重组酶聚合酶扩增:扩增原理及性能分析
2020-12-23李显明侯贤灯
李显明,郑 婷,高 露,李 峰,,侯贤灯,,吴 鹏,
(1.四川大学分析测试中心;2.化学学院,成都610064)
1 Introduction
DNA amplification is essential to most nucleic acid testing strategies,and thus is in the central of current medical diagnosis.Among the existing nucleic acid amplification protocols,polymerase chain reaction(PCR)is the most widely used technique,which in fact revolutionizes the modern molecular biology[1—3].However,the requirement of cyclic heating and cooling processes largely restricts its use in centralized laboratories.Isothermal nucleic acid amplification protocols are thus appealing,particularly for point-of-care testing(POCT)applications[4,5].Compared with other isothermal technologies,recombinase polymerase amplification(RPA)features rapid kinetics,high efficiency(up to 1012-fold amplification),low temperature and lyophilized format,thus is considered as an ideal platform for POCT[6—8].
Principally,RPA is generally similar to PCR,except that the thermal cycling process in PCR is replaced by recombinase and accessary proteins in RPA(Fig.1).Therefore,RPA can be potentially used in places that are currently performed with PCR[9].Since it was first proposed by Armeset al.[10]in 2006,the developments of RPA grow rapidly in the past decade[8].For diagnosis applications,although promising and commercializing in step[6],few RPA-based testings have been approved by authoritative organizations.On one hand,the batch robustness of the amplification is still limited due to difficulty in precise control of the primer recombination and extension,making the amplification susceptible to environmental variations.On the other hand,the low working temperature may produce multiple non-target amplification,thus resulting in unexpected noise signals.Currently,several strategies aimed at improving the performance of RPA are emerged,such as introducing labels,designing unique primer structures,and specific readout technologies for amplicons.Nevertheless,the ultimate performance in RPA promoting is still limited,which delayed its theoretical status in nucleic acid amplification.

Fig.1 Process of single cycle of recombinase polymerase amplification(RPA)(A)and polymerase chain reaction(PCR)(B)
Herein,we make a summary on RPA,with particular interest in the process of the primer recombination and the dynamic balance of ATP that guide the eventual performance.It should be noted that there are already several reviews introducing the principle and applications of RPA,but the process of primer recombination and its dynamic environment that heavily influence the performance is seldom discussed[6—8,11—14].In addition,the basic rules for selection of ideal primer pairs and nucleic acid probes are included,according to the RPA instructions and also recent references.Moreover,readout technologies for amplicons,particularly those with high specificity,are also discussed.
2 Mechanism and Dynamics of RPA Amplification
As shown in Fig.1,the amplification cycle of RPA starts with the binding of primer with recombinase to form the active nucleoprotein filaments,which quickly scan the dsDNA library to recognize the homologous sequence.Upon hybridizing with primer,the nucleoprotein filaments invade the selected dsDNA with a D-loop formation.Subsequently,DNA polymerase is loaded onto the 3′-end of primer and start to extend the primer through displacing the complementary parent strand.Finally,a new dsDNA containing one parent strand is formed.In a full cycle of RPA,a pair of primers would generate two same dsDNAs,thus resulting in exponential amplification.Apparently,RPA employs recombinase for primer loading,thereby eliminating the thermal cycling process in PCR.Since the primer recombination is a crucial and rate-limited step for RPA,it is easily suffering from fluctuations from the reaction environment as well as operations[15].
2.1 Recombinases-triggered Primer Recombination
Recombinases is the key protein that catalyzes the DNA exchange reactions between the primer and the homologous dsDNA.Generally,there are two types of recombinases(Table 1),namely uvsX from bacteriophage T4 and RecA fromEscherichia coli.To accelerate the formation of the nucleoprotein filament,ssDNA binding proteins(SBPs)and accessory proteins are typically used[15].In the uvsX system,gp32 and uvsY act as the corresponding SBP and accessory protein,respectively.While in the RecA system,it employs single strand DNA-binding protein(SSB),RecF and RecO.
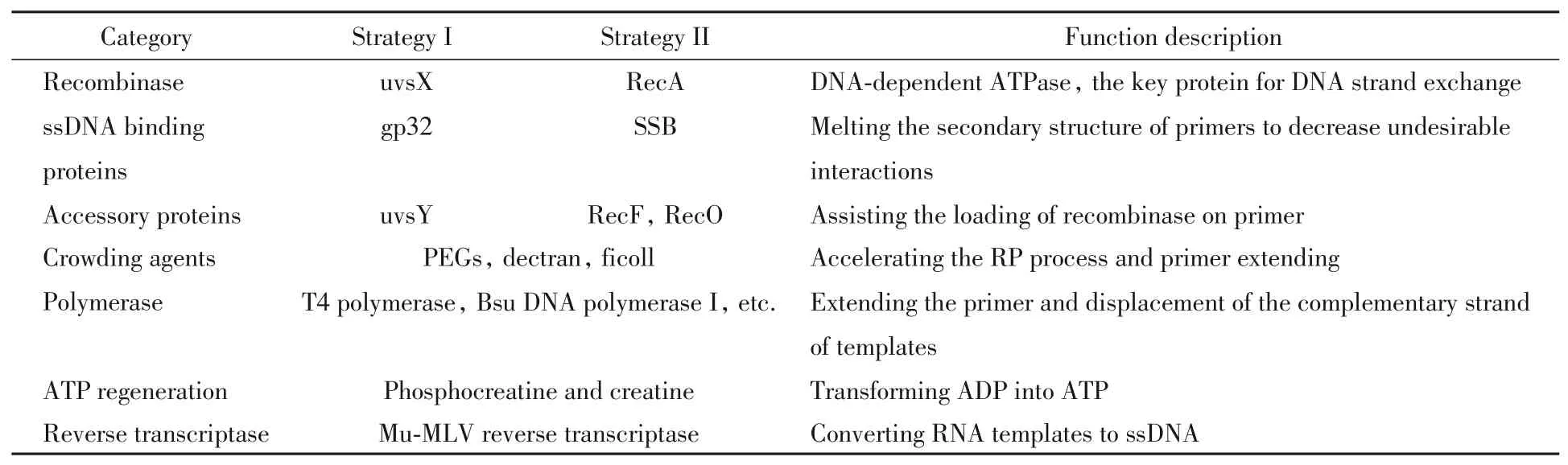
Table 1 Functional substances involved in recombinase polymerase amplification
The formation of the active nucleoprotein filament proceeds in three steps(Fig.2):(1)primer binding with SBPs;(2)loading of accessory protein;(3)recombinase displacing SBPs to form the active nucleoprotein filament.Without SBPs,primers tend to form undesirable secondary structures,which may restrict the access of recombinase[16—19].Besides,SBPs can weaken undesirable interaction between oligonucleotides(nontarget binding)[15,20]and facilitate the displacement of the outgoing strand during primer extension(binding with the displaced strand)[10],thus is crucial for RPA.However,the binding product of primer and SBPs is in fact not easily accessible to recombinase.The introduction of accessory protein changes the conformation of the above complex,thus weakening the above binding and facilitating the subsequent recombinase loading[21—24].Subsequently,the nucleoprotein filament formation begins with the nucleation of recombinase in the presence of ATP.Afterwards,the propagation of recombinase on the primer continues.The cooperative interaction between the loaded recombinase monomer can increase the affinity between recombinase and primer,thereby disassembling SBPs and forming active nucleoprotein filament.In this manner,homology search(>103bp per second)begins and strand exchange occurs after sequence matching,accompanied by ATP hydrolysis and release of related proteins for next cycle of amplification[25,26].
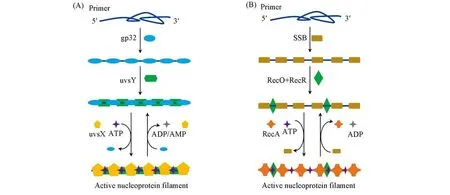
Fig.2 Nucleoprotein filament formation from uvsX(A)and RecA(B)
2.2 Factors Guiding the Efficient Primer Recombination
From Fig.2,it is apparent that the cofactor ATP fuels the recombination process,which can adjust the dynamic assembly(ATP-assisted)and disassembly of recombinase(ATP hydrolysis).Therefore,to maintain the recombination process,an ATP refresh system is indispensable.Such refresh is typically achieved through the creatine kinase-catalyzed reaction(with an equilibrium constant ofca.140)[27]:

However,for the uvsX system,hydrolysis of ATP can also generate AMP,which cannot be transferred back to ATP by the above reaction.Therefore,chicken myokinase is added to convert AMP to ADP[15]:

Since pyrophosphate(PPi)from ATP hydrolysis is detrimental for RPA,the uvsX system(high activity for ATP hydrolysis)is preferred for fast amplification(20 min),while the RecA system(low activity for ATP hydrolysis)is thus suitable for stable and long-time amplification(<30 min)[15,28].
Besides ATP,SBPs also contribute to the dynamic equilibrium of the recombination process.On one hand,SBPs is necessary for melting the secondary structure of the primer.On the other hand,it also competes with recombinase to inhibit the nucleation and propagation of recombinase.Owing to the higher affinity of SBPs with the primer over the recombinase,the active nucleoprotein filament may be deactivated due to the displacement of recombinase by SBPs,especially when the cofactor ATP cannot be efficiently refreshed[29].Accessory proteins can regulate the balance through destroying the cooperativity of the monomer SBPs[30],thus accelerating the nucleation of recombinase and maintaining the integrity of nucleoprotein filament[31,32].
2.3 Selection of DNA Polymerase and Other Reagents
Many DNA polymerases have been approved to be suitable for RPA,such as Sau polymerase,Klenow fragment ofE.coliDNA polymerase I,Bst DNA polymerase frombacillus stearothermophilus,and so on.Overall,there are three basic rules for the selection of the polymerase.First,polymerases with high processi-vity are desirable,as the loop or branch migration in RPA can cause the disassembly of polymerase with low processivity,resulting in poor efficiency and the accumulation of undesired short products.Second,the extra exonuclease activity of the polymerase should be taken into consider.Polymerases with 5′→3′exonuclease activity is not suitable for RPA,because they can digest the complementary strand of the template,resulting in low amplification efficiency.Although the 3′→5′exonuclease activity of polymerases may be beneficial for RPA since it can increase the fidelity of polymerase by incising misincorporations.However,the 3′→5′exonuclease activity of polymerases may also increase undesired extension,given that the unpaired bases from non-specific hybridization is excised.Last,the activity of polymerase in accessing the 3′-end of the recombination intermediates is important for efficient RPA.The recombination intermediates with multiple binding substances are different from those in PCR,thus may inhibit the loading of polymerases,especially in the uvsX system[15].Generally,two polymerases,one for accessing the 3′-end of the primer and the other for processivity,are recommended in RPA.
Besides the above proteins,there are also some ingredients that contribute to RPA,including crowding reagents,dNTPs,and buffer.Obviously,the reagents required for RPA are much more than those in PCR,which makes the RPA process complicated and difficult to be precisely controlled.Overall,compared with PCR,the standardization of RPA operation is relatively difficult,and thus demands further endeavor for future optimization.
3 Primer Design and Selection
Generally,the optimal length of RPA primer should be 30—35 bases,which is suitable for the formation of stable nucleoprotein filament.Longer primers can work,but possible intra-or inter-primer interactions at low temperature would increase primer artifacts.While the signal-to-noise ratio of shorter primers is generally higher,but the mean amplification kinetics is typically slow.Due to the three hydrogen bonds between G-C base pair,primer containing over 70%and less than 30%GC should be avoided.Particularly,providing a GC clamp at the 3′-end of primer may be beneficial[12].Although DNA-based primers are preferred for RPA,but RNA,PNA(peptide nucleic acid)and LNA(locked nucleic acid)can also work[15].Last,the length of the amplicon would influence the performance of primer.Although up to 1.5 kb amplicons can be obtained,shorter ones(80—400 bps,especially 100—200 bps)are preferred.
Non-targeted amplification,as typically encountered in other amplification protocols,is relatively serious in RPA due to the low working temperature.Elegant designing of the primer can alleviate non-targeted amplification to some extent.It’s suggested that primer with non-hydrolysable backbones at the 3′-end,such as phosphonothioate,morpholino and LNA,could efficiently reduce noise arising from mispairing[15].Self-avoiding molecular recognition(SAMRs)oligonucleotides,which form only one unstable H-bond between SAMRs primers but two stable H-bond with templates,can reduce the hybridization between primers but not the base pairing with the template[Fig.3(A)],thus largely avoiding the formation of primer dimers[33].
Undesired extension can also be reduced through incorporating short competitor oligonucleotides that can hybridize with the 3′-end of the primer[15].Generally,there are two design types for the competitor oligonucleotides:primer-independent and primer-dependent[Fig.3(B)].In the independent design[Fig.3(B)a],the competitor oligonucleotide is 3′-end blocked(e.g.,dideoxy sugar and biotin)to inhibit the extension of polymerase.Besides,a non-phosphate linkage at the ultimate and/or penultimate backbone is necessary to restrict the exonuclease activity of polymerase(3′→5′).In real working condition,the hybridization between the primers and the competitor oligonucleotides(OFF state)can efficiently suppress the noise.However,the short length of such competitor oligonucleotides(6—15 bases)may be also detached from the primer(ON state),thus endowing highly efficient amplification.While in the primer-dependent protocol[Fig.3(B)b],the unlabeled competitor oligonucleotide is directly linked to the 5′-end of primer.Subsequent formation of a hairpin structure can restrict undesirable extension,while the dynamic equilibrium from the short oligonucleotide length(6—10 bases)also permits efficient amplification.It should be noted that primer-dependent protocol can form plenty of self-primers in the next cycles of amplification,which is advantageous and preferable for RPA-based diagnosis.
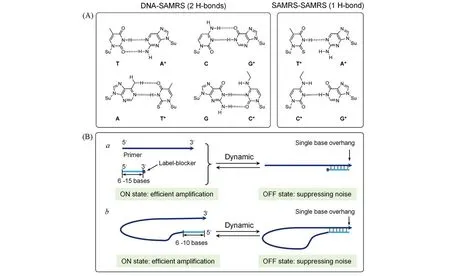
Fig.3 Primers for reducing nose products
For diagnosis applications,amplification efficiency and specificity are crucial.However,RPA is prone to suffer from subtle changes in primers,such as one base migration,addition,or excision.Although the primer can be designed in corresponding software,it is less efficient for RPA as compared with PCR,due to the complexity and incomplete understanding of the primer recombination process[34].Therefore,experimental verification seems to be inevitable for verification of the primer pair.
4 Readout Technologies
Currently,RPA kits are exclusively supplied by TwistAmp™.For highly sensitive detection of RPA amplicons,TwistAmp™has recommended two real-time fluorescent probes[exo probe and fpg probe,Fig.4(A)and(B)],the designing principle of which can be referred from the websites of TwistAmpTM.Besides,TwistAmp™also provides a lateral flow(LF)probe design suitable for end-point testing(POCT).Those probes have been widely used in RPA assays[7,8],but the performances of TwistAmp™probes are less predictable.
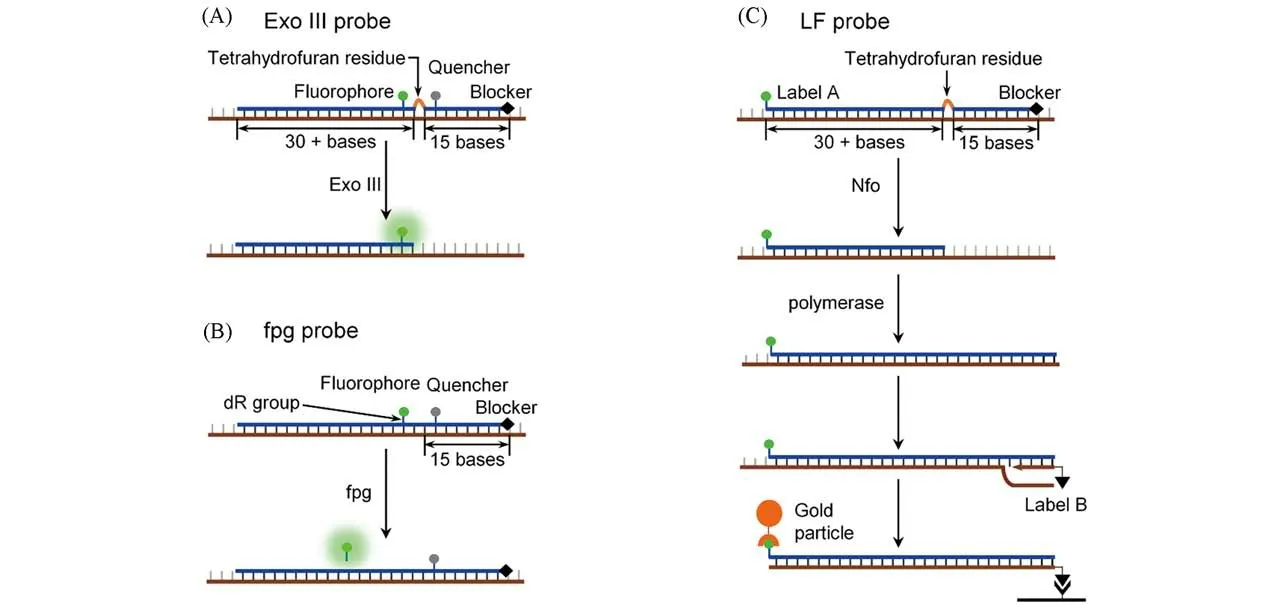
Fig.4 Nucleic acid probes for RPA
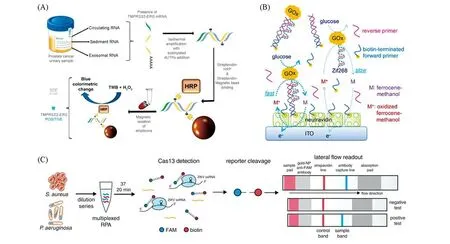
Fig.5 New amplicon testing methods
To ease the RPA readout,several new types of probes are emerged recently[35—43].Colorimetric readout based on labeled AuNPs or catalytic oxidation of chromogenic substrates is relatively simple and less instrument-dependent[44,45].The most popular mode for colorimetric assays is the“sandwich”type[Fig.5(A)],the testing results of which are probe to be influenced by the primer dimers.Electrochemical chemical detection approach usually has high sensitivity and easily be implanted in portable devices[Fig.5(B)],but differentiation signals between target amplicons and side products needed further attention[46].CRISPR/Cas-based system seems a perfect match with RPA,since the CRISPR/Cas probe added a further recognition for the target amplicons by base-paring between the sgRNA and target amplicon,which largely increased the specificity of RPA-based detection[47-49].In addition,some Cas proteins(e.g.,Cas13a)possesses high cleavage activity for the probes,resulting in significant sensitivity improvement of RPA-based assays[47,48,50].The successful implement of CRISPR/Cas-based lateral flow assay largely simplified the signal readout,thus facilitating such technology for POCT applications[Fig.5(C)][48].However,CRISPR/Cas-based assay currently is only an end-point detection for RPA.The complexity of both technologies thus demands further care for successful coupling.Other technologies,such as chemiluminescence[51—53]and Raman scattering[54,55],are also been explored in detection of RPA amplicons.Although the sensitivity of these strategies are appealing,the requirement of specific devices for signal readout are not desirable in the applications of POCT.
5 Performances of RPA-based Assays
The request for decentralized nucleic acid testing(NAT)increases greatly in recent years.Isothermal amplifications,which feature less instrument-dependent and easier access to NAT-based POCT as compared with PCR,thus draw much attention.There are several isothermal nucleic acid amplification protocols(Table 2),among which RPA exhibits obvious advantages,including short reaction time(20 min)and low amplification temperature(30—42℃)[10,56~73].In addition,RPA components can be lyophilized with high stability[7],thus paving the way to resource-limited areas.Another advantage is the robustness of the reagents,since RPA can tolerate potential inhibitors presented in samples or during sample preparations[8,14].

Table 2 Comparison of current isothermal amplification methods
The excellent performances of RPA have permitted successful detection of pathogenic bacterium[44,48,49]and genetically modified organisms and genetic alterations[74—77],with limit of detection(LOD)down to even 1 copy of DNA or RPA target.However,it should be noted that the LODs and reaction time varied with the primer and the length of amplicons,thus demanding more effort for improving and standardizing the performance of RPA.Thanks to the rapid developments of RPA in recent years,several RPA-based kits for pathogens,includingCampylobacter,Listeria monocytogenes,Red snapper,andSalmonella,are already commercially available.
Benefiting from its low-temperature amplification,RPA is easily accessible to portable devices for fielddeployable diagnostics,especially for resource-limited areas[78,79].Microfluidic chip is one of the ideal carriers for RPA-based portable diagnosis devices,because of its small size and multi-functionality[80—82].Particularly,disc-[83—86]and paper-based microfluidics[87,88]are promising due to their low cost and easy-to-automation.In addition,smartphone-based or specific signal-readout devices are also developed for field diagnosis[51,89].
6 Conclusions and Future Perspectives
Due to the similarity between RPA and PCR,RPA has the potential to supplement PCR in some applications.Meanwhile,RPA has unique advantages over other isothermal amplifications,such as low temperature,high efficiency,stable lyophilized format,and less instrument-dependent,which make RPA suitable and popular in the POCT area.In this review,we made a brief summarize on RPA from its basic principle to performance.We hope such information may be helpful for researchers to better understand RPA and promote its development and improvement.
To further improve the performances of RPA,here we proposed several critical issues and potential points.First,the recombination kinetics of recombinase is far from excellent understanding[17,90,91],which demands further endeavor of basic research.In this manner,ideal primer pair and probes that are as simple and efficient as those in PCR will be expected[92].For example,caged-ATP that cannot directly fuel RPA,was reported to be activated by light,which may be potentially useful in precise regulation of RPA dymamic[15,93].Second,the undesirable artifacts due to low amplification temperature should be avoided.Although methods such as SMARS primer or introduction of short competitor oligonucleotides are introduced,the performances are far from satisfactory.Thus,new approaches that can destabilize the undesirable hybrids or increase the selectivity of primer are demanded,especially in diagnosis applications.Third,the performances of RPA are susceptible to multiply factors,and improving the robustness of RPA is thus urgently needed.Third,optimization of reaction conditions or engineering relative proteins may be also important for improving the performance of RPA.For example,RecO and RecR can greatly enhance the recombination of RecA bothin vivoandin vitro[32,94],but not applicable in typical RPA assays[15].Therefore,the current working conditions may be far from optimal for RecA in PRA.Besides,regulating the activity of related proteins by protein engineering has already been reported[15,21,32],but not yet in PRA.Last,the introduction of microfluidics or other portable devices will make RPA easily accessible to POCT.However,the robustness of these devices and potential cross contamination in amplification should be considered.Thus,portable devices with stable and enclosed environment for RPA reaction and proper signal readout are preferred and recommended.
Overall,RPA may not replace PCR or other isothermal amplification techniques in recent years.However,the potential of RPA in diagnosis are already been demonstrated[95].With continuously development and improvement,we believe that RPA will ultimately mature as a benchmark technology in near future.
This paper is supported by the National Natural Science Foundation of China(No.21874093)and the Fundamental Research Funds for the Central China Universities(No.2018SCUH0075).
