Systematic review of robust experimental models of rheumatoid arthritis for basic research
2021-02-14LINYeHUMingyueZHANGFengDAIZongshunXIEYingCAIXiongLIULing
LIN Ye, HU Mingyue, ZHANG Feng, DAI Zongshun, XIE Ying, CAI Xiong*, LIU Ling*
a.Institute of Innovation and Applied Research in Chinese Medicine, Hunan University of Chinese Medicine, Changsha, Hunan 410208, China
b.State Key Laboratory of Quality Research in Chinese Medicine, Macau University of Science and Technology, Macau 999078, China
Keywords Rheumatoid arthritis Animal models Pathological features Drug screening Traditional Chinese medicine (TCM)
ABSTRACT Rheumatoid arthritis (RA) is a common autoimmune disease characterized by progressive joint inflammation and destruction,deformity, loss of mobility, and permanent disability.Although the cellular and molecular mechanisms involved in RA are understood in detail, no drugs or therapies can completely cure RA.Many long-term efforts have been directed towards a better understanding of RA pathogenesis and the development of new classes of therapeutics.Thus, the ongoing elucidation of pathogenic events underlying RA mostly relies on studies of animal models.Herein, we comprehensively review and discuss the characteristics, challenges, and unresolved of issues of various experimental models of RA to provide a basis and reference for the rational selection of experimental RA models for basic investigations into traditional Chinese medicine (TCM).
1 Introduction
Mice and rats can serve as models that mimic human diseases.They are extensively applied to investigate the pathogenesis of rheumatoid arthritis (RA)and develop potential anti-arthritic agents.Small experimental animals are easy to maintain and handle,their reproductive cycles are short, they have similar genomic and physiological characteristics to humans, and their genetics can be manipulated[1].The pathogenesis of human RA is complex, involving genetic susceptibility and environmental factors, and many models of RA have pathological characteristics that resemble those of human diseases[2].Animal models have a shorter disease onset than humans,which contributes to the overall knowledge of inflammation, cartilage destruction, and bone resorption.However, animal models of RA still have some limitations, as they cannot fully mimic human RA, although some can be close to specific clinical manifestations or pathological features of RA.Therefore, the choice of a model should be appropriate to the aims of studies.
RA is a systemic autoimmune disease characterized by inflammation and extra-articular involvement[3].The pathogenesis of RA is mainly mediated by the activation of macrophages by autoreactive T cells, which leads to the release of key pro-inflammatory cytokines, such as tumor necrosis factorα(TNF-α) and interleukin (IL)-1, IL-6, and IL-17[4].Early RA is mainly characterized by synovitis, which presents with the classic inflammatory manifestations of redness, swelling, heat, and pain.Persistent synovitis leads to the rapid division and proliferation of synovial cells and the gradual formation of thickened pannus.Innumerable immune cells and related enzymes invade RA joints in the late stage,causing cartilage and bone destruction and impaired joint function (Figure 1)[5,6].The ultimate goal of RA treatment is to relieve joint swelling and pain, inhibit the malignant progression of the disease, and improve the quality of life.Current anti-RA medications mainly comprise disease-modifying antirheumatic drugs (DMARDs), non-steroidal anti-inflammatory drugs (NSAIDs), and glucocorticoids, but the side effects of long-term use of these drugs are substantial.Traditional Chinese medicines (TCM) have a long history of treating RA, and they offer the advantages of effective improvement of clinical symptoms, low side effects, and various treatment methods, but many traditional formulas and therapies for RA have not been validated in experimental animals.Animal models are essential for developing more effective agents and new therapies for human RA.This report summarizes contemporary representative animal models of RA.Construction methods, immune mechanisms, pathogenic targets, and disease characteristics are comprehensively and systematically discussed.A comprehensive comparison provides a basis and reference for the rational selection of experimental inducible animal models (Table 1)[7-25]for basic studies and should result in novel strategies for treating RA using TCMs.
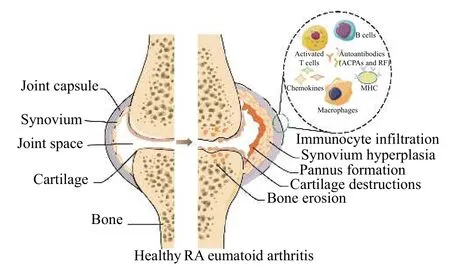
Figure 1 Healthy joints compared with joints in RA
2 Adjuvant-induced arthritis (AIA)
The AIA model was originally induced by inoculating various strains of rats with a Freund-type water-in-oil emulsion[26].Since then, inducible AIA has become a popular model of human RA and other arthritic diseases.Adjuvant-induced arthritis is an aggressive,monophasic, sub-chronic type of arthritis that is usually very severe and eventually leads to complete ankylosis and permanent joint deformity[27].It manifests clinically and serologically (erythrocyte sedimentation rate, ESR; C-reactive protein, CRP), and the histopathological, radiological, and immune changes in AIA are quite similar to those in human RA[7,8].Therefore, AIA is most commonly applied as a model for screening and testing anti-arthritic drugs[28].Atractylodes oil gentiopicroside, and other Chinese medicinal materials have proven effective against the progression of arthritis in the AIA model[29,30].Compared with other experimental models of autoimmune arthritis, AIA is relatively unique in that a joint-associated target autoantigen has been defined.Thus, the basic mechanism of how external triggers lead to undesirable self-recognition can be studied using this model.
A single injection of incomplete (IFA) and complete (CFA) Freund adjuvant withMycobacterium tuberculosis(MTB) into the base of the tail is likely to induce AIA in rats.Rat strains have different genetic sensitivities to AIA.Lewis rats are popular for AIA studies because they generally have a higher incidence of relatively severe and consistent disease[8];Sprague-Dawley (SD) rats are less genetically susceptible to AIA and have a less consistent disease onset[8].However, the AIA model is hindered by several unfavorable features.For example, even with inbred Lewis rats, the incidence and severity of arthritis greatly varies, and the number of susceptible rat product coefficients is limited.SD rats are less costly and more readily available than Lewis rats, and they have a heterologous background, so the SD rat model of AIA might better mimic the genetic characteristics
of human RA than other experimental models of autoimmune arthritis[8].In addition, the intensity of the disease might significantly impact the response of the AIA model to different classes of agents.Therefore, changing the disease severity through optimal operating techniques and the arthritogenic conditions of models might be of interest when testing new anti-arthritis drugs.
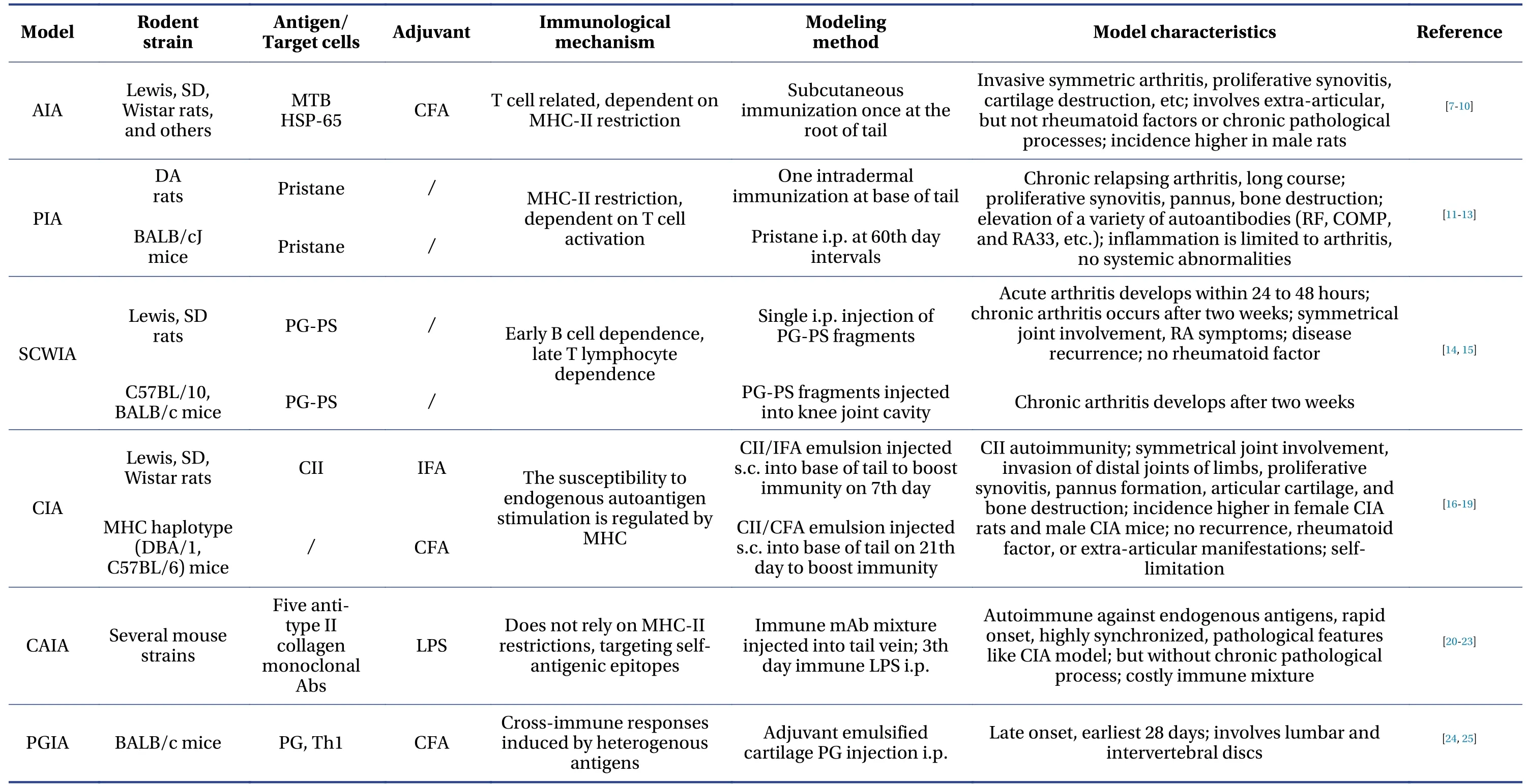
Table 1 Basic characteristics of experimental models of rheumatoid arthritis
3 Pristane-induced arthritis (PIA)
An intraperitoneal (i.p.) injection of the synthetic mineral oil(2,6,10,14-tetramethylpentadecane;pristane) induces a severe, chronic inflammatory type of arthritis (PIA) in mice and rats[31,32].Arthritis was originally induced by injecting pristane i.p.into BALB/cJ mice.The incidence and time of onset of arthritis considerably vary among susceptible strains.Dark Agouti rats are highly sensitive to PIA and a PIA model is easily formed.Acute arthritis occurs 2 – 3 w after a single intradermal (i.d.) injection of pristine at the base of the tail, and this causes dysmotility of the small joints of the limbs, recurrent attacks, and chronic arthritis after 6 – 8 w.The mass formation of osteoclasts and inflammatory cell infiltration, bone erosion, and new bone formation occur at a rate close to 100%[11].A prolonged, delayed clinical course of joint inflammation starts between 60 and 180 days after pristane administration[33].Evolving histological features include synovial hyperplasia, polymorphonuclear infiltration, periostitis, cartilage erosion, and progressive marginal erosion[32].Serologically,rheumatoid factor (RF), antibodies against heat shock proteins and type I and type II collagen have also been detected[32,34].Pristane-induced arthritis is unique because it is induced by a non-infectious,non-antigenic oil.In addition, the delay between exposure to irritants and disease development is long.The exact mechanism of PIA is unknown, but pristane promotes autoimmune responses through immune activation in response to antigens found on microorganisms prevalent in the environment[35].In fact, a specific pathogen-free (SPF) environment hinders PIA development, and returning these mice to a normal environment re-establishes their susceptibility[34].
Pristane-induced arthritis is erosive, chronic, and it specifically and symmetrically evokes pannus formation, major histocompatibility complex (MHC)class II expression, and T lymphocyte infiltration in peripheral joints[36].In addition, pristane can induce lupus-related arthritis[37].The severity and chronicity of arthritis varies between MHC haplotypes in MHC homologous Lewis strains.Rats with RT1f haplotypes are significantly more susceptible to PIA.The variability in arthritis susceptibility between strains with the same MHC haplotype also suggests a powerful effect of non-MHC genes; the most and least sensitive rat strains are dark Agouti (DA) and E3[38].The ratio of CD25+/CD4+T cells in PIA rats is significantly higher compared with controls[39].The initial development and chronic stage of PIA can be improved by antibodies againstα,β-T cell receptors, indicating that the disease is T cell-dependent cell dependent[31].
4 Streptococcal cell wall-induced arthritis (SCWIA)
Bacterial cell wall peptidoglycans have many pro-inflammatory properties relevant to rheumatic diseases.Bacterial cell wall components can directly or indirectly stimulate or regulate immune or inflammatory mechanisms.For example, they can trigger acute inflammation by directly activating the bypass pathway of the complement system[40].Streptococcal cell wall (SCW)-specific antibody responses and cartilage and bone destruction are dependent on tolllike receptor (TLR)-4[41]and the progression from innate to adaptive immune involvement is consistent with a shift from TLR-2, to TLR-4 dependence.The direct activation of macrophages by SCW through TLR-2 during the acute inflammatory phase leads to an innate immune response against SCW fragments,a process that is gradually replaced by an adaptive immune response during the chronic phase as SCWspecific B and T cell responses develop[41].Notably,the structure of bacterial peptidoglycan (PG) plays a decisive role in chronic SCWIA.The degradation of only those PGs with A3αand A4αsubtypesin vivoleads to the generation of large pro-inflammatory bacterial cell wall (CW) fragments that persist in tissues[42].The T cell-dependent chronic phase of SCW arthritis is significantly suppressed in TLR9-/- mice,indicating that TLR9 is involved in regulating the T cell-dependent phase of chronic SCW arthritis[43].Furthermore, SCWIA clinically, histologically, and radiologically resembles RA in humans[44,45].Arthritis is symmetrical and involves peripheral, rather than axial joints.Histological and radiological findings have revealed a period of acute exudation, followed by erosive synovitis, which leads to cartilage and subchondral bone destruction, and fibrous ankylosis of the joints[15,46,47].Classical AIA and SCWIA are similar, but they differ in several ways; for example, AIA is monophasic, whereas SCWIA manifests as a biphasic clinical course.
Since infection and stress can affect the induction of arthritis, a pathogen-free environment and widely spaced housing are important for the successful SCWIA induction.The genetic and immunological mechanisms underlying the pathogenesis of SCWIA have shown that arthritis susceptibility is straindependent in rats, and the effects are hormonally mediated sex-linked.The incidence of SCWIA in female Lewis (LEW/N) rats is almost 100%, whereas male Lewis rats, MHC-compatible males, female Fisher (F344/N), WKY, BUF and other strains are relatively resistant and develop no, or less severe disease after exposure to SCW[48].Both arthritis-susceptible and resistant strains mount a vigorous mononuclear cell inflammatory reaction to SCW, but the response is mainly limited to the spleen of arthritis-resistant rat strains.These findings suggest that the host genetic background plays an important role in determining susceptibility to arthritis in this model[48,49].The development of chronic erosive polyarthritis in this model depends to varying degrees on the propagation, deposition, and persistence of SCW in synovial tissue, as well as the relative inability of susceptible rats to neutralize the pro-inflammatory properties of cell walls[50,51].However, given the biphasic clinical course of SCWIA, the development of chronic disease might reflect more than just toxininduced pro-inflammatory effects.
5 Collagen-induced arthritis (CIA)
Immunization with collagen type II (CII), the main component of articular cartilage, can induce CIA in sensitive strains of rodents (rats and mice) and nonhuman primates[52].After immunization, these animals develop autoimmune-mediated polyarthritis,which is very similar to human RA in terms of clinical,histological, radiological, and immunological characteristics and genetic linkage.Herbal formulas to treat RA such as Soufeng Sanjie Formula (搜风散结方), Guizhi Shaoyao Zhimu Decoction (桂枝芍药知母汤)[53,54]have been experimentally validated in CIA models.
B cells play an important role in the development of CIA, and B cell-deficient mice do not develop type II CIA[55].The immune response to CII is characterized by stimulating collagen-specific T and B cells to produce high titers of antibodies specific to immunogens (heterologous CII) and autoantigens (mouse or rat CII)[56].Immunization with various type II collagens sourced from different species and emulsified in CFA can induce CIA in susceptible mouse strains[57].However, the sensitivity of H-2q and H-2r mouse strains to these collagens differs[57].Although mouse models of CIA are popular because of their characterized genetic system and an abundance of immune substances, the rat model has many features that make it ideal[58,59].More rat than mouse strains are sensitive to CIA, and Wistar rats are the most sensitive.Wistar rats have a high incidence ( >83%) of severe arthritis, followed by Wistar Furth, and SD rats[18].Furthermore, rats injected subcutaneous(s.c.) with CII emulsified in IFA are prone to develop arthritis, thus avoiding the use of potent immune adjuvants, such as MTB, that provoke the immune system to mount an immune response to antigens other than CII.Rats that are highly responsive develop symptoms of arthritis sooner than mice (9 – 16 d vs.24 – 42 d), which decreases the length of experiments and the amount of time the animals need to be maintained.In addition, the larger rats allow easier procedures, such as extracting primary cells from arthritic joints and the accurate and reproducible measurement of claw volumes by hydroplethysmography.
6 Collagen antibody-induced arthritis (CAIA)
Collagen antibody induces arthritis with many characteristics of CIA, such as macrophage infiltration and polymorphonuclear inflammatory cells, but it does not affect T and B cell responses[60].Arthritis is induced in mice by the systemic administration of a mixture of arthritogenic monoclonal antibodies against CII.These antibodies target various types of type II collagen epitopes[61,62].The development of CIA mainly depends on MHC alleles, of which the MHC class II allele, I-Aq is highly susceptible, whereas CAIA is independent of these alleles.This suggests that CAIA is not associated with MHC-restricted T cells or T cell-dependent B cells[63].Therefore, CAIA cannot summarize the complexity of immune and tissue remodeling responses during human RA.
This model is induced by injecting type II collagen antibodies, then the incidence and severity of the disease is increased by a later injection of lipopolysaccharides.This indicates that bacterial toxins and autoantibodies play important pathogenic and nonspecific roles and contribute to the induction and progression of arthritis.Unlike CIA, CAIA is primarily a rapid model in which joint inflammation is mainly acute, appearing 24 – 48 h after immunization, with a peak at 5 – 7 d, followed by a decrease in the severity of arthritis over the next few days lasting about two weeks[56].Almost all mouse strains modeled in this way are susceptible.The influence of genes, age, sex,and effector cells in the pathogenesis of advanced arthritis has been investigated in this model.The inflammatory process of arthritis and common mechanisms involving many antibody-mediated diseases can also be investigated using CAIA models, and candidate drugs for controlling the stage of joint inflammation can be screened[60].
7 Proteoglycan-induced arthritis (PGIA)
This model of arthritis is specifically induced in BALB/c mice by immunization with deglycosylated human or canine chondroprotein polysaccharide[24,64].No other mouse strains or other laboratory animals such as rats, hamsters, guinea pigs, rabbits,and dogs are sensitive to PGIA.Clinical and histopathological evidence has shown that female BALB/c mice develop polyarthritis and ankylosing spondylitis resembling human RA when injected i.p.ABC fetal human chondroprotein polysaccharide without chondroitinase and CFA[24,65].The onset of arthritis takes a relatively long time and manifests approximately 28 days after immunization.The first external signs of joint inflammation are swelling and redness associated with edema of the synovial and periarticular tissues, and massive cell proliferation that peaks 7 – 9 w after immunization[66].Mononuclear cell infiltration, perivascular concentration, and small-vessel occlusion develop, and arthritis affects increasing numbers of joints.Repeated inflammatory episodes lead to the complete degradation of articular cartilage, bone erosion, and severely deformed peripheral joints[66].The proximal intervertebral discs of the lumbar spine and tail also become inflamed and degenerate.
The development of arthritis is based on crossreactive immune responses between immune allogeneic and autologous (mouse) PGs[67]and is associated with the production of autoreactive T cells and autoantibodies against murine PG[68,69].These autoantibodies enter joints and bind to cartilage PG,forming IgG immune complexes (ICs).The FcγR is associated with the development of PGIA[70-72].One of the main differences between PGIA and other arthritis models is its dependence on interferon (IFN)-γ,which is an important pro-inflammatory cytokine released by Th1 cells that is essential for the development of PGIA[67,73].IFN-γcan induce the expression of FcγRI and enhance the secretion of IgG2a through class conversion, whereas abundant FcγRI expression enhances IgG2a binding to IC[70,74,75].
8 Animal model of integrated diseases and TCM syndromes
TCM has great advantages in the treatment of RA.It is necessary to develop RA animal models based on TCM syndromes.It can not only provide a basis for the development of new drugs, but also provide basic research tools for the treatment of RA in TCM.Treatment of diseases based on syndrome differentiation is the core feature of the diagnostic and therapeutic practices of TCM, which offers a unique advantage in RA[76].Therefore, an animal model with characteristic TCM syndromes is needed to facilitate investigation into the effects of TCM.The relationship between RA and TCM symptoms is not simulated well by pure animal models of Western diseases.Therefore, only the development of animal models that comply with TCM evidence can truly reflect the effects of TCM.Animal models of integrated diseases and syndromes combine the benefits of mimicking “diseases” in modern medicine and “syndromes” in TCM.These are more appropriate animal models when the biological basis of syndromes has not been clarified.Pathogenic TCM factors have been added to AIA and CIA models based on characteristic TCM symptoms, such as wind-cold-damp[77],wind-damp-heat[78], kidney-deficiency[79], spleen-deficiency[80], and phlegm-stasis[81].In general, the development animal models of integrated diseases and syndromes has achieved good preliminary results compared with previous models, but further investigation is still needed.
At present, animal models of integrated diseases and TCM syndromes are only a simple superposition of several factors based on the “disease” of modern medicine and the “evidence” of TCM.Hence, unified modeling methods and clear criteria for judging modeling effects are not presently available.A successful animal model of integrated diseases and syndromes must be able to mimic the main cause of the symptoms created by the etiology, and the pathological manifestations caused by the etiology must have the characteristics of the symptoms of the model.Therefore, the development of animal models of integrated diseases and syndromes will require more understanding of the mechanism and evolution of combined diseases and evidence to achieve an appropriate model.
9 Discussion
RA is an autoimmune disease mediated by T cells,and the present development of animal models of RA tends to focus on specific factors.However, to create stably reproducible, standardized animal models that precisely reflect human RA is challenging.Being able to replicate animal models of RA is an important part of RA research, and a good RA model needs to meet the requirements of mature technology, simple methodology, low cost, and a high success rate.Here,we summarized the characteristics of all popular inducible RA animal models, potential biological mechanisms (Table 1), modeling methods, and common pathological features (Figure 2).Future models should be as close as possible to the human disease state, such that relevant mechanisms and clinical symptoms of RA pathogenesis can be investigated.Such models should also be strengthened from the perspectives of pathology and immunity.Modern medical imaging technology can be used to improve the accuracy of observations.The implications of TCM symptoms should be investigated, starting with the etiology and pathogenesis of symptoms, and these should fit modern medical etiology to ensure scientific and standardized model replication.Models of RA models that meet the requirements of western pathology and TCM diagnosis should be explored.
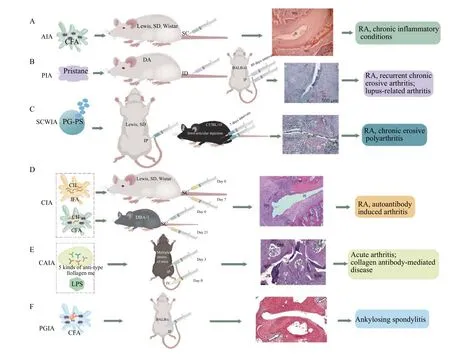
Figure 2 Creation of animal models and pathological features of different types of arthritis
Acknowledgements
We thank for the funding support from the Science and Technology Innovation Program of Hunan Province (No.XKJ [2021]43-2021RC4035), and was supported by the Hunan Furong Distinguished Scholar Program (No.XJT [2020]58) and the Chinese Academy of Engineering Academician LIU Liang’s Workstation of Hunan (No.XKXT [2020]34).
Competing interests
The authors declare no conflict of interest.
杂志排行
Digital Chinese Medicine的其它文章
- P-glycoprotein mediated interactions between Chinese materia medica and pharmaceutical drugs
- Traditional Chinese medicine compounds for the treatment of functional dyspepsia: an updated meta-analysis of randomized,double-blind, placebo-controlled trials
- Rational drug design, synthesis, and biological evaluation of novel N-(2-arylaminophenyl)-2,3-diphenylquinoxaline-6-sulfonamides as potential antimalarial, antifungal, and antibacterial agents
- Study on differential gene expression profile of serum exosomes in patients with acute cerebral infarction
- Effects of Fuke Qianjin Formula on hormones and their receptors and metabonomics study in uterine fibroids model rats
- Protective effects of Fufang Ejiao Jiang against aplastic anemia assessed by network pharmacology and metabolomics strategy
