NOx storage and reduction assisted by non-thermal plasma over Co/Pt/Ba/γ-Al2O3 catalyst using CH4 as reductant
2021-02-27TaoZHU竹涛XingZHANG张星NengjingYI伊能静HaibingLIU刘海兵andZhenguoLI李振国
Tao ZHU (竹涛), Xing ZHANG (张星), Nengjing YI (伊能静),Haibing LIU (刘海兵) and Zhenguo LI (李振国)
1 Institute of Atmospheric Environmental Management and Pollution Control,China University of Mining& Technology (Beijing), Beijing 100083, People’s Republic of China
2 Solid Waste and Chemicals Management Technology Center, China Ministry of Ecology and Environment, Beijing 100029, People’s Republic of China
3 National Engineering Laboratory for Mobile Source Emission Control Technology, China Automotive Technology & Research Center Co., Ltd., Tianjin 300300, People’s Republic of China
4 State Key Laboratory of Organic Geochemistry, Chinese Academy of Sciences, Guangzhou 510640,People’s Republic of China
Abstract NOx storage and reduction (NSR) technology has been regarded as one of the most promising strategies for the removal of nitric oxides(NOx)from lean-burn engines,and the potential of the plasma catalysis method for NOx reduction has been confirmed in the past few decades.This work reports the NSR of nitric oxide (NO) by combining non-thermal plasma(NTP) and Co/Pt/Ba/γ-Al2O3 (Co/PBA)catalyst using methane as a reductant.The experimental results reveal that the NOx conversion of NSR assisted by NTP is notably enhanced compared to the catalytic efficiency obtained from NSR in the range of 150°C–350°C,and NOx conversion of the 8%Co/PBA catalyst reaches 96.8%at 350°C.Oxygen (O2) has a significant effect on the removal of NOx, and the NOx conversion increases firstly and then decreases when the O2 concentration ranges from 2%to 10%.Water vapor reduces the NOx storage capacity of Co/PBA catalysts on account of the competition for adsorption sites on the surface of Co/PBA catalysts.There is a negative correlation between sulfur dioxide (SO2) and NOx conversion in the NTP system, and the 8% Co/PBA catalyst exhibits higher NOx conversion compared to other catalysts, which shows that Co has a certain SO2 resistance.
Keywords: non-thermal plasma, NOx storage and reduction, Co/Pt/Ba/γ-Al2O3 catalyst NOx conversion, influence parameter
1.Introduction
The wanton emission of nitric oxides(NOx)could lead to many environmental pollution problems, such as acid rain, photochemical smog and so on.NOxhas long been perceived as a harmful pollutant that poses a serious threat to human health.Therefore, the mitigation emission of NOxis of paramount importance on account of its negative impacts.During the past decades, numerous efforts have been devoted towards finding adequate solutions to remove NOxin order to fulfill the stricter regulations [1–6].Compared to other internal combustion engines, the lean-burn engine has attracted widespread attention because it could improve the fuel efficiency by up to 20%–30%.However, owing to the presence of excess oxygen (O2) in exhaust gas,the NOxproduced by the lean-burn engine cannot be degraded effectively by three-way catalysts[7,8].Therefore,the removal of NOxfrom lean-burn engines is one of the most challenging issues, and has received much attention in the academic and industrial community.
NOxstorage and reduction (NSR) technology has been regarded as one of the most promising strategies for the removal of NOxfrom lean-burn engines.It was first introduced by Toyota and has been used widely to remove NOxfor several years [9, 10].NSR technology works between the fuel-lean and fuel-rich cyclic stage.In the fuel-lean stage,the NOxis adsorbed by the NSR catalyst.Afterwards, it is reduced to N2by reductants in the fuel-rich stage.NO2is more easily stored on the NSR catalyst than NO during the NOxstorage process.Therefore, the NO oxidation capacity(NOC)is one of the important indicators to evaluate the NSR reaction [11–14].In the past few decades, a large variety of catalysts have been reported to be active for NSR, and Pt/Ba/γ-Al2O3(PBA) is one of the most commonly used catalysts, which has a superior catalytic performance in the removal of NOx.However, the exhaust temperature of the lean-burn engine ranges from 180 °C to 350 °C in practice,and the PBA catalyst exhibits low NOxconversion below 300 °C.Therefore, it is of crucial importance to develop a new solution to improve low-temperature NOxconversion.
Recently,non-thermal plasma(NTP)has emerged as one of the most promising technologies to overcome barriers in conventional catalysis, and it has been widely applied in the treatment of gaseous pollutants, especially in the removal of NOx.The potential of the plasma catalysis method for NOxreduction has been confirmed, and NTP could be conducted within wide ranges of gas space velocity and temperature [15–20].NSR assisted by NTP in the fuel-rich stage has been extensively investigated and there are two NTP catalysis methods,namely plasma-driven catalysis(PDC)and plasma-enhanced catalysis (PEC), which can remove NOxefficiently according to the relevant reports.The catalyst is placed at the discharge area in the PDC system,and can make full use of the short-lived intermediates created by NTP.In contrast, PEC involves placing the catalyst downstream from the NTP, and the by-products are easy to deposit on the surface of the catalyst [21–25].Wanget alcombined NTP with NSR over an H-ZSM-5 (Si/Al = 22) catalyst in the PDC system, and they revealed that NOxcould be adsorbed by H-ZSM-5,and then it was reduced to N2and N2O at room temperature[26].Zhanget alproposed an NTP-assisted NSR process on cobalt (Co)-containing catalysts, and the experimental result showed that NOxconversion was improved significantly in the range of 150°C–350°C[27].Beyond that,Kimet alreported that the NOxstorage capacity(NSC)of Cocontaining catalysts was higher than that of Co-free catalysts[28].This research reports the observations of dielectric barrier discharge (DBD) plasma and Co/PBA catalyst in the PDC system, and discusses the influence parameters of NSR assisted by NTP,such as oxygen(O2),water vapor(H2O)and sulfur dioxide (SO2).It is of great significance to investigate NOxremoval using a combination of NSR and NTP, which could provide an alternative technology to remove NOxfrom lean-burn engines.
2.Experiment
2.1.Catalyst preparation
The catalyst support γ-Al2O3was commercially obtained from Sinopharm Chemical Reagent Co., Ltd, and the Co/PBA catalysts were prepared by the wetness impregnation method.The loadings of Ba and Pt were 20 wt% and 10 wt% during the preparation process of Co/PBA catalysts, and the loadings of Co were 0 wt%, 2 wt%, 4 wt%, 6 wt% and 8 wt%, respectively.Firstly, the γ-Al2O3was impregnated with aqueous Ba(O2CCH3)2.Next, the Ba-loaded γ-Al2O3was impregnated with aqueous Co(NO3)2· 6H2O, and then dried at 100 °C for 12 h and calcined at 500 °C for 5 h.In the second step, the solid samples were impregnated with aqueous H2PtCl6· 6H2O,followed by drying and calcination.Finally, the Co/PBA catalysts were pressed, crushed and sieved to 20–40 meshes,and the obtained samples were denoted as PBA,2%Co/PBA,4% Co/PBA, 6% Co/PBA and 8% Co/PBA, respectively.
2.2.Catalyst characterization
The specific surface area of the Co/PBA catalyst was determined by the Brunauer–Emmett–Teller (BET) method, and the pore volume and average pore diameter of Co/PBA catalysts were obtained from the desorption curve using the Barrett–Joyner–Halenda (BJH) method.Prior to the measurements, all samples were outgassed for 12 h at 200 °C in a vacuum.The x-ray diffraction (XRD) measurement was carried out on an XRD-7000 S (Shimadzu Corporation, Japan), which was operated at 40 kV and 40 mA using Cu Κα radiation.The scanning angle(2θ)scans ranged from 10°to 80°with a step of 0.02°,and the scanning speed was 5° min−1.The hydrogen temperatureprogrammed reduction (H2-TPR) measurements of Co/PBA catalysts were conducted on the TP-5080-B (Xianquan Corporation, Tianjin), which was equipped with a thermal conductibility detector.The 100 mg catalyst was preprocessed at 200 °C for 1 h under Ar atmosphere and then cooled to room temperature.After that,the temperature was raised to 700°C at 10 °C min−1with a flow of H2(10%)/Ar (50 ml min−1).Furthermore, the thermal stability of adsorbed NOxspecies is important for the Co/PBA catalysts,which was evaluated by the temperature-programmed desorption of NOx(NOx-TPD) measurements.Firstly, the Co/PBA catalysts (100 mg) were pretreated at a flow of 2%H2and Ar at 500°C for 1 h prior to the NOx-TPD measurements.After that,the Co/PBA catalysts were cooled down to 200 °C under Ar atmosphere.Then, NOxadsorption was carried out at 200 °C for 1 h under Ar atmosphere, and the feed gas contained 500 ppm NO and 6% O2.Finally, the temperature was increased to 800 °C with a ramp rate of 10 °C min−1under Ar atmosphere.
2.3.NOC and NSC experime nts
The NO oxidation capacity (NOC) and NOxstorage capacity(NSC) of Co/PBA catalysts were measured on a microreactor under the fuel-lean stage,and the NOxconcentration was detected by a chemiluminescence NOxanalyzer (Ecotech ML9841AS).The Co/PBA catalysts (200 mg) were preprocessed under an atmosphere of 5%H2and Ar at 500°C for 1 h.NO was used as the feed gas, which was kept at 500 ppm, and Ar served as the balance in the range of 150°C–350°C.The NOC experimental equilibrium NO conversion was calculated by HSC Chemistry V7.0 software.The NSC measurements were carried out under the same atmosphere as the NOC experiment, and the storage time was 50 min.The weight hourly space velocity (WHSV) of the NSC and NOC experiments was stabilized at 30 000 ml · g−1· h−1, and the experimental results were obtained at a steady state.Equations(1)and(2)were used to evaluate the NOC and NSC performance.
whereNO2,outis theNO2concentration in the outlet,NOinis the NO concentration in the inlet,tsrefers to the storage time,mcatrefers to the mass of Co/PBA catalyst,NOx,inrepresents theNOxconcentration in the inlet, andNOx,outrepresents theNOxconcentration in the outlet.
2.4.Catalyst evaluation
The diagram of NSR assisted by NTP is shown in figure 1.The simulated gas flowed into the buffer tank after being regulated by the mass flow controller, and the H2O was generated by a vapor chamber using a microscale digital injector.The DBD plasma reactor was adopted in this research, which could work at atmosphere pressure with the advantage of high security and easy reactivity.The Co/PBA catalyst (200 mg, 20–40 meshes) was placed at the center of the discharge region and the WHSV was kept at 30 000 ml · g−1· h−1.The Fourier transform infrared spectrometer (FT-IR) gas analyzer (GASM ET DX 4000, Finland) was used to analyze the NOxconcentration in the outlet and the alkali absorption device was used to absorb exhaust gas.The DBD plasma was powered by an alternating current with afrequency of 10 kHz and a voltage of 20 kV, and the output waveform was observed on a Tektronix-TBS1102 digital oscilloscope.The reaction property of Co/PBA catalysts was investigated in the fuel-lean and fuel-rich cyclic stages.The experimental gas composition of NSR assisted by NTP is summarized in table 1.
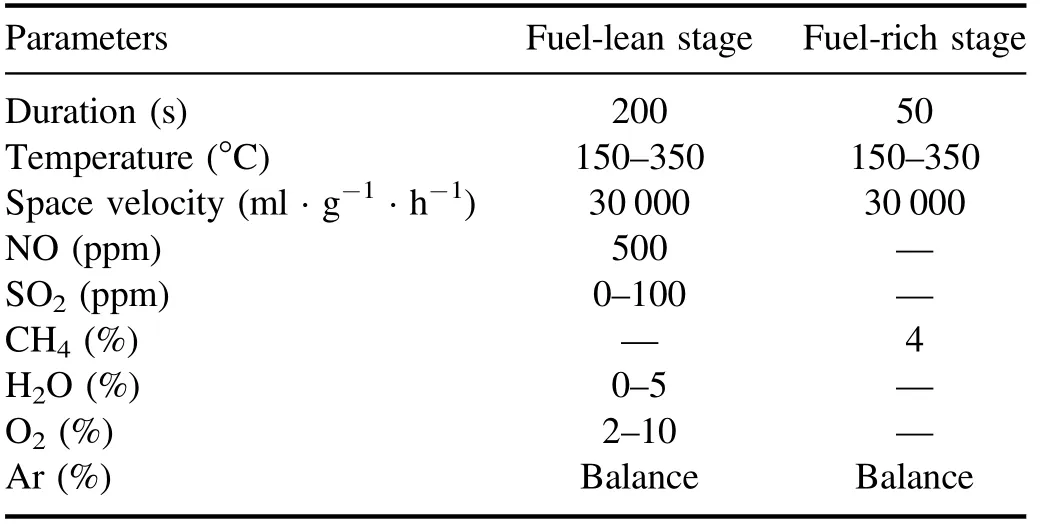
Table 1.Experimental gas composition of NSR assisted by NTP.
A diagram of the combined DBD plasma and NSR system is shown in figure 2.The plasma reactor has a coaxial line structure, the dielectric tube adopts quartz and the sealing gaskets are made of polytetrafluoroethylene.The dielectric tube is wrapped by copper mesh, which serves as a ground electrode.The thicknesses, diameter and length of the dielectric tube are 2 mm, 20 mm and 500 mm, respectively.
The NOxconcentration was averaged over five cycles and then a mean conversion was obtained.Equation (3) was used to estimate the NOxconversion.

whereNOx,inis theNOxconcentration in the inlet,NOx,outis theNOxconcentration in the outlet.
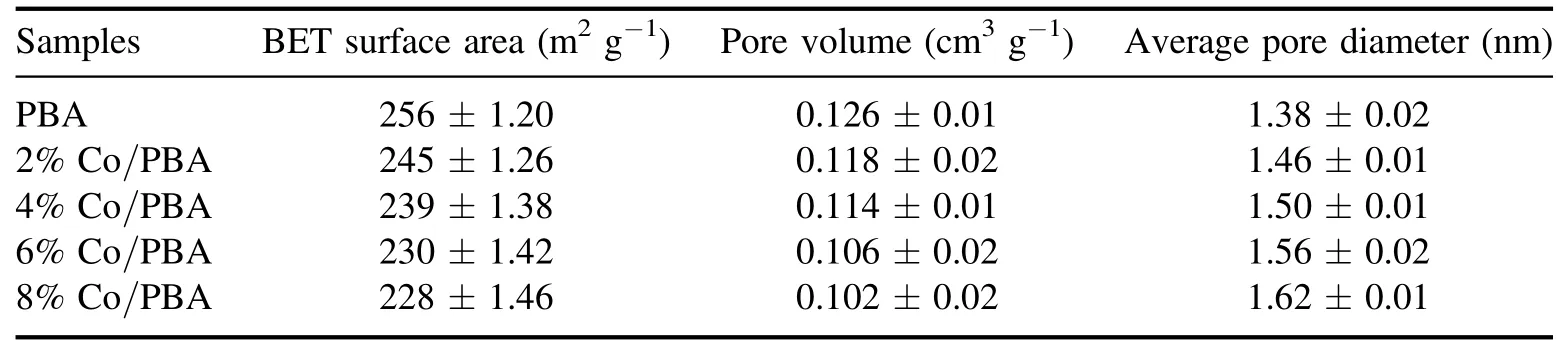
Table 2.Physical characteristics of the Co/PBA catalysts.
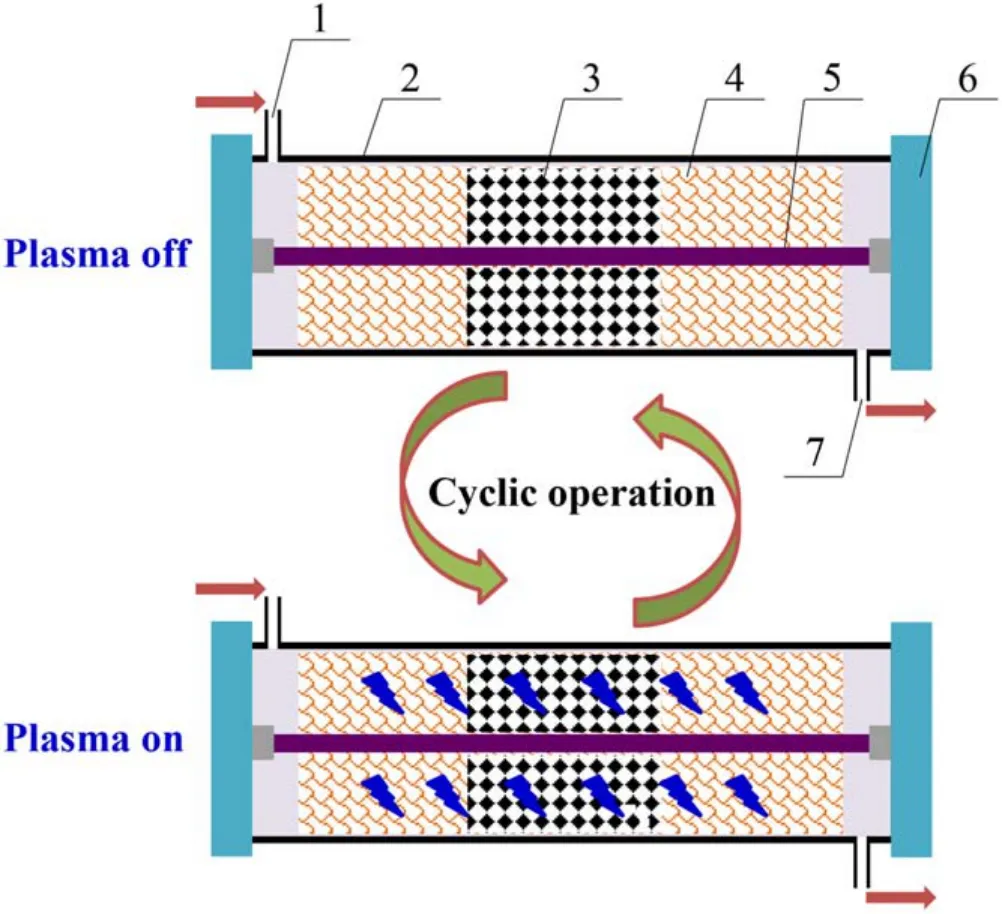
Figure 2.Diagram of the combined DBD plasma and NSR system.1 – inlet, 2 – dielectric tube, 3 – Co/PBA catalyst, 4 – ground electrode, 5 – internal electrode, 6 – sealing gasket, 7 – outlet.
3.Results and discussions
3.1.Characterizations of Co/PBA catalysts
The results of the BET surface area,pore volume and average pore diameter of Co/PBA catalysts are summarized in table 2.Obviously, the BET surface area of Co/PBA catalyst ranges from 228 m2g−1to 256 m2g−1,and PBA has the largest BET surface area.The specific surface area of the Co/PBA catalysts decreases slightly because the PBA is impregnated with Co metal element.Liet alrevealed that the reaction performance of the catalyst was closely related to the specific surface area, and found that a larger specific surface area could provide more active sites.Furthermore, it significantly promoted NOxadsorption [29].The pore volume and average pore diameter of Co/PBA catalysts are about 0.11 cm3g−1and 1.50 nm, respectively, which indicates that the pore properties of Co/PBA catalysts are little affected by the loading of Co.
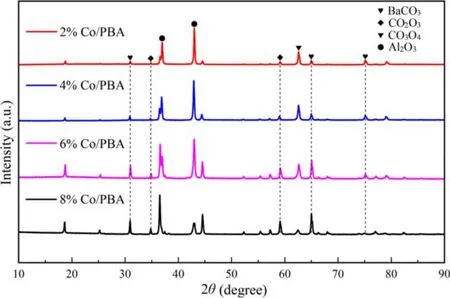
Figure 3.The XRD pattern of Co/PBA catalysts with different Co loadings of 2%, 4%, 6% and 8%, respectively.
As depicted in figure 3, the main phases of the Co/PBA samples are BaCO3and γ-Al2O3, which confirms that Ba(O2CCH3)2is decomposed into crystalline BaCO3during the calcination process.In addition, trace amounts of the Co2O3phase and Co3O4phase are observed in the XRD patterns besides BaCO3and Al2O3.This conclusion is consistent with the research of Baiet al[30]; their results revealed that the major diffraction peaks presented in PBA + CoMn and PBA + CoMn-K were BaCO3, γ-Al2O3and Co3O4, a new phase Ba3CoO5appeared, and the peak intensity of BaCO3weakened after mechanical mixing,which indicated that there was a strong chemical interaction between Ba/γ-Al2O3and CoMn during the process of calcination.
The H2-TPR profiles of the Co/PBA catalysts with different Co loadings are shown in figure 4, and two major H2consumption peaks can be observed for the Co/PBA catalysts.But beyond that, the H2consumption amounts in the range of 200°C–500°C are summarized in table 3.It can be concluded that the H2consumption amounts increase with the increase of the Co loading amount.The H2consumption of the 8% Co/PBA catalyst is notably higher than that of the others, in accordance with the data in table 3.The peak at 260 °C could be attributed to the reduction of Co3+to Co2+,while the peak at 455°C belongs to the reduction of Co2+to Co0according to the research of Baiet al[30].
3.2.NOC performance

Figure 4.The H2-TPR profiles of Co/PBA catalysts with different Co loadings of 2%, 4%, 6% and 8%, respectively.
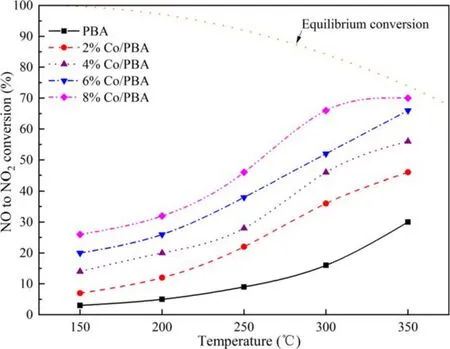
Figure 5.The conversion efficiency of NO to NO2 over Co/PBA catalysts in fuel-lean stage.
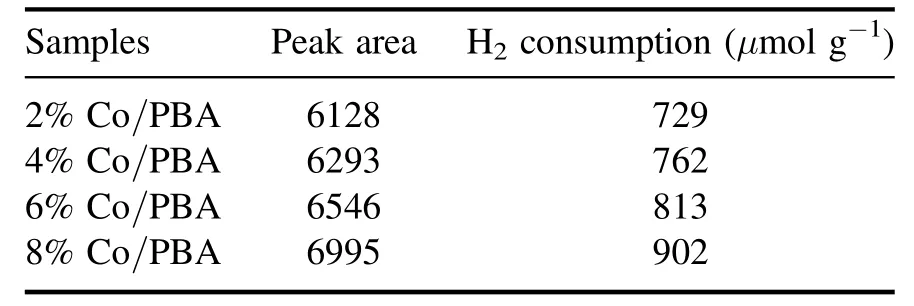
Table 3.The H2 consumption amounts of Co/PBA catalysts.
Figure 5 displays the conversion efficiency of NO to NO2over Co/PBA catalysts.Baiet alrevealed that NO2was easier to store on the catalyst than NO during the NOxstorage process.Therefore,NOC has an important impact on the NSR reaction [31].As shown in figure 5, the NO conversion increases with the increase of temperature from 150 °C to 350 °C for all Co/PBA catalysts.Wanget alrevealed that PBA was a common NO oxidation catalyst,which exhibited a significant catalytic performance at high temperature [32].Compared to Co/PBA catalysts, NO conversion over the PBA catalyst is relatively low, and the Co component of Co/PBA catalysts contributes significantly to NOC,which is consistent with the research of Zhanget al[33], who found that the surface-adsorbed oxygen played a major role in the process of NO oxidation,and the Co/PBA catalysts contained higher amounts of surface oxygen than PBA,so it exhibited a higher NOC.

Figure 6.NSC over Co/PBA catalysts measured in fuel-lean stage.
3.3.NSC performance
Figure 6 presents the NSC over Co/PBA catalysts,and shows that the NSC of Co/PBA catalysts increases when the temperature ranges from 150 °C to 350 °C.On the contrary,the PBA catalyst did not show such significant improvement in NSC, which indicates that temperature has little effect on the NSC of PBA.Zhanget alrevealed that NOxwas mainly stored as nitrates on BaO, and the strong surface basicity of BaO made the thermodynamic properties of nitrates more stable[34].The 8%Co/PBA catalyst has the highest NSC of 456.6 μmol g−1at 300 °C, and then it decreases at 350 °C.According to the research of Baiet al[35], the thermal stability of nitrate may be reduced because of the presence of Co oxides,and they reported that the majority of transition metal oxides as the NO oxidation component and barium as the chief NOxstorage component could improve the NSC significantly.
3.4.Thermal stability of adsorbed NOx
The thermal stability of adsorbed NOxfor the Co/PBA catalysts is shown in figure 7.It was determined by the NOx-TPD measurement performed from 100 °C to 800 °C and it revealed the properties of adsorbed NOxspecies on Co/PBA catalysts.A broad NOxdesorption peak can be seen from figure 7,which starts at about 400°C and ends at about 600°C.It is reasonable to ascribe the broad peak at high temperature to the overlap of different adsorbed NOxspecies on Ba according to a relevant report[33].In addition,there is a marked change in NOxdesorption to higher temperature due to the incorporation of Co compared with the NOxdesorption curve of PBA,and the amount of NOxdesorption on Co/PBA catalyst is increased significantly,which further indicates that Co has an obvious influence on the thermal stability of adsorbed NOx.This conclusion is consistent with the result that the NSC of Co/PBA catalyst is enhanced remarkably due to the presence of Co.
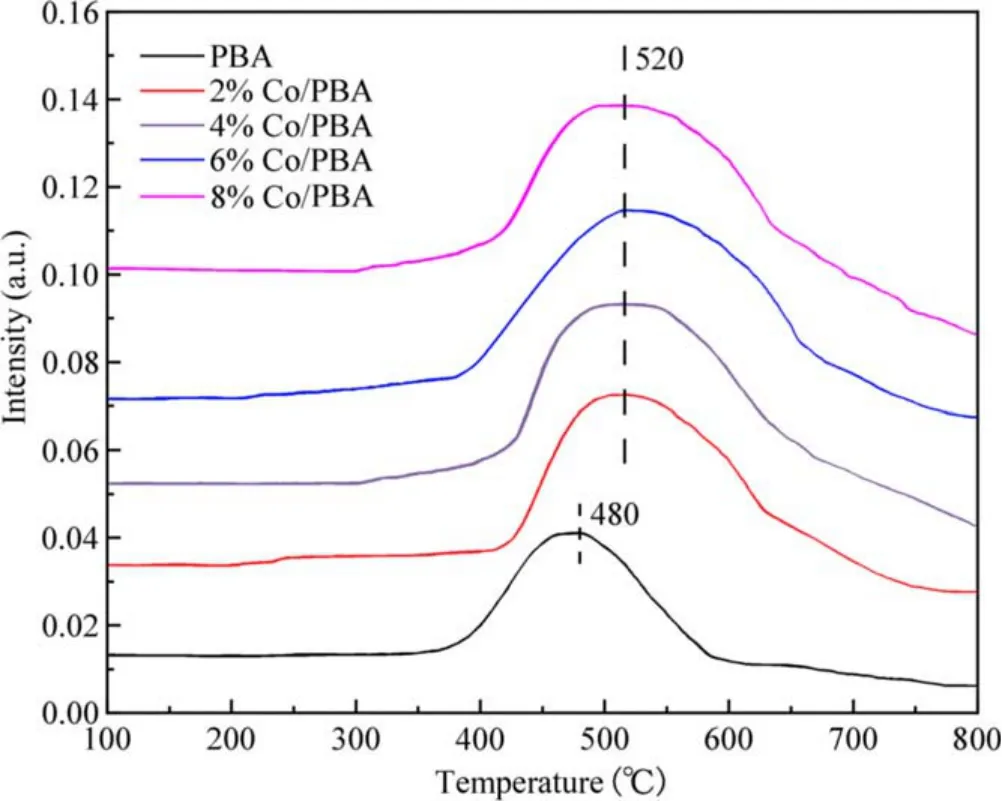
Figure 7.The thermal stability of adsorbed NOx for the Co/PBA catalysts.
3.5.NSR performance assisted by NTP
Figure 8 shows the NSR performance in the fuel-rich stage in the range of 150 °C–350 °C.Generally speaking, NOxconversion has a positive correlation with the temperature and Co loading amount, and PBA displays lower NOxconversion at all temperatures compared with the Co/PBA catalysts.In addition, NTP enhances the NSR performance significantly.As shown in figure 8(a), the NOxconversion increases obviously with the increase of temperature, and the highest NOxconversion of 90%is obtained at 350°C.As indicated in figure 8(b), the NOxconversion of Co/PBA catalysts in the range of 150 °C–350 °C is enhanced obviously compared to the performance obtained in the catalysis-only condition.What is more, the NOxconversion of 8% Co/PBA reaches 96.8% at 350 °C.Overall, PBA exhibits the poorest activity even in the CH4-NTP-assisted NSR process, which indicates that Co plays a crucial role in CH4activation even when NTP is present during the fuel-rich stage.This result is consistent with the conclusion made by Zhanget al[33], who revealed that high NOxconversion could be obtained over a broad temperature window of 150 °C–350 °C by combining the high NSC of Pd/Co/Ba/Al in the fuel-lean stage with NTPassisted activation of the reductant in the fuel-rich stage.According to the relevant research, the following chemical reactions could occur in the NTP system and the decomposition of NO may be as follows [27, 30, 34].


CH4*,Ar*and H2*are excited molecules of CH4,Ar and H2.O*is excited O atom.
3.6.Effect of O2 on NSR assisted by NTP
In recent years, NOxfrom lean-burn engines has become a great challenge, and diverse internal combustion engines result in different O2concentrations (<10%) in exhaust gas[36, 37].According to practice and experience, the O2concentration has a significant effect on NSR performance in the NTP system.Therefore, it is of great necessity to investigate the effect of O2concentration on the performance of NSR assisted by NTP.The reaction temperature is set at 350 °C.Figure 9 presents the effect of O2on NSR assisted by NTP.As depicted in figure 9, NOxconversion increases firstly and then decreases when the O2concentration ranges from 2%to 10%, and the O2concentration of 6% has an optimal NOxconversion of 96.8%.In the NTP system,O2could react with N2to generate different varieties of NOx, which is similar to the research of Wanget al[38].They reported that the change curve of NOxto N2conversion efficiency with O2concentration was an asymmetric parabola, which had a maximum value at an O2concentration of 5%,and the conversion of NOxto N2O was opposite to the change trend of O2concentration.Due to the presence of O2in the NTP system, the following chemical reactions would occur, according to the relevant reports [30, 34, 38].
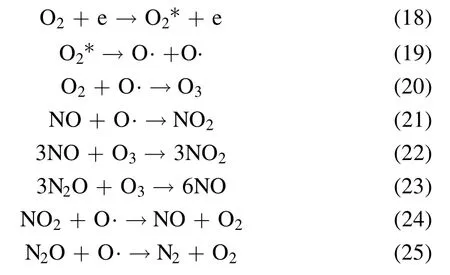
where O2*is an excited O2molecule.
3.7.Effect of H2O on NSR assisted by NTP
In the realistic situation, the NSR performance could be affected greatly by the large quantities of H2O in exhaust gas[39].Therefore, it is of vital importance to investigate the effect of H2O for NSR in the NTP system.The reaction temperature is kept at 350 °C, and the O2concentration is stable at 6%.Figure 10 displays the effect of H2O on NSR assisted by NTP.The NSC decreases sharply with the increase of H2O, which indicates that the H2O cut down the NSC of Co/PBA catalysts on account of the competitive adsorption between H2O and NOxon the surface of Co/PBA catalysts.In addition, H2O exhibits a similar performance in depressing the NOxreduction, which results in a decrease in NOxconversion with an increase of H2O in the NTP system.The experimental result is similar to the research of Wanget al[38], who discovered that H2O had a negative effect on the adsorption and discharge stage.The adsorbed H2O molecule could consume high-energy electrons due to its electronegativity, and the energy density of the NTP system declined significantly.Meanwhile, they put forward that increasing the concentration of the reducing agent and enhancing the energy density of the NTP system could improve the NOxconversion under a high H2O condition.
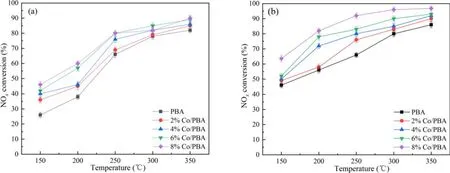
Figure 8.NSR performance during lean and rich cycling in the range of 150 °C–350 °C.(a) NSR performance without NTP, (b) NSR performance with NTP.
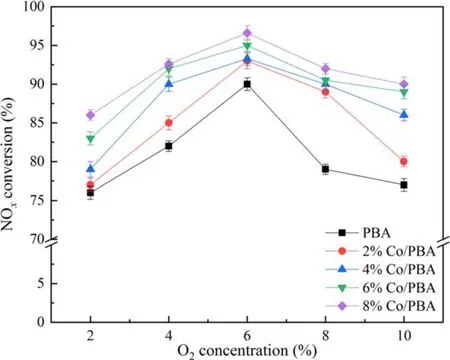
Figure 9.Effect of O2 on NSR assisted by NTP.
3.8.Effect of SO2 on NSR assisted by NTP
SO2is a by-product of diesel engine exhaust, and it is worth noting that the SO2concentration is normally more than 50 ppm in marine exhaust gas.It is derived from the fuel and results in catalyst poisoning during the NSR application process.The reason for deactivation is that the S-containing species are easy to deposit on the surface of the NSR catalyst,and it is difficult to recover its initial catalytic activity using conventional methods [40–42].Therefore, SO2resistance is an important index to evaluate the NOxconversion of Co/PBA catalysts, and the effect of SO2on NSR assisted by NTP is discussed in this section.Figure 11 shows the effect of SO2on NSR assisted by NTP.Clearly,the NSC is decreasing with the increase of SO2concentration, which illustrates that SO2has adverse effects on NSC.Similarly,there is a negative correlation between SO2and NOxconversion in the NTP system, which indicates that Co/PBA catalysts are deactivated under the influence of SO2.What is more, it can be clearly seen that the 8% Co/PBA catalyst exhibits a higher NOxconversion compared to other catalysts, which shows that Co has a certain SO2resistance.
4.Conclusions
The aim of the presented work is to investigate the removal of NO in a combination of NSR and NTP,which exhibits a high catalytic performance in NOxconversion.It could serve as an alternative solution to remove NOxfrom lean-burn engines.The influence parameters of NOxconversion were investigated, adopting NSR technology assisted by NTP over Co/PBA catalysts, and the following conclusions could be drawn from this research.
(1) NOxconversion increases with the increase of reaction temperature, and NOxconversion of NSR assisted by NTP is enhanced obviously compared to the catalytic efficiency obtained from NSR in the range of 150 °C–350 °C.What is more, the NOxconversion of 8%Co/PBA catalyst could reach up to 96.8% at 350 °C.
(2) O2has a significant effect on the removal of NOx, and the NOxconversion increases firstly and then decreases when the O2concentration ranges from 2%to 10%.The O2concentration of 6%shows optimal NOxconversion.

Figure 10.Effect of H2O on NSR assisted by NTP.(a) NSC over Co/PBA catalysts, (b) NSR over Co/PBA catalysts.
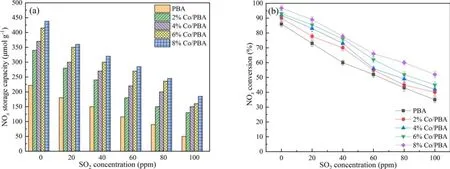
Figure 11.Effect of SO2 on NSR assisted by NTP.(a) NSC over Co/PBA catalysts, (b) NSR over Co/PBA catalysts.
(3) H2O reduces the NSC of Co/PBA catalysts significantly on account of the competition for adsorption sites on the surface of Co/PBA catalysts.Therefore, it leads to a decrease in NOxconversion with the increase of H2O in the NTP system.
(4) NSC decreases with the increase in SO2concentration,there is a negative correlation between SO2and NOxconversion in the NTP system, and the 8% Co/PBA catalyst exhibits a higher NOxconversion compared to other catalysts, which shows that Co has a certain SO2resistance.
Acknowledgments
This work was supported by the National Engineering Laboratory for Mobile Source Emission Control Technology(No.NELMS2019A13), the National Key Research and Development Project of China (No.2019YFC1805505), the Shanxi Province Bidding Project (No.20191101007), the Major Science and Technology Projects of Shanxi Province(No.20181102017), and State Key Laboratory of Organic Geochemistry (No.SKLOG -201909).
杂志排行
Plasma Science and Technology的其它文章
- Effect of edge turbulent transport on scrapeoff layer width on HL-2A tokamak
- An investigation on improving the homogeneity of plasma generated by linear microwave plasma source with a length of 1550 mm
- Spatio-temporal evolution characteristics and pattern formation of a gas–liquid interfacial AC current argon discharge plasma with a deionized water electrode
- Turbulent boundary layer control with a spanwise array of DBD plasma actuators
- Plasma activation towards oxidized nanocarbons for efficient electrochemical synthesis of hydrogen peroxide
- Spatio-temporal evaluation of Zr plasma parameters in a single-beam-splitting double-pulse laser-induced plasma
