Prevalence and risk factors of nonalcoholic fatty liver disease in patients with inflammatory bowel diseases: A cross-sectional and longitudinal analysis
2021-01-15PeterHoffmannVictoriaJungRouvenBehnischAnnikaGauss
Peter Hoffmann, Victoria Jung, Rouven Behnisch, Annika Gauss
Abstract
Key Words: Crohn’s disease; Ulcerative colitis; Inflammatory bowel disease; Nonalcoholic fatty liver disease; Nonalcoholic steatohepatitis; Ultrasound
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is a common disorder with a prevalence of 25% worldwide[1]and 22.9% in the German population. Its prevalence in Germany is expected to reach 26.4% by the year 2030[2]. In patients with inflammatory bowel diseases (IBDs) – consisting of Crohn’s disease (CD) and ulcerative colitis (UC) – an even higher NAFLD prevalence of up to 40% has been described[3-5].
The main risk factor for NAFLD is the metabolic syndrome[6]. Ultrasound of the liver is the most common diagnostic tool to detect NAFLD with a sensitivity of 85% and specificity of 94%[7]. Hepatic steatosis may progress to nonalcoholic steatohepatitis (NASH), which can result in the development of cirrhosis and hepatocellular carcinoma. NAFLD is associated with an increased overall mortality and independently predicts the risk for future cardiovascular disease events[8,9].
IBD patients develop NAFLD based on fewer metabolic risk factors in comparison with non-IBD NAFLD patients[10]. In IBD patients, hypertension, obesity, small bowel surgery, and use of corticosteroids at the time of imaging were associated with NAFLD, while among patients receiving anti-TNFα therapy, NAFLD has been shown to be less prevalent[10]. In contrast, another study found a high prevalence of NAFLD among IBD patients under anti-TNFα treatment[11].
Thus it may be assumed that the risk factors for NAFLD in IBD patients differ from those in the general population. Even underweight IBD patients showed an increased prevalence of NAFLD[12]. A history of bowel resection(s) and parenteral nutrition have also been described as risk factors for NAFLD[13,14]. Treatment with high doses of corticosteroids–as still frequently applied in IBD patients–has been related to hepatic steatosis as well[15].
The key goal of this study was to investigate the prevalence and risk factors of NAFLD in patients suffering from IBD. The superordinate aim of this project is to optimize preventive and therapeutic measures of NAFLD in the identified population at risk, and thus to reduce the incidence of NASH or hepatocellular carcinoma in IBD patients.
MATERIALS AND METHODS
Study design and data extraction
This is an uncontrolled, monocentric retrospective study including patients diagnosed with IBD at a South German tertiary referral center for the care of IBD. The Ethics Committee of the University of Heidelberg (protocol number: S-299/2018) approved the study. Inclusion criteria were as follows: (1) Age ≥ 18 years; (2) Diagnosis of IBD according to European Crohn’s and Colitis Organisation criteria[16]; (3) Therapy at the IBD outpatient clinic of the University Hospital Heidelberg; (4) At minimum one visit to the IBD outpatient department between June 1, 2014 and May 31, 2018; and (5) Availability of at least one documented abdominal ultrasound examination including profound assessment of the liver performed prior to May 31, 2018, as this date was defined as the cut-off time point for data acquisition. Exclusion criteria were: (1) Diagnosis of any infectious liver disease; (2) Suspected alcoholic fatty liver disease; or (3) Documented abuse of alcohol (daily intake of alcohol of > 10 g/d in women and > 20 g/d in men). All data were collected from computerized medical course records. The available daily consultation lists of the IBD outpatient clinic between June 1, 2014 and May 31, 2018 were screened, to identify eligible patients.
All data concerning NALFD diagnosis and grading of NAFLD severity were extracted from the liver ultrasound reports of the patients. NAFLD severity was categorized into four groups: (1) No steatosis; (2) Mild steatosis; (3) Moderate steatosis; and (4) Severe steatosis, as specified in the ultrasound report.
Demographic and disease-related parameters of all included patients were entered into a Microsoft Excel spreadsheet. As standard at the IBD outpatient department, all IBD patients who were under immunosuppressive treatment underwent a routine abdominal ultrasound every year. Patients who visited the clinic for the sole purpose of a second opinion usually did not receive an abdominal ultrasound examination. In cases of diagnosed NALFD, the patients were routinely advised about lifestyle modifications, including nutrition and physical training.
Definitions
The Montreal classification for CD and UC[17]was used to categorize disease extent. Disease activity was graded using the Harvey Bradshaw Index (HBI)[18]for CD patients and the Simple Clinical Colitis Activity Index (SCCAI)[19]for UC patients. A HBI and SCCAI of > 4, respectively, were considered to represent active inflammation. HBI and SCCAI scores have been routinely used at our outpatient clinic since 2017 to monitor disease activity and evaluate therapeutic effects. For data collected prior to 2017, the indices were retrospectively calculated on the basis of all written information available in the patients’ records.
Studied variables included age at diagnosis of IBD, age at index abdominal ultrasound, body mass index (BMI), sex, presence of diabetes mellitus, dyslipidemia or hypertension, smoking status, CD location and phenotype, extent of UC, laboratory results [plasma concentrations of aspartate aminotransferase, alanine aminotransferase, gamma-glutamyltransferase (γGT), C-reactive protein, white blood cell count, fecal calprotectin concentration], HBI, SCCAI, inflammation detected by intestinal ultrasound or colonoscopy, medications, history of intestinal surgery, parenteral nutrition, concomitant diseases, and infections.
A sonographically measured bowel wall thickness of > 3 mm in the small bowel and of > 4 mm in the colon were considered to represent inflammation. Endoscopically active disease was considered whenever the mucosa was not described as normal.
Liver ultrasound
In the cross-sectional component of this study, ultrasound results from May 31, 2017 through May 31, 2018 were included. In the longitudinal part of the study, all results of any ultrasound examination ever performed at our center in the included patients were considered.
Study endpoints
The primary study end points were (1) To determine the prevalence of NAFLD in the cross-sectional part of the study; (2) To reveal factors associated with changes of the presence and severity of NAFLD during the further disease course in the longitudinal part of the study.
The secondary aim of the study was to identify potential risk factors for NAFLD in the cross-sectional component of the study.
Statistical analyses
All statistical analyses were performed using “R” Version 3.5.2 (2018-12-20). Normality of data distribution was first assessed using the Shapiro-Wilk test. None of the data were normally distributed, so the results were calculated as medians and interquartile ranges.
To identify potential risk factors for NAFLD, the Mann-Whitney-U-test and Kruskal-Wallis-test were used for ordinal and continuous variables, andX²-tests for categorical variables. For the multivariable assessment of risk factors for NAFLD, a logistic regression analysis was performed. A backwards elimination was used with the following parameters: BMI, age, sex, diabetes mellitus, hypertension, current smoking, dyslipidemia, activity index showing inflammation (HBI and SCCAI), elevated C-reactive protein level, inflammation in colonoscopy, previous bowel resection(s), as well as therapy with steroids, azathioprine, infliximab, adalimumab, vedolizumab, ustekinumab, mesalazine or sulfasalazine. A linear mixed model for repeated measurements was applied for the identification of potential risk factors which influence the changes in NAFLD: The change of NAFLD status was the dependent variable, while the previously identified and known confounders of BMI, age, sex, diabetes mellitus, hypertension, dyslipidemia, current smoking, and use of steroids were fixed factors. Parameter estimates were determined together with 95% confidence intervals and associatedPvalues.
In the linear mixed model for repeated measurements, all ultrasound examinations were included. The model considered the different numbers of ultrasound examinations per patient as well as changes in clinical, laboratory and treatment parameters and their impact on NAFLD. Because of the exploratory character of the study, there was no adjustment for multiple testing. The interpretation ofPvalues is descriptive.Pvalues < 0.05 were considered as statistically significant.
RESULTS
Characteristics of Crohn’s disease patients
651 different CD patients attended the IBD outpatient clinic between June 1, 2014 and May 31, 2018. Among these, 184 patients did not undergo any ultrasound examination, which was mainly due to their receiving a second opinion for therapy of CD. Exclusion of 22 patients was due to the diagnosis of other liver diseases, and five patients due to alcohol abuse. Thus, 440 CD patients who underwent at least one abdominal ultrasound examination were included. Among these 440 patients, 138 patients were excluded from the cross-sectional part of the study because they did not undergo any ultrasound examination between May 31, 2017 and May 31, 2018, leaving 302 patients included in the cross-sectional part of the study. In the longitudinal part of the study, 1620 abdominal ultrasound results from 440 patients were included. Figure 1A shows a flow diagram of all patient inclusions and exclusions.
Forty-nine percent of the included CD patients in the cross-sectional study were male, the median age at the time of the ultrasound examination was 42 years, and the median BMI was 24.5 kg/m².
Results of the cross-sectional study in CD patients
In the cross-sectional study, fifty-two percent of the CD patients did not suffer from NAFLD. Mild NAFLD was revealed in 18%, moderate NAFLD in 16%, and severe NAFLD in 14% of the patients.
Comparison of demographic and disease-related parameters between the subgroups with and without a diagnosis of NAFLD determined by the ultrasound results is shown in Table 1.
The BMI differed significantly between the subgroups: 23.0 kg/m² was the median BMI of patients without NAFLD as compared to 28.0 kg/m² in patients with NAFLD (P< 0.001). Age at abdominal ultrasound was 35 years in patients without NAFLD as compared to 49 years in patients with NAFLD (P< 0.001). An increased HBI > 4 was found in 15% of the patients without NAFLD, compared to 40% with NAFLD (P< 0.001). Other significant differences were revealed in dyslipidemia, hypertension, γGT, disease duration, history of bowel resection(s), and treatment with sulfasalazine. Laboratory results and endoscopic or ultrasound results suggesting active inflammation were not associated with the presence of NAFLD, while elevated plasma liver enzyme concentrations tended to be more frequent in NAFLD patients (P= 0.069).

Table 1 Comparison of baseline characteristics between the subgroups of patients with nonalcoholic fatty liver disease versus those without nonalcoholic fatty liver disease in Crohn’s disease
Using a multivariable logistic regression model, age at abdominal ultrasound, increased HBI, and increased BMI were identified as independent risk factors for NAFLD in CD patients (Table 2).
Using a multivariable logistic regression model considering BMI and disease duration, disease duration was also significantly associated with NAFLD (P< 0.001). These results were evaluated in a separate model, as age and disease duration are too similar to be included in the same model.
Time-dependent course of NAFLD and relation to CD therapy
In the 440 included CD patients, 1620 abdominal ultrasound examinations were performed (Figure 1A). The minimum number of examinations per patient was one, while the maximum number of examinations per patient was 12 (Table 3). Of 1180 time intervals, a NAFLD improvement was found in 141 time intervals (11.9%), while NAFLD deteriorated in 159 intervals (13.5%), 880 time intervals showed no change (74.6%). The mean length of a time interval was three years and seven months. Using a linear mixed model for repeated measurements, the parameters at the time intervals between the ultrasound examinations were inspected. In Table 4, the results of the linear mixed model for repeated measurements analyzing the NAFLD status are shown.
First, potential confounders as BMI, age, sex, diabetes mellitus, hypertension, dyslipidemia, current smoking, and steroid use were included in the model. BMI [Odds Ratio (OR): 1.32,P< 0.001] age (OR: 1.07,P< 0.001) and dyslipidemia (OR: 10.82,P= 0.049) were statistically associated with progression of NAFLD. In a second step, an additional linear mixed model including all parameters plus BMI and age was calculated. Increased HBI, inflammation in colonoscopy, history of bowel resection(s), and azathioprine use were significantly associated with NAFLD progression.
Characteristics of ulcerative colitis patients
400 different UC patients attended the IBD outpatient department between June 1, 2014 and May 31, 2018. Of these, 128 patients suffering from UC did not undergo any ultrasound examination at our clinic mainly due to the fact that they came to receive a second opinion. Sixteen patients were excluded due to other liver diseases, and two patients due to alcohol abuse. Thus, 254 patients who underwent more than one abdominal ultrasound examination at the IBD outpatient clinic were included in the study. Of these 254 patients, 101 patients were excluded from the cross-sectional part of the study because they did not undergo any ultrasound examination between May 31, 2017 and May 31, 2018. Thus, 153 patients were included in the cross-sectional part of the study. In the longitudinal part of the study, 847 abdominal ultrasound results from 254 patients were considered. Figure 1B shows a flow diagram of inclusions and exclusions for all UC patients.
Fifty percent of the UC patients included in the cross-sectional part of the study were male, the median age at the time point of ultrasound was 40 years, while the median BMI was 25.4 kg/m².
Results of the cross-sectional study in UC patients
In the cross-sectional study, fifty-six percent of the included UC patients showed no signs of NAFLD, while mild NAFLD was diagnosed in 20%, moderate NAFLD in 16%, and severe NAFLD in 8%.
Demographic and clinical parameters were compared between the patients who were diagnosed with NAFLD and those who were not. Table 5 demonstrates the differences of demographic and clinical parameters of patients with NAFLD compared to patients without NAFLD.
The BMI diverged significantly between patients with NAFLD and those without: patients without NAFLD had a median BMI of 22.8 kg/m² as compared to 28.0 kg/m² in patients with NAFLD (P< 0.001). Age at abdominal ultrasound was 37.0 years in patients without NAFLD as compared to 48.5 years in patients with NAFLD (P= 0.005). In patients with NAFLD, hypertension and smoking in genereal were more common compared to patients without NAFLD (P< 0.001). An SCCAI > 4 was found in 14% of the patients without NAFLD as compared to 24% in those with NAFLD (P= 0.156). Another significant difference was revealed in plasma γGT concentrations, which were 23 U/L in NAFLD patients and 18 U/L in patients without NALFD (P= 0.007). There was no association of laboratory results as well as endoscopic or ultrasound results proving inflammation with the occurrence of NAFLD.
Making use of a multivariable logistic regression model, increased BMI was identified as the only independent risk factor for NAFLD in UC patients (Table 6).
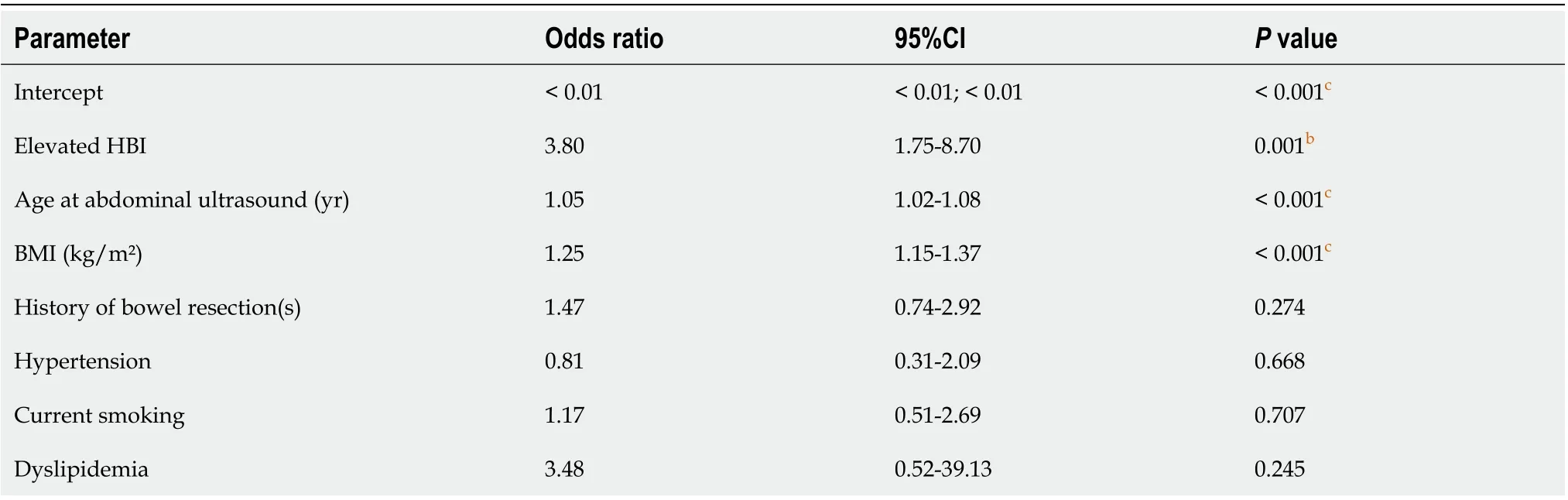
Table 2 Results from multivariable logistic regression model (backwards elimination) to identify risk factors for nonalcoholic fatty liver disease in Crohn’s disease patients

Table 3 Number of abdominal ultrasound examinations per patient
A multivariable logistic regression model considering BMI and disease duration did not identify disease duration as a risk factor of NAFLD in UC patients (P= 0.066).
Time-dependent course of NAFLD and relation to UC therapy
In the cohort of the 253 UC patents, 847 ultrasound examinations were performed in total (Figure 1B). The minimum number of examinations per patient was one, while the maximum number of examinations per patient was 12 (Table 3). In 593 time intervals, NAFLD improved over 77 time intervals (13.0%), while it worsened over 72 intervals (12.1%), and 444 time intervals showed no change (74.9%). The mean length of a time interval was three years and nine months. A linear mixed model for repeated measurements analyzing NAFLD change over time showed the results summarized in Table 7.
In a first step, the potential confounders BMI, age, sex, diabetes mellitus, hypertension, dyslipidemia, current smoking, and steroid use were included in the model. BMI and hypertension were statistically associated with progression of NAFLD (OR: 1.28,P< 0.001; OR: 2.89,P= 0.037), while age was not (OR: 1.01,P= 0.667). In a second step, a linear mixed model including all the different parameters plus BMI and age was calculated. Significant associations were revealed between progression ofNAFLD and endoscopically detected inflammation.
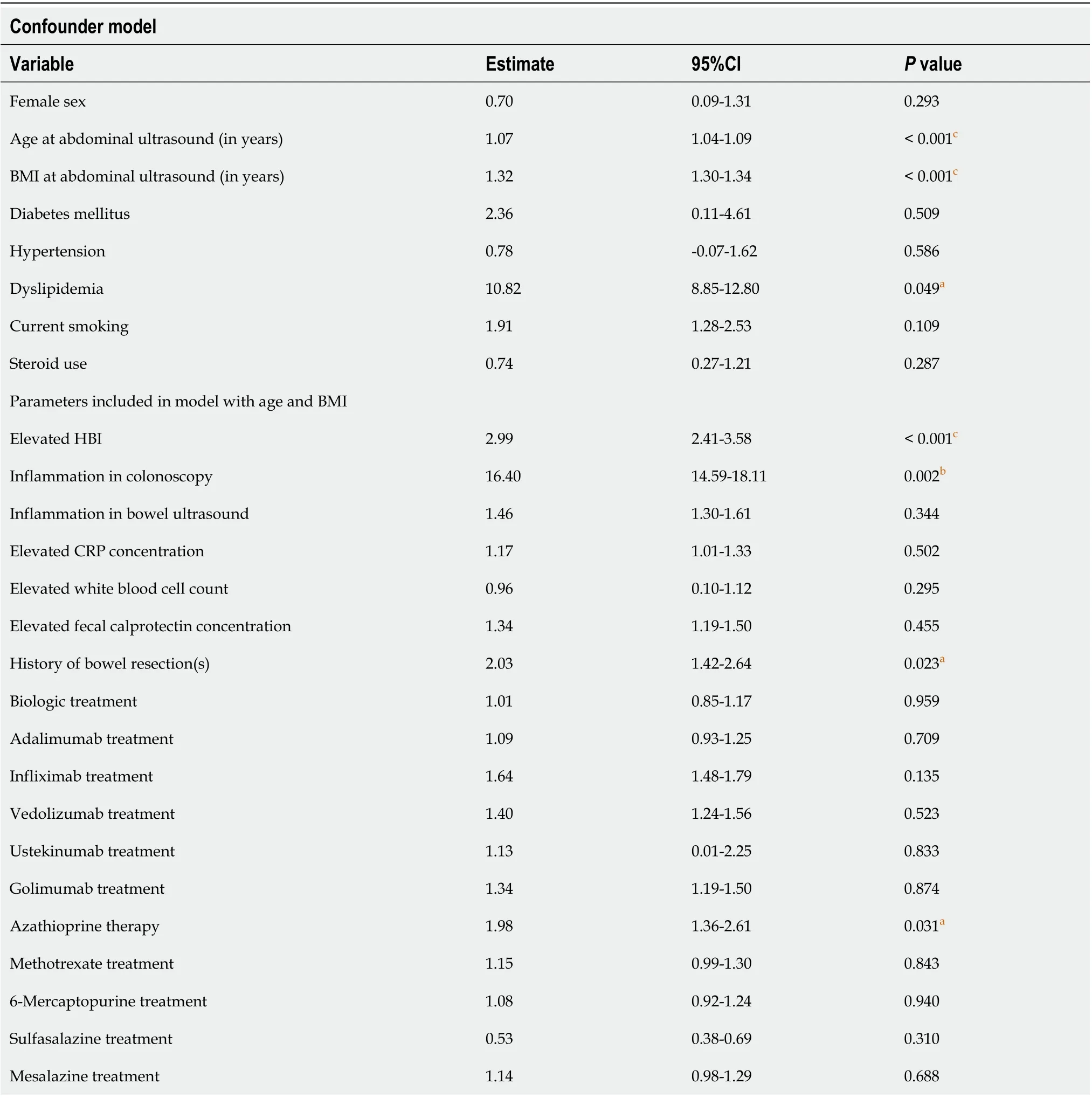
Table 4 Results of mixed model analysis to identify factors associated with nonalcoholic fatty liver disease in Crohn’s disease patients
DISCUSSION
Our study is one of the first large retrospective studies also including longitudinal analyses to explore changes of NAFLD severity during the course of IBD in up to 18 years of follow-up. As key findings, we confirmed that higher BMI and age are risk factors for the development of NAFLD in IBD patients, while clinical disease activity as expressed by higher HBI scores proved to be another independent risk factor in CD patients.
In patients with steatosis of > 33% of hepatocytes, liver ultrasound is a very reliable tool to detect steatosis. However, the method is not suitable to exclude mild fatty infiltration of the liver[20]. Therefore, we are aware that our results may underestimatethe prevalence of NAFLD.
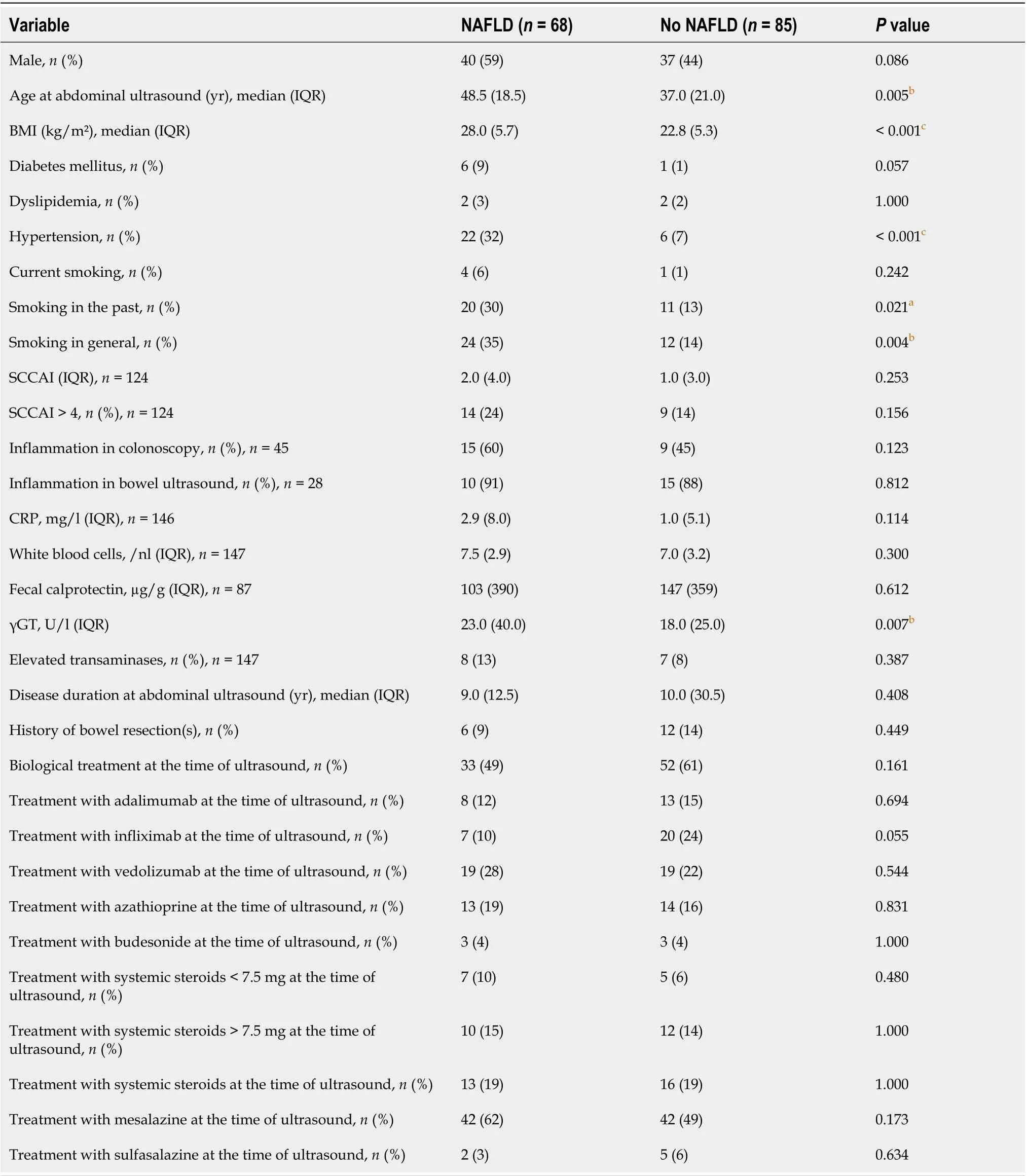
Table 5 Comparison of baseline characteristics between the subgroups of patients with nonalcoholic fatty liver disease versus those without nonalcoholic fatty liver disease in ulcerative colitis
In the cross-sectional part of the study, 157 (52%) of the CD patients had no NAFLD, while mild NAFLD was diagnosed in 54 (18%), moderate NAFLD in 50 (16%), and severe NAFLD in 41 (14%) of the patients. In UC patients, corresponding results were 85 (56%), 30 (20%), 25 (16%), and 13 (8%).
In the longitudinal part of this study, 2467 hepatic ultrasound results were included.Age, dyslipidemia and BMI in CD patients and BMI and hypertension in UC patients were identified as risk factors in the mixed logistic regression model. Also, in CD patients, higher HBI, endoscopic disease activity, history of bowel resection(s), and use of azathioprine were risk factors for NAFLD. In UC patients, endoscopic disease activity was significantly associated with NAFLD in the mixed logistic regression model.
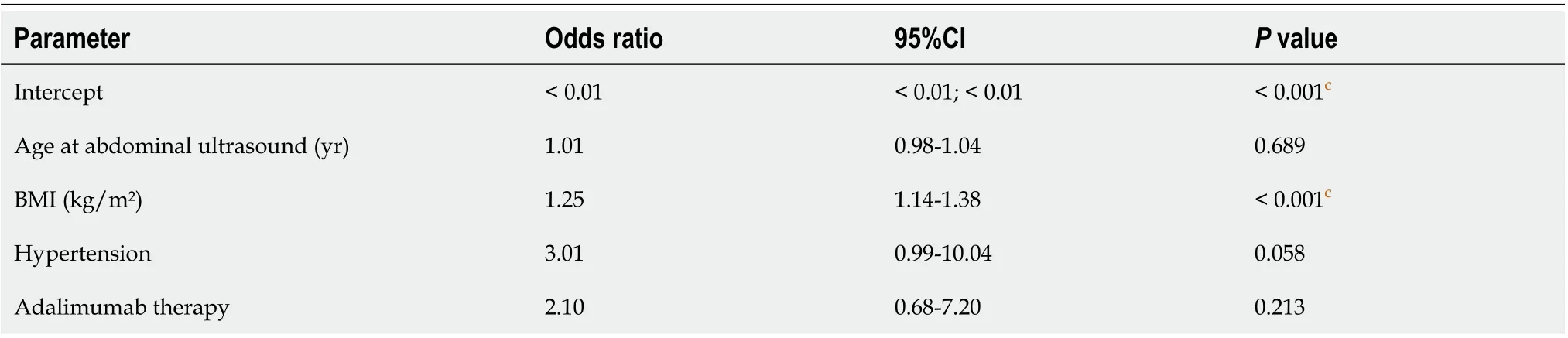
Table 6 Results from multivariable logistic regression model (backwards elimination) to identify risk factors for nonalcoholic fatty liver disease in ulcerative colitis patients
In a recent smaller cohort of Greek IBD patients, NAFLD was revealed in 14.2% of the included CD patients and in 26.2% of the included UC patients[21]. In comparison, our data identify a higher prevalence of NAFLD in both patient groups. The same is the case if we compare our results with those of Bargiggiaet al[3]from 2003. Concerning the Greek study, the discrepancy might be explained by diverging dietary habits. Looking at the study by Bargiggiaet al[3], it should be noted that nutrition has relevantly changed over the last 15 years. One more important factor to bear in mind is the improvement of IBD therapies and thus the better management of malnutrition in IBD patients over time.
A recently published meta-analysis included one study from Japan and 18 studies from Western countries: It found an overall prevalence of NAFLD in 27.5% of IBD patients and identified type 2 diabetes, hypertension, obesity, insulin resistance, metabolic syndrome, chronic kidney disease, methotrexate use, surgery for IBD, and longer duration of IBD as risk factors for its development[22].
We also identified greater age in CD patients as a risk factor for NAFLD. This finding may be due to a higher prevalence of metabolic risk factors with rising age.
In Germany, the mean BMI in the general population in the year 2013 was 26.5 kg/m² in females and 27.2 kg/m² in males[23]. Median BMI in our study as compared to the mean BMI in Germany was lower: 25.5 kg/m² in CD patients and 25.7 kg/m² in UC patients. That is why higher BMI alone cannot explain the high prevalence of NAFLD in our IBD patients. Furthermore, metabolic syndrome was only diagnosed in 23% of IBD patients in the United States[24].
In contrast to the results of the above-cited meta-analysis, we found in our cohort no association between methotrexate use and NAFLD, but between azathioprine use and an increase of NAFLD severity (OR: 1.98). In our cohort, azathioprine was prescribed far more frequently than methotrexate. All other drug therapies of IBD including corticosteroids were not associated with the course of NAFLD in our patients. Interestingly, in contrast, a study from 2013 identified corticosteroids as potential risk factor for NAFLD[10], while a more recent study showed no difference of steroid use in NAFLD and non-NAFLD IBD patients[25].
The positive correlation of clinical and endoscopic disease activity with NAFLD deterioration in the longitudinal part of the study leads us to the assumption that the systemic, extraintestinal inflammation caused by IBD may promote the development of NAFLD. Also, a history of bowel resection(s) resulted in a progression of NAFLD over time. This observation supports the hypothesis that bowel inflammation may aggravate NAFLD, as surgery is usually performed in patients with aggressive disease courses.
Adipokines also play a role in intestinal inflammation[26]. There may be a link between visceral fatty tissue, NALFD and intestinal inflammation, all factors influencing one another in a detrimental way. For example, apelin is upregulated in the intestinal epithelial cells of IBD patients and highly expressed in the mesenteric adipose tissue of Crohn’s disease patients[27,28]. Another study group showed that apelin is promoting hepatic fibrosis[29].

Table 7 Results from multivariable logistic regression model (backwards elimination) to identify risk factors for nonalcoholic fatty liver disease in ulcerative colitis patients
There are several limitations of the study. Due to its retrospective, uncontrolled nature, risk of bias is given. We focused on patients visiting a university IBD outpatient clinic. 184 CD and 128 UC patients did not undergo any abdominal ultrasound examination due to only getting a second opinion, which may produce a selection bias. Patients who did not receive immunosuppressive therapy did not undergo routine ultrasound examinations as well. Another limitation is the examiner dependency of the ultrasound examination results. Further limitations are that vibration controlled transient elastography or MR spectroscopy were not routinely performed to grade steatosis and fibrosis; nor was liver histology examined. Differentiation between NAFLD and NASH is only possible by histology. Also, nothing is known about the nutrition or the exact metabolic status of the patients. However, elevated plasma γGT concentrations and the tendency of elevated transaminases in CD patients in our study support the correct discrimination of NAFLD by liver ultrasound.

Figure 1 Flow diagram of inclusion and exclusion. A: Crohn’s disease patients; B: Ulcerative colitis patients.
One of the strengths of our study is the multitude of liver ultrasound examinations performed over a long duration. Thus, our study implies the possibility to reveal the influence of parallel therapies for IBD and the course of NAFLD under real-world conditions. Our data is relatively homogeneous, due to restriction to patients treated at a university center for IBD. In the cross-sectional component of our study, the data was retrieved exclusively from May 2017 until 2018, when data documentation at our center had been optimized by new standard operation procedures for the outpatient clinic.
CONCLUSION
We conclude from our results that NAFLD is very common in IBD patients, especially in older patients and patients with higher BMI. Disease activity and azathioprine use in CD may increase the prevalence of NAFLD. As visceral fat seems to perpetuate inflammation, IBD patients with NAFLD may be at risk of more aggressive IBD course, so they may require more intense anti-inflammatory treatment strategies. Our study provides a rationale for regular ultrasound screening of the liver in IBD patients. Further prospective studies are necessary to detect the role of disease activity and the influence of different medication in NAFLD in IBD patients.
ARTICLE HIGHLIGHTS

杂志排行
World Journal of Gastroenterology的其它文章
- Evolving role of artificial intelligence in gastrointestinal endoscopy
- Contrast-enhanced ultrasound in association with serum biomarkers for differentiating combined hepatocellular-cholangiocarcinoma from hepatocellular carcinoma and intrahepatic cholangiocarcinoma
- Colonic vitamin D receptor expression is inversely associated with disease activity and jumonji domaincontaining 3 in active ulcerative colitis
- Prognostic value of the preoperative fibrinogen-to-albumin ratio in pancreatic ductal adenocarcinoma patients undergoing R0 resection
- Infliximab is effective in the treatment of ulcerative colitis with dermatomyositis: A case report
- Challenges in COVID-19 drug treatment in patients with advanced liver diseases: A hepatology perspective
