hours, 600℃, in the air) were more active than the catalysts prepared based on other transition metals (Figs. 6 and 7). Therefore, these two groups of catalysts will be considered separately below.
CatalystsbasedonPt-groupmetals
As can be seen from the data presented in Table 5 in order of ascending temperature values, at which the degree of N2O decomposition on the catalyst reaches the values of 0.05 and 0.2 (for comparison, the catalysts containing 13%-15% AC were selected), the activity of the samples is higher at the second determination. According to thermal gravimetric studies, this is due to further oxidation of the metal surface, i.e. the activity of Pt-group metal oxides is higher than that related to pure metals.
The comparison of data on the activity of various catalysts in the N2O decomposition reaction made it possible to arrange the studied samples in the following series:Rh>Ru>Ir>Pt>Pd.
The obtained series was confirmed experimentally by the differential method.

Table 4 Activity of various active components applied to the aluminum oxide carrier in the N2O d ecomposition reaction (second heating)
Rhodium (15%Rh/Al2O3), as the most active metal, begins to decompose N2O at 250℃, and at 320℃, the decomposition degree reaches a value of 0.05 (first determination). At a temperature of 350℃, the N2O decomposition degree reaches 20%. At re-starting, N2O decomposition begins at 200℃, and the decomposition degree of 0.05 and 0.2 is reached at 260 and 305℃, respectively.
The experiments showed that Pd is the least active of Pt-group metals. The decomposition reaction based on it (first determination) begins at a temperature of 360℃, and the decomposition degree of 0.05 is reached at 455℃. In a repeated experiment, the initial decomposition temperature decreases by 50℃ and the decomposition degree of 0.05 is reached at 420℃ (Tables 4 and 5). The obtained results are confirmed by data on the activation energy (E) and pre-exponential multiplier (K0), which were calculated by the decomposition degree up to 0.3 at a temperature of 350℃ and not complicated by autocatalysis processes. To compare the catalysts by the degree of their activity, an intermediate temperature of 500℃ was selected, for which, using experimental data onEandK0, the value of the rate constantK500was calculated with the Arrhenius equation.
As can be seen from Table 5, the studied catalysts differ in these indicators significantly. The activation energy value for the studied catalyst samples is in the range from 174kJ/mol (for Pd) to 118 kJ/mol (for Ir). The comparison of the activation energy values, obtained under the conditions of the first and second experiments,and data on changes in the weight of the sample during the tests allows us to conclude that the oxidation of the catalyst surface leads to a decrease in the activation energy. For a rhodium catalyst, a decrease in the activation energy by 60 kJ/mol leads to the fact that already at 260℃ the degree of N2O decomposition on a “trained” (oxidized) sample reaches the value ofx=0.05, and at 305℃ -0.2 (second determination). The same behavior is typical for all platinum metals. For a ruthenium catalyst, a decrease in the activation energy after training in N2O is 90kJ/mol. Accordingly,the onset temperature of the decomposition reaction decreases by 100℃.
The study of the influence of Pt-group metals concentration on the activity showed (Table 4) that the maximum activity is achieved when their content is different in the catalyst. Thus, Ir catalysts are characterized by a continuous increase in activity up to an increase in the AC content up to 36%.
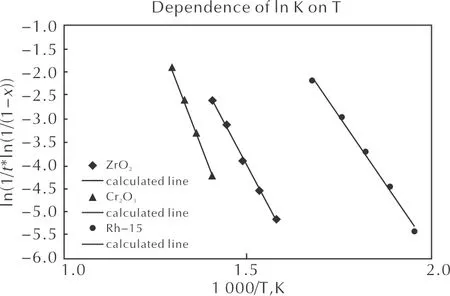
Fig. 7 Dependence of ln k on the temperature for the following samples: Rh2O3/Al2O3;Cr2O3/Al2O3;ZrO2

Table 5 Activity of supported catalysts based on platinum group metals in the N2O decomposition reaction; first and second determinations; AC content (13%-15%); carrier-aluminum oxide
For Rh catalysts, the maximum activity is reached at the values of up to 20% AC, and it does not depend much on the AC concentration. It should be noted that according to the results of Rh spectral analysis, the catalysts contained copper impurities, which were apparently introduced into the catalyst from the original AC salt. Removal of copper compounds by washing with weak nitric acid increased the catalyst activity significantly. It seems that this abnormal behavior of rhodium catalysts with an increase in the Me concentration is due to the presence of Cu impurity, which, as shown by spectral analysis methods, is located on the catalyst surface and, thus, blocks its activity.
We also determined the influence of the initial salt on the activity of palladium catalysts: the catalysts prepared from the palladium nitrate are more active than those obtained from the palladium chlorides. The maximum activity of Ru samples (Table 6) is observed in a sample containing 7% AC. At the second determination, the onset temperature of decomposition is 220℃, and at 270℃, the decomposition degree reaches 0.2. Pt-containing catalysts are characterized by a slight increase in activity: the onset temperature of decomposition decreases by 20℃ with an increase in the AC concentration from 5 to 13%.
Table 7 shows the physical and chemical properties of the catalyst samples after their testing in N2O. Since the final temperature of aluminum oxide carrier heat treatment is 750℃, tests in N2O up to a temperature of 600℃ did not significantly affect the value of the total pore volume and specific surface area, while the pour density increased slightly, which indicates the oxidation of the AC.

Table 6 Influence of metal (Ru, Pt) concentration on the catalyst activity in the N2O decomposition reaction

Table 7 Physical and chemical properties of the supported catalysts before and after testing in the N2Odecomposition reaction; AC content 13%-15% wt; carrier-aluminum oxide
Catalystsbasedonmetaloxides
Transition metal (Co, Ni, Mn, Cu, Cr, Fe) oxides are quite active in the N2O decomposition reaction and have high thermal and chemical stability[2]. To select the AC catalyst, we prepared a series of oxide catalysts containing up to 30% AC in terms of metal on the same aluminum oxide carrier as the Pt-group catalysts. As the test results showed, the behavior of oxide catalysts is fundamentally different from the behavior of catalysts based on platinum group metals: for example, their activity does not increase after the first experiment in N2O up to a temperature of 600℃. The activation energy from experiment 1 to experiment 2 almost does not change, which allows us to conclude that the phase composition of the catalyst surface is constant, which is confirmed by the data on the properties of the catalyst after the test (Table 7). The exception is a sample based on manganese oxide. After the first experiment in N2O, its activation energy decreases by 30 kJ/mol, thus, resulting in an increase in the onset temperature of the decomposition reaction by 15℃.
Table 8 presents experimental values of the activity of the catalysts in the N2O decomposition reaction (experiment 2), which shows that by the minimum onset temperature of N2O decomposition, the oxides can be arranged in the following series:
Co3O4(415℃) Table 8 Activity of metal oxides applied to the aluminum oxide carrier in the N2O decomposition reaction The highest activation energy is observed in samples based onCuO and Cr2O3(187-176kJ/mol), followed by a catalyst based on Fe2O3(163kJ/mol); activation energies for samples based on MnO2, NiO and Co3O4are close to each other (133-143kJ/mol). Therefore, when moving to higher temperatures, the activity series changes slightly. And already at 500℃, we get the following sequence (the value of the rate constant correlates to the temperature, at which the degree of conversion reaches 0.2): CuO>MnO2, Co3O4,Cr2O3>NiO>Fe2O3 Thus, in terms of activity in the N2O decomposition reaction, the studied supported oxide catalysts are inferior to samples based on rhodium, iridium, and ruthenium. It seems that their activity is largely determined by the properties of the aluminum oxide carrier. Oxides of the following metals were selected as active components for bench testing of the composition of the supported catalyst in a simulated engine: Rh,Ir, Co. It is recommended to use the following carriers as catalyst carriers: Al2O3-SiO2; Al2O3-AlN; ZrO2; Al2O3. Pt and its alloys with Rh and Ir, as well as Ni and its alloy with chromium (nichrome) in the form of a wire are recommended as precursors for catalysts of the “metal rubber” type in the output layer. In the future, it is recommended to produce enlarged samples of the selected catalytic systems for bench testing of simulated engines. After the tests, it is required to conduct a comprehensive examination of the tested catalyst samples to finally select catalyst formulation and composition. Conflictofinterest The author confirms that the provided information contains no conflicts of interest.
4 Conclusion
